ABSTRACT
Little or no attention has so far been paid to using sweet sorghum malt for commercial beer brewing. Thus, three sweet sorghum varieties (SSV) and four sorghum varieties (SV) were analyzed for brewing quality. Variations were observed in their thousand grain weights (22.8 to 58.7 g), grain moisture contents (12.5 to 20.5%), germination energies (99.0 to 100%) and germination capacities (99.7 to 100%). After 4-day germination, radicle lengths of seedlings were 2 to 5 fold of plumules. Remarkable variations existed in their water sensitivity (7.1 to 27.6%) and grain protein contents (7.0 to 11.8%). Malts moisture contents (8.6 to 13.8%), malting losses (16.3 to 26.0%) and malts protein contents (12.2 to 19.5%) differed among cultivars. Cold water extracts (CWE) (3.8 to 8.8%) and hot water extracts (HWE) (8.8 to 17.5%) varied with cultivars. HWE were 1.5 to 3-fold of CWE. Diastatic powers (DP) were substantially higher in SSV (123.7 to 136.7º) compared to SV (111.8 to 117º). Amyloglucosidase (AMG) activities were detected in SSV malts but not in SV. α-Amylases activities in both SSV and SV malts were about 2 to 4-fold of β-amylases. Generally, DP in SV malts = α-amylase + β-amylase activities. But DP in SSV malts >α-amylase + β-amylase + AMG activities, thus, suggesting synergism between the enzymes. SSV showed similar wort yields with SV. Reducing sugars in wort of SSV (12.3 to 15.6 mg/ml) were higher than those of SV (6.2 to 10.5 mg/ml). Malts and worts analyses suggest that SSV have greater beer brewing potentials than SV.
Key words: Diastatic power, amylase, amyloglucosidase, sweet sorghum, malt, wort.
The potential of sorghum as a viable alternative substrate for beer brewing, particularly in the tropics where barley does not thrive well, has been recognized (Palmer et al., 1989; Owuama, 1999). In Nigeria, improved sorghum varieties have been cultivated in large scale over several years to address brewery industry demands for sorghum. This approach has helped Nigerian government conserve large amounts of valuable foreign exchange reserves spent on importing barley malt. Presently, malts from sorghum varieties (Sorghum vulgare) are used for commercial beer brewing in Nigeria and little or no attention has been paid to using malts from sweet sorghum varieties (Sorghum bicolor [L] Moench). Traditionally in Northern Nigeria, sweet sorghum varieties
are cultivated in limited quantities, in small portions of land, usually close to farmer’s residence, and commonly harvested for their sugary stalks and consumed. Although, there are some reports on malting S. bicolor grains (Hassani et al., 2014; Bekele et al., 2012), much more extensive literature exists on large scale commercial cultivation of sweet sorghum varieties, because of their sugary stalks which are exploited for bioenergy and ethanol production (Regassa and Wortmann, 2014). Sorghum varieties with acceptable malt qualities such as, diastatic power, malting loss and total soluble nitrogen are desirable for brewing beer (Owuama, 1999). Diastatic power is a reflection of the activities of diastatic enzymes in malt that comprise activities of α-amylase, β-amylase and amyloglucosidase (AMG) (encompasses α-glucosidase and limit dextrinase) (MacGregor et al., 1999). Malts with high levels of diastatic enzymes are known to yield increased reducing sugar levels in wort and enhance its fermentability (Delcour and Verschaeve, 1987). α-Amylases (endo-acting) hydrolyze α-1,4-glycosidic linkages in starch molecules yielding dextrins, oligosaccharides, maltose and glucose. β-Amylases (exo-acting) hydrolyze β-1,4 linkages yielding beta-limit dextrins and maltose, while AMG or glucoamylases (gamma-amylases) (exo-acting) hydrolyze both α-1,4 and branching α-1,6-linkages yielding glucose. Limit dextrinase hydrolyses alpha-limit dextrins, amylopectin and beta-limit dextrins, and has been purified from sorghum malt (Hardie et al., 1976; Sanni and Fatoki, 2017). Alpha glucosidase in sorghum malt contributes to glucose production in wort by hydrolyzing terminal α-1, 4 linked D-glucose residues to release glucose (Agu and Palmer, 1997). Among the diastatic enzymes, AMG has been shown to exhibit synergistic activity with α-amylase in starch hydrolysis, yielding glucose (PreseÄki et al., 2013; Zhang et al., 2013). As well, limit dextrinase and α-glucosidase are known to have synergistic activity with β-amylase and α-amylase respectively, in solubilizing starch (MacGregor et al., 1999).
Due to the use of malts from sorghum varieties for commercial beer brewing with little or no attention paid to sweet sorghum varieties, this work therefore reports on the assessment of brewing potentials of some sweet sorghum varieties based on their grain characteristics, malt qualities and wort properties vis-à-vis those of sorghum varieties.
Three sweet sorghum varieties, sweet ‘Takanda’, sweet mace Maikanya, and sweet white Moskwa and 4 sorghum varieties, Farin dawa, Jigari, Fara fara, and Red Moskwa were obtained from sorghum farms in Girei and Guyuk, Adamawa State, Nigeria.
Nitrogen content of grain was determined using Kjeldahl method while protein content was obtained by multiplying the nitrogen content by 6.25. Germination energy, germination capacity and water sensitivity were determined using a modification (that is, at 30°C) of ASBC method (Anon, 1997).
Malts were produced (by steeping grains for 18 h, germinating for four days at 30°C and kilning at 55°C for then 6 h and 65°C until the rootlets became friable. Rootlets were removed by rubbing between palms), and percentage malting loss determined as described by Owuama and Asheno (1994).
Beta-amylase activity was determined by slight modification of diastatic method. Briefly, 0.1M ammonium oxalate (inhibits α-amylase activity) (Delcour and Verschaeve, 1987), was added into buffered starch solution and mixed prior to the addition of malt infusion. Ammonium oxalate was also added in the Blank correction solution. Titrations were done following Fehling’s (or Soxhlet) solution procedure and the malt β-amylase activity, in degree, was calculated (both ‘As is’ and ‘Dry basis’), using the methods of JECFA (Anon, 1971).
Amyloglucosidase activity was determined by slight modification of diastatic method. Briefly, 0.5 × 10-3 mg/ml mercuric chloride and 0.1M ammonium oxalate were added into buffered starch solution and mixed prior to the addition of malt infusion. Mercuric chloride and ammonium oxalate were also added in the Blank Correction Solution. Titrations were done following Fehling’s (or Soxhlet) solution procedure and the malt amyloglucosidase activity, in degree, was calculated (both ‘As is’ and ‘Dry basis’), using the methods of JECFA (Anon, 1971).
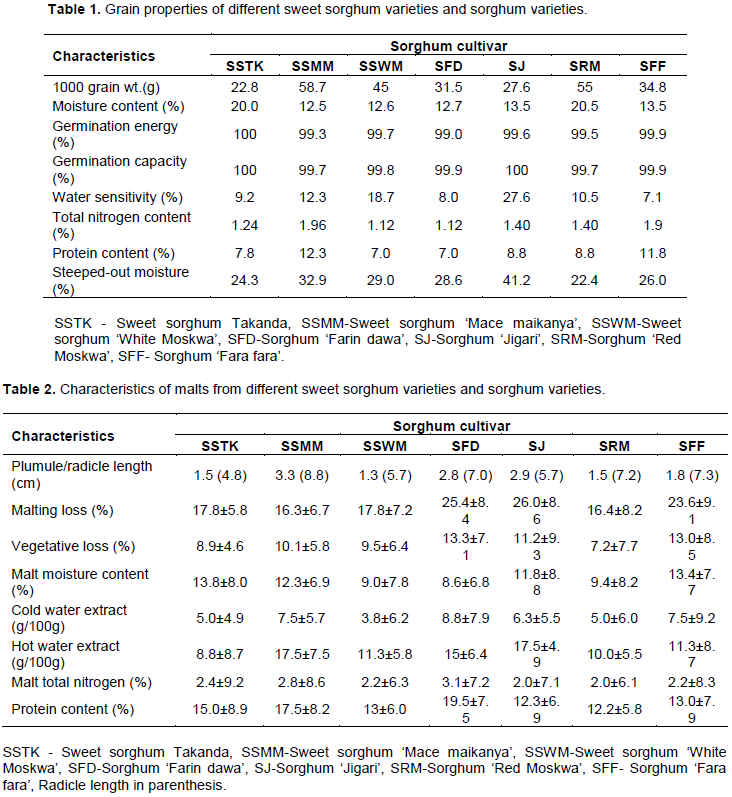
The results are the mean values of three experiments. The different varieties of sorghum showed varying grain properties (Table 1). The thousand grain weight of both SSV and SV ranged from 22.8 to 58.7 g, germination energy (GE) (measures percentage of grains expected to germinate fully at the time of test) fell between 99.3 and 100%, and germination capacity [GC] (used to determine if seeds that did not germinate in the GE test are dormant or dead, that is, measures percentage of viable corns in a sample) was within 99.7 to 100 %. Water sensitivity of the grains varied among SSV (9.2 to 18.7%) and SV (7.1 to 27.6%). The SSV grains showed percentage moisture contents of 12.5 to 20% while those of SV were between 13.5 and 20.5%. The total nitrogen contents of SSV varied slightly from those of SV, and fell within 1.12 to 1.96%, while their proteins values were between 7.0 and 12.3%. Percentage steep-out moisture varied considerably with different SSV and SV and ranged from 22.4 to 41.2% (Table 1).
Sorghum malts characteristics varied among SSV and between SSV and SV (Table 2). Vegetative outgrowths or sprouts after 4-day germination period of the grains revealed remarkably different plumule and radicle lengths for the various SSV and SV. The radicles were about 2 to 5-fold longer than the plumules among SV and about 3 to 4.5-fold longer among SSV. Vegetative outgrowths in both SSV and SV showed no clear relationship with the size of grains (as reflected by 1000 grain weight), vegetative loss and malting loss (metabolic plus vegetative loss). However, the vegetative loss correlates with DP (dry basis) of SSV, but not with those of SV (Table 3). The percentages malting loss differed among the varieties and generally lower among SSV (16.3 and 17.8%) than those of SV (16.4 to 26.0%). Percentage malt moisture content of SSV and SV showed virtually similar variations, and ranged from 9.0 to 13.8%. HWE and CWE (which are soluble products including sugars and amino acids of enzyme hydrolysis from malting process) varied with the SSV and SV. There were substantial differences between CWE and HWE of malts among various sorghum cultivars. HWE values were about 1.5 to 3 fold higher than CWE in both SSV and SV. Percentage malt total nitrogen in both SSV and SV malts varied considerably between 2.0 and 3.1% while their proteins ranged from 12.2 to 19.5%. CWE apparently correlated with total nitrogen and protein contents in malts from SSV but not those from SV (Table 2).
In Table 3, the diastatic powers, α-amylases and β-amylases differed among and between SSV and SV. There were remarkable differences in diastatic powers as well as α-amylase activities of malts between various sorghum cultivars. SSV had higher diastatic powers (123.7 to 136.7°) compared with those of SV (111.8 to 117.0°). The α-amylase activities of SSV (71.8 to 83.2°) were greater than their β-amylase activities (26.6 -27.2°). Similarly, α-amylase activities in SV malts (78.8 to 85.2°) were higher than their β-amylase activities (25.2 to 28.0°). α-Amylase activities in SV were 70 to 75% of DP and substantially higher than 56 to 61% of DP in SSV. Also, β-amylase activities in SV were 22 to 25% of DP and slightly higher than 19 to 22% of DP in SSV. However, malts of SSV showed amyloglucosidase (AMG) or glucoamylase activities of 14.5 to 21.3° while no AMG activity was detected in SV malts. AMG activities in SSV malts were 12 to 16% of their DP. α-Amylases activities in both SSV and SV malts were 2 to 4-fold those of β-amylases. In SSV, α-amylases activities were 3.6 to 5-fold those of AMG while the β-amylase activities were 1.2 to 1.9 fold those of AMG activities. Generally, DP in SV malts were equal to α-amylase plus β-amylase activities, but in SSV, the sum of α-amylase, β-amylase and AMG activities were less than the DP.
In Table 4, the percentage wort yields (dry basis) of both SSV and SV fell within 93.0 and 95.4%, and were 1.2 to 1.5-fold of the percentage wort yields (wet basis) (61.2 to 78.7%). The reducing sugars in SSV (12.3 to 15.6 mg/ml) were remarkably higher than those from SV (6.2 to 10.5 mg/ml). As well, the total sugars in SSV (16.3 to 22.0 mg/ml) were substantially higher than those in SV (12.3 - 13.2 mg/ml). The non-reducing sugars ranged from 2.2 to 6.4 mg/ml and differed considerably in both SSV and SV, but had no clear correlation with reducing sugars. The total soluble nitrogen contents in SSV worts (0.16 to 0.19%) were slightly higher than those in SV worts (0.08 to 0.16%). Similarly, the protein contents in SSV worts (1.0 to 1.2%) were higher than those in SV worts (0.5 to 1.0%).
There were remarkable differences among and between SSV and SV thousand grain weights (22.8 g to 58.7 g), however they differed slightly from earlier observations (Subramanian et al., 1995), apparently due to varietal differences in grain sizes, storage period and conditions (Owuama, 1999). Thousand grain weights of the various sorghum grains did not correlate with percentage grain moisture content, and percentage steep-out moisture apparently due to morphological and physiological variations of the different sorghum cultivars (Badau et al., 2006). The variations in vegetative outgrowth or sprouts (plumule and radicle lengths) after germination from SSV and SV seedlings showed unclear correlation with the size of the grains (as reflected by 1000 grain weight), and this could be attributed to different physiological activities among the various cultivars of sorghum used in this work (Svenson et al., 2011). The percentage moisture contents which varied among and between SSV and SV grains (12.5 to 20.5%), compare favourably with earlier report by Owuama and Asheno (1994). The variations in moisture content of grains may be attributable to differences in sorghum cultivar, storage conditions, maturity and age of grains (Owuama, 1999). The grains percentage germination energies (99.3 to 100%) and germination capacities (99.7 to 100 %) fell within acceptable viability level for sorghum grains used in malting (Agu and Palmer, 2013). The water sensitivity for SSV (9.2 to 18.7%) and SV (7.1 to 27.6%), were less than 30% indicating that the grains were not water sensitive (Davidson et al., 1976; Anon, 1997). There seems to be no clear relationship between grain moisture content and water sensitivity among the different varieties of sorghum.
Higher percentage malt nitrogen as well as protein contents observed in the sorghum malts vis-à-vis the percentage grain nitrogen and proteins are consistent with earlier reports and are attributable to carbohydrase activity during germination resulting in increased nitrogen content (Agu and Palmer, 2013). Specific protein level and high grain size are usually required by maltsters to produce good quality malt from barley grains (Magliano et al., 2014). Malting loss of SV and SSV ranged from 16.3 to 26.0% and fell within those reported for sorghum malts (Owuama and Asheno, 1994; Subramanian et al., 1995). The lower malting loss among SSV is desirable for producing brewing malt as more carbohydrates are available for malt enzymes hydrolysis during mashing that would increase wort sugar content (Agu and Palmer, 2013). The malting loss among the different sorghum varieties showed no direct correlation with vegetative loss which considerably varied among SSV and between SSV and SV. As well, vegetative outgrowths showed no proportional relationship with vegetative loss. However, the vegetative loss correlates with DP of SSV malts, but not with those of SV. These observations may be due to physiological variations among different sorghum cultivars as partly reflected in different respiratory activity and sizes of vegetative outgrowths during germination. Imbibed sorghum grains are known to accumulate in their embryo higher levels of gibberellic acid (GA) which controls α-amylase and protein syntheses, and also the function of endosperm during germination (Shakoor et al., 2016).
Diastatic enzymes in malt comprise α-amylase, β-amylase and AMG or glucoamylases (α-glucosidase and limit dextrinase) (Evans et al., 2010). In sorghum malts, diastatic power has been shown to vary with varieties of sorghum and usually comprise mainly α-amylase and β-amylase (Muoria et al., 1998). However, in this work, DP of SSV comprised AMG, α-amylase and β-amylase activities and were substantially higher than DP of SV (comprising α-amylase and β-amylase activities). This difference is apparently due to the presence of AMG in SSV. Although a wide range of DP have been observed among varieties of sorghum (Subramanian et al., 1995), the higher DP in SSV may be attributed to AMG synergistic activity with amylases. Addition of AMG in mash has been shown to increase diastatic power and increase wort glucose and total fermentable sugars equivalents (Pozo-Insfran et al., 2004), apparently because of the synergistic activity between α-glucosidase and α-amylases (Wong et al., 2007), and between limit dextrinase activity and β-amylases activity (MacGregor et al., 1999). However, in SV malts, the sum of the activities of α-amylases and β-amylases, is virtually equal to DP, thus suggesting additive reaction. Although, there were remarkable differences in α-amylase activities of malts from SV (70 to 75% of DP) and SSV (56 to 61% of DP), the α-amylase activities in both sorghum cultivars were generally 2 to 4-fold those of β-amylase activities. This observation compares favourably with that of Taylor (1992). The observed β-amylase activities in SV (22 to 25% of DP) and in SSV (19 to 22% of DP) are consistent with the report of Aisien et al. (1983). In SSV malts, DP was 6 to 9-fold of AMG activities compared with activities of α-amylases (3.5 to 5-fold of AMG) and β-amylases (1.2 to 1.9-fold of AMG). The disparity in the DP of different SSV may be a manifestation of varying synergistic activities influenced by ratios of their AMG to amylases activities (Wong et al., 2007; MacGregor et al., 1999).
Diastatic power in SSV is directly proportional to wort yield (dry basis) apparently due to hydrolytic activities of diastatic enzymes breaking down mash carbohydrates and bringing more hydrolysates into solution. Generally, DP of both sorghum types showed correlation with the total soluble nitrogen (TSN) in wort although the nitrogen values were higher in SSV than SV. The higher TSN in the malts of SSV and SV compared with their worts is consistent with the release of nitrogen during mashing by carbohydrase activity (Agu and Palmer, 2013). However, no clear relationship was observed between DP and total soluble nitrogen in sorghum wort (Agu and Palmer, 2013). The variation in observations may be attributable to varietal differences among the sorghum cultivars used. HWE increased with total sugars in wort and this could be attributed to temperature of extraction for HWE which encouraged enzymic activities and consequently the hydrolysis of more malt starch and protein (Agu and Palmer, 2013). However, HWE had no clear relationship with malting loss or DP. These observations are consistent with earlier report by Subramanian et al. (1995). CWE related positively with non-reducing sugar in SSV but not in SV. It is not clear why it is so but it may be a reflection of the differences in the nature of their starch granules. Sweet sorghum varieties have been shown to have larger granule size, higher water solubility index, lower amylose content and lower swelling power than grain sorghum. As well, the amylose content of sweet sorghum is lower than that of grain sorghum (Ahmed et al., 2016).
In conclusion, SSV have higher DP and reducing sugars than SV. Unlike SV, SSV had AMG activity which apparently contributed to higher DP, perhaps due to synergistic activity with amylases. The α-amylases activities in malts of SSV and SV were virtually similar, and higher than the β-amylases activities in both sorghum cultivars. SSV generally had less malting loss compared with SV. Thus, SSV apparently have greater potential for producing quality malts for brewing beer than SV. It is expected that the production of good quality SSV malts will greatly reduce if not completely eliminate the need to combine barley malts with SV malts in brewing beer, as is presently the case in Nigeria. Eventual use of 100% SSV malt for beer brewing would inevitably enable Nigeria conserve foreign exchange reserve spent on barley malt importation. Given the use of SSV stalks for commercial production of sugar, the additional use of their grain for producing quality malt for beer brewing will invariably encourage farmers in Northern Nigeria to produce more SSV to meet industrial demands and consequently increase their profit margins.
The author has not declared any conflict of interest.
The author wish to acknowledge the technical assistance of Aguh S., Ishaku J., Ahinda D. J. and Thompson J. in carrying out this work. There is no research grant or any other funding for this research.
REFERENCES
|
Agu RC, Palmer GH (1997). α-Glucosidase activity of sorghum and barley malts. Journal of the Institute of Brewing 103(1):25-29.
Crossref
|
|
|
|
Agu RC, Palmer GH (2013). Evaluations of the potentials of millet, sorghum and barley with similar nitrogen contents malted at their optimum germination temperatures for use in brewing. Journal of the Institute of Brewing 119(4):25-29.
Crossref
|
|
|
|
Ahmed AM, Zhang C, Liu Q (2016). Comparison of physicochemical characteristics of starch isolated from sweet and grain sorghum. Journal of Chemistry 2016.
Crossref
|
|
|
|
Aisien AO, Palmer GH, Stark JR (1983). The development of enzymes during germination and seedling growth in Nigerian sorghum. Starch Starke 35(9):316-320.
Crossref
|
|
|
|
Anon (1971). Malt carbohydrase prepared at 15th JECFA, published in NMRS 50B (1972) and in FNP 52 (1992). An ADI 'not limited' was established at the 15th JECFA 1971.
|
|
|
|
Anon (1997). Simultaneous determination of germination energy, water sensitivity and germination capacity in barley. Journal of American Society of Brewing Chemists 55(4):179-182.
Crossref
|
|
|
|
Badau MH, Nkama I, Jideani IA (2006). Steep-out moisture, malting loss and diastatic power of pearl millet and sorghum as affected by germination time and cultivar. International Journal of Food Properties 9(2):261-272.
Crossref
|
|
|
|
Bekele A, Bultosa G, Belete K (2012). The effect of germination time on malt quality of six sorghum varieties (Sorghum bicolor) grown at Melkassa, Ethiopia. Journal of the Institute of Brewing 118(1):76-81
Crossref
|
|
|
|
Davidson D, Eastman MA, Thomas JE (1976). Water uptake during germination of barley. Plant Science Letters 6(4): 223-230.
Crossref
|
|
|
|
Delcour JA, Verschaeve SG (1987). Malt diastatic activity Part II: A modified EBC diastatic power assay for selective estimation of β-amylase activity and temperature dependence of the release of reducing sugars. Journal of the Institute of Brewing 93:296-310.
Crossref
|
|
|
|
Etokakpan OU, Palmer GH (1990). A simple di-amylase procedure for the estimation of α-amylase and diastatic activity. Journal of the Institute of Brewing 96:89-91.
Crossref
|
|
|
|
Evans DE, Li C, Eglinton JK (2010). The properties and genetics of barley malt starch degrading enzymes in Genetics and improvement of barley malt quality, pp. 143-189, (Springer: Berlin).
Crossref
|
|
|
|
Hardie DG, Manners DJ, Yellowlees D (1976). The limit dextrinase from malted sorghum (Sorghum vulgare). Carbohydrate Research 50(1):75-85.
Crossref
|
|
|
|
Hassani A, Zarnkow M, Becker T (2014). Influence of malting conditions on sorghum (Sorghum bicolor (L.) Moench) as a raw material for fermented beverages. Food Science and Technology International 20(6):453-463.
Crossref
|
|
|
|
MacGregor AW, Bazin SL, Macri LJ, Babb JC (1999). Modelling the contribution of alpha amylase, beta amylase and limit dextrinase to starch degradation during mashing. Journal of Cereal Science 29(2):161-169.
Crossref
|
|
|
|
Magliano PN, Prystupa P, Gutierrez-Boem FH (2014). Protein content of grains of different size fractions in malting barley. Journal of the Institute of Brewing 120(4):345-354.
Crossref
|
|
|
|
Muoria JK, Linden JC, Bechtel PJ (1998). Diastatic power and alpha amylase activity in millet, sorghum and barley grains and malts. Journal of American Society of Brewing Chemists 56(4):131-135.
Crossref
|
|
|
|
Owuama CI (1999). Brewing beer with sorghum. Journal of the Institute of Brewing 105(1):23-34.
Crossref
|
|
|
|
Owuama CI, Asheno I (1994). Studies on malting conditions for sorghum. Food Chemistry 49:257-260.
Crossref
|
|
|
|
Palmer GH, Etokakpan OU, Igyor MA (1989). Sorghum as brewing material. MIRCEN Journal of Applied Microbiology and Biotechnology 5:265-275.
Crossref
|
|
|
|
Pozo-Insfran DD, Urias-Lugo D, Hernandez-Brenes C, Serna-Saldivar SO (2004). Effect of amyloglucosidase on wort composition and fermentable carbohydrate depletion in sorghum beers. Journal of the Institute of Brewing 110:124-132.
Crossref
|
|
|
|
PreseÄki AV, Blaževic ZF, VasiÄ-RaÄki D (2013). Complete starch hydrolysis by synergistic action of amylase and glucoamylase: impact of calcium ions. Biotechnology and Bioprocess Engineering 36(11):1555-1562.
Crossref
|
|
|
|
Regassa TH, Wortmann CS (2014). Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenergy 64:348-355.
Crossref
|
|
|
|
Sanni DM, Fatoki TH (2017). Evaluation of malting properties and activities of here enzymes from sorghum (Sorghum bicolor) during malting. African Journal of Food Scence and Technology 8(6):90-98.
Crossref
|
|
|
|
Shakoor N, Ziegler G, Dilkes BP, Brenton Z, Boyles R, Connolly EL, Kresovich S, Baxter I (2016). Integration of experiments across diverse environments identifies the genetic determinants of variation in Sorghum bicolor seed element composition. Plant Physiology 170(4):1989-1998.
Crossref
|
|
|
|
Subramanian N, Sambasiva Rao N, Jambunathan R, Murty DS, Reddy BVS (1995). The effect of malting on the extractability of proteins and its relationship to diastatic activity in sorghum. Journal of Cereal Science 21:283-289.
Crossref
|
|
|
|
Svenson B, Denyer K, Field RA, Smith AM (2011). Role of α-glucosidase in germinating barley grains. Plant Physiology 155(2):932-943.
Crossref
|
|
|
|
Taylor JRN (1992). Mashing with malted grain sorghum. Journal of American Society of Brewing Chemists 50(1):13-18.
Crossref
|
|
|
|
Taylor JRN, Daiber KH (1988). Effect of calcium ions in sorghum beer mashing. Journal of the Institute of Brewing 94:68-70.
Crossref
|
|
|
|
Wong DW, Robertson GH, Lee CC, Wagschal K (2007). Synergistic action of recombinant α-amylase and glucoamylase on the hydrolysis of starch granules. Protein Journal 26(3):159-164.
Crossref
|
|
|
|
Zhang B, Dhital S, Gidley MJ (2013). Synergistic and antagonistic effects of α-amylase and amyloglucosidase on starch digestion. Biomacromolecules 14(6):1945-1954.
Crossref
|