ABSTRACT
Oral Candida infections are common among the people who are immunocompromised, with poor oral hygiene, diabetics and advanced age. Even though several effective antifungal agents are available for treating oral Candida infections, on prolonged and frequent treatment with these drugs, they may result in development of drug resistant organisms. This leads to looking for an effective, inexpensive and simple alternative. Therapeutic products of plants play an important role in human health since ancient times. The present study was conducted to determine the bioactive components from the ethanol extract of Aloe vera by gas chromatograph-mass spectrometer (GC-MS) and to evaluate the antimicrobial effects of A. vera extracts on Candida albicans. GC-MS analysis revealed that ethanol extract of A. vera contains 26 bio active compounds. The results of in vitro antimicrobial activity of ethanol, methanol and aqueous extracts of A. vera against Candida albicans showed high inhibitory growth in yeast after treatment with ethanol extract followed by methanol extract with an inhibition zone of 23 and 12 mm, respectively. Aqueous extract did not show any zone of inhibition. The minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) of the extracts were also determined. From this study, it is concluded that, A. vera has potent antifungal activity against C. albicans.
Key words: Aloe vera, gas chromatograph-mass spectrometer (GC-MS), antifungal, Candida albicans, minimum inhibitory concentration (MIC), the minimum fungicidal concentration (MFC).
The dimorphic fungus, Candida albicans is the normal inhabitant of oral microbiota in 30 to 50% of healthy people. The transition of C. albicans from normal flora to pathogenic organism is decided by various predisposing factors like suppression of immunity, poor oral hygiene, high carbohydrate diet, old age, infancy, dentures, nutritional deficiency, etc (Akpan and Morgan, 2002). Oral candidiasis can be treated with topical anti-fungal drugs, such as fluconazole and amphotericin B. Topical therapy is given as an oral suspension which is washed around the mouth and then swallowed by the patient. Recently, C. albicans develops resistance against these antifungal agents on long-term treatment and leads to the development of drug-resistant C. albicans (Azmi and Tamer, 2014). So it is high time to look for an e ffective adjunct antifungal therapeutic
strategy. Medicinal plants are the integral part of human life and rich source of novel drugs (Botelho et al., 2007). Aloe vera Linne or Aloe barbadensis Miller belongs to the family Liliaceae under which it comprises about 360 species. Historical evidence indicates that A. vera originated in the warm, dry climate of Southern and Eastern Africa, and was subsequently introduced into Northern Africa, the Arabian Peninsula, China, Gibraltar, the Mediterranean countries and the West Indies. It is perennial succulent xerophytes with thick leaves that supplies water for the plant to survive in dry areas for longer periods (Botelho et al., 2007). The Aloe plant has two parts, each of which produces substances that are completely varying in compositions and therapeutic properties. The inner portion is made up of parenchymal and forms the A. vera gel (or mucilage), a clear, thin, tasteless, jelly-like material. The other portion is made up of specialized cells known as the pericyclic tubules, which occur just beneath the outer green ring of the leaf. An exudate produced from that cells, consists of bitter yellow latex with powerful laxative-like actions (Heggers and Kucukcelebi, 1996).
It has numerous therapeutic potentials and it is claimed to cure ailments like wounds, burns, immune modulation, inflammation and has antiseptic, antimicrobial, antifungal and antiviral properties (Richa et al., 2014). More than 75 active ingredients from inner gel have been identified including vitamins, minerals, enzymes, sugars, anthraquinones “phenolic compounds, lignin, saponins, sterols, amino acids and salicylic acid” (Nandal and Bhardwaj, 2012). Active ingredients include vitamin B12, magnesium, manganese, copper, zinc, calcium, potassium, folic acid, sodium, chromium, selenium and amino acids. It also contains lipases and proteases, which are enzymes that help with digestion; long-chain polysaccharides that boost the immune system and detoxify the body; fatty carboxylic acids that work as an anti-inflammatory; saponins that work as anti-viral, anti-fungal and anti-microbial agents; emodin and aloin that work as pain relievers as well as many other powerful medicinal agents (Nandal and Bhardwaj, 2012). In a study conducted by Bernardes et al. (2012), it was demonstrated that A. vera fresh leaves plant extract can inhibit both the growth and the germ tube formation by C. albicans.
Hence the present investigation was conducted to determine the bioactive components from the ethanol extract of A. vera by GC-MS and to determine the antifungal activity of A. vera extract against C. albicans and also the minimum inhibitory concentration (MIC) and the minimum fungicidal concentration (MFC) of whole leaf A. vera extract.
Test microorganisms
The test organism, C. albicans (MTCC 227) was obtained from Microbial Type of Culture Collection & Gene Bank (MTCC), Institute of Microbial Technology, Chandigarh. The test strain was maintained on Sabouraud’s Dextrose agar plates (Hi‑Media Laboratories Pvt. Limited, Mumbai, India) at 4°C and sub cultured on to yeast extract, peptone, dextrose (YPD) broth for 24 h prior to testing. This fungus served as test pathogen for antifungal activity assay.
Preparation of A. vera extract
Fresh A. vera leaves were collected from Coimbatore, Tamilnadu, India. The whole leaf was chopped into pieces and air dried. All the dried parts of the leaves were ground into powdered form using mortar and pestle and solubilized with ethanol, methanol and water solvents at 0.2 g/ml concentration. These mixtures were maintained overnight in a shaker at 120 rpm for proper extraction of the active ingredients at (25°C) room temperature (Harbourne, 1991). The mixtures were then filtered using Whatman No. 1 filter paper (Pore size 0.47 µm). The solvents were evaporated using water bath at 40°C to ensure proper concentration and to avoid the influence of those solvents in the anti-fungal effect of A. vera.
Phytochemical screening of the extracts
The phytochemical screening of the extract was carried out in order to ascertain the presence of its secondary metabolites such as saponins, alkaloids, flavonoids, steroids, terpenoides, anthraquinones, phlobatanins, tannins, cardiac glycosides, glycosides and phenols by gas chromatograph-mass spectrometer (GC-MS) analysis.
GC-MS analysis
GC-MS analysis of this extract was performed using GC SHIMADZU QP 2010 system and gas chromatograph interfaced to a mass spectrometer (GC-MS) equipped with Elite-1 fused silica capillary column (Length : 30.0 m, Diameter : 0.25 mm, Film thickness : 0.25 μm composed of 100% dimethyl poly siloxane). For GCMS detection, an electron ionization energy system with ionization energy of 70eV was used. Helium gas (99.999%) was used as the carrier gas at a constant flow rate of 1.5 ml/min and an injection volume of 1 ml was employed (split ratio: 10). Injector temperature was 240°C and ion source temperature 200°C. The oven temperature was programmed from 70°C (isothermal for 2 min.), with an increase of 300°C for 10 min. Mass spectra were taken at 70 eV; a scan interval of 5 min with scan range of 40 – 1000 m/z. Total GC running time was 35 min. The relative percentage amount of each component was calculated by comparing its average peak area with the total areas.
The antimicrobial screening of the extracts
The antimicrobial activity of extracts was determined in vitro in response to the C. albicans. The activities were measured using agar well diffusion method and broth dilution method, as previously described by the Clinical and Laboratory Standards Institute (CLSI; formerly known as the National Committee for Clinical Laboratory Standards) (NCCLS, 1997; Fothergill, 2011).
Agar diffusion method
The organism was sub-cultured into prepared normal saline and incubated at 37°C for 30 min, the concentration of each organism was increased to form a turbidity that matched with 0.5 McFarland’s standard by visual comparison at which it was assumed that the number of cells was 1.5 × 108 CFU/ml. Inoculum containing 1.5 × 108 CFU/ml of fungal suspension to be tested was spread on Sabouraud’s dextrose agar plates with a sterile swab moistened with the fungal suspension. Subsequently, wells were then made using sterile cork borer of 6 mm in diameter on the surface of pre seeded agar plates and filled with 100 µl (0.2 g/ml) of each plant extracts and allowed to diffuse at room temperature for 2 h for proper diffusion. Wells containing the same volume of corresponding solvent served as negative control while standard antibiotic discs of fluconazole (50 mg/ml) used as the positive control. All the plates were incubated at 37°C for 24 h. The presence of zone of inhibition was regarded as the presence of antimicrobial action. From the inhibition zones seen, antimicrobial activity was expressed in terms of average diameter of the zones of inhibition measured.
Minimum inhibitory concentration (MIC)
The minimum inhibitory concentrations of the extracts were determined using broth micro dilution method approved by the National Committee for Clinical Laboratory Standards (M27- A) (NCCLS, 1997). The yeast were cultured overnight on Sabouraud’s dextrose agar (SDA) and then resuspended in normal saline to obtain a final concentration of 1.5 × 108 cfu/ml. 200 mg/ml of each of the extracts were reconstituted into Sabouraud’s dextrose broth and serially diluted using Sabouraud’s dextrose broth forming concentrations of 100, 50, 25, 12.5, 6.25 and 3.125 mg/ml. 0.1 ml of the cell suspension was inoculated into each of the tubes with varied concentrations. All the tubes were incubated at 37°C for 24 h. After incubation, the MIC was determined as the lowest concentration of extract for which there was no visible growth compared with the control (CLSI, 2008).
Minimum fungicidal concentration (MFC)
The MFC was determined by inoculating 0.1 ml of negative growth in MIC onto sterile SDA plates. The plates were incubated at 35°C for 48 h. The lowest concentration of plant extract that did not demonstrate growth of the tested organism was considered as the MFC; the negative control was a plate grown with media only (Ernst et al., 2002; Wiegand et al., 2008).
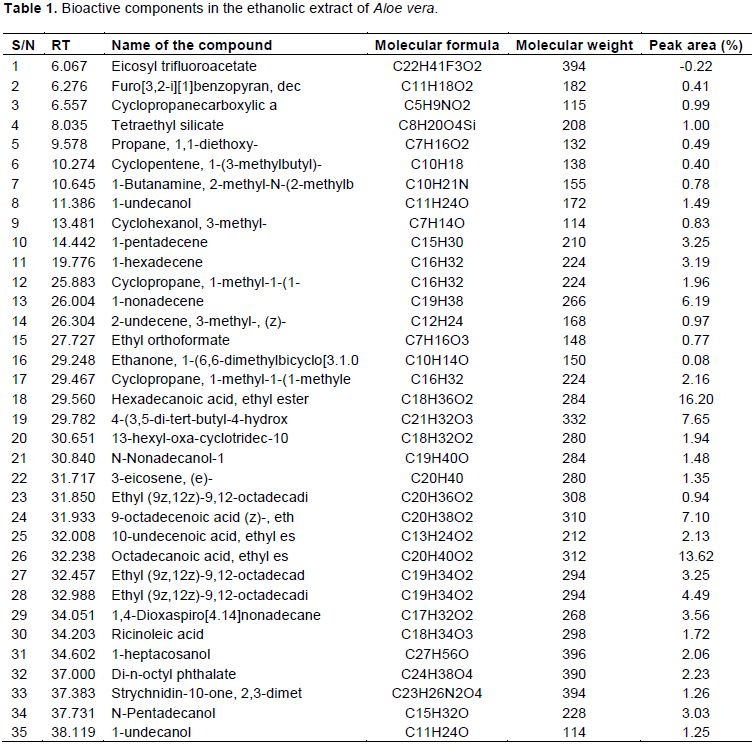
The components present in the extract was identified based on the spectrum obtained using the data base of National Institute of Standard and Technology (NIST). The name of the compound, retention time, molecular formula and structure were determined. The area percentage of each component was calculated by comparing its average peak area with the total areas.
GC-MS analysis of A. vera revealed the presence of 35 compounds, of which 26 were found to have biological activities. These compounds were confirmed based on their peak area (%), retention time, molecular formula and molecular weight (MW) and are represented in Table 1 and Figure 1. Their reported biological activities are given in Table 2. The prevailing compounds of ethanol extract of A. vera were hexadecanoic acid, ethyl ester (16.20%), octadecanoic acid, ethyl ester (13.62%), 4-3,5-di-tert-butyl-4-hydroxyphenyl)butyl acrylate (7.65%), 9-octadecenoic acid (z)-, ethyl ester (7.10%), 1-nonadecene (6.19%), ethyl (9z,12z)-9,12-octadecadienoate (4.49%), 1,4-dioxaspiro[4.14]nonadecane (3.56%), 1-pentadecene (3.25%), 1-hexadecene (3.19%), n-pentadecanol (3.03%), di-n-octyl phthalate (2.23), cyclopropane, 1-methyl-1-(1-methyle thyl)-2-nonyl- (2.16%), 10-undecenoic acid, ethyl ester (2.13%), 1-heptacosanol (2.06%), cyclopropane, 1-methyl-1-(1- (1.96%), 13-hexyl-oxa-cyclotridec-10-en-2-one (1.94%), ricinoleic acid (1.72%), 1-undecanol (1.49%), n-nonadecanol-1 (1.48%), 3-eicosene, (e)- (1.35%), strychnidin-10-one, 2,3-dimethoxy- (1.26%), 1-undecanol (1.25%), tetraethyl silicate (1.00%), cyclopropane carboxylic acid (0.99%), 2-undecene, 3-methyl-, (z)- (0.97%), cyclohexanol, 3-methyl- (0.83%), propane, 1,1-diethoxy- (0.49%), 1-butanamine, 2-methyl-n-(2-methylb (0.78%), ethyl orthoformate (0.77%), furo[3,2-i][1]benzopyran decahydro-, [3as-(3a.alpha.,6a.beta.,10as*)]- (0.41%), ethanone, 1-(6,6-dimethylbicyclo[3.1.0]hex-2-en-2-yl)- (0.08%) and eicosyl trifluoroacetate. A. vera is reported to contain carbohydrates, tannins, saponins, flavonoids and alkaloids (Yebpella et al., 2011). Phyto constituents have been found to inhibit bacteria, fungi, viruses and pests. The presence of phyto constituents in the A. vera extracts may be responsible for the antifungal activity of the plant (Cowan, 1999).
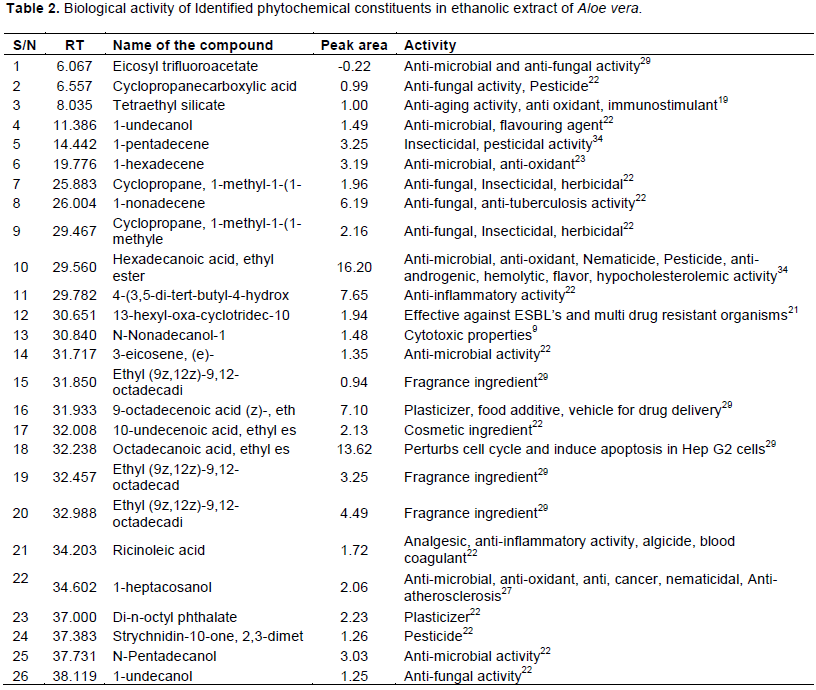
The antimicrobial activity of the ethanol, methanol and aqueous extracts against C. albicans had shown varying degrees of zones of inhibition and the results are depicted in Figure 2 and Table 3. The ethanol extracts exerted highest activity on C. albicans as compared to the methanol extract. The ethanol extract at the concentration of 0.2 g/ml showed 23 mm diameter zone of inhibition against C. albicans, methanol extract showed 12 mm diameter whereas aqueous extract showed no activity. The minimal inhibitory concentration of the ethanol, methanol and aqueous extracts against C. albicans was determined by broth dilution method and the results are depicted in Table 4. The MIC value of ethanol extract was found to be 50 mg/ml and methanol extract was 100 mg/ml. The MIC and the MFC were in the range of 25 to 100 mg/ml and 50 to 100 mg/ml, respectively. The growth of C. albicans in ethanol extract was inhibited at 25 mg/ml while the minimum concentration that showed no growth of C. albicans by methanol extract is 50 mg/ml.
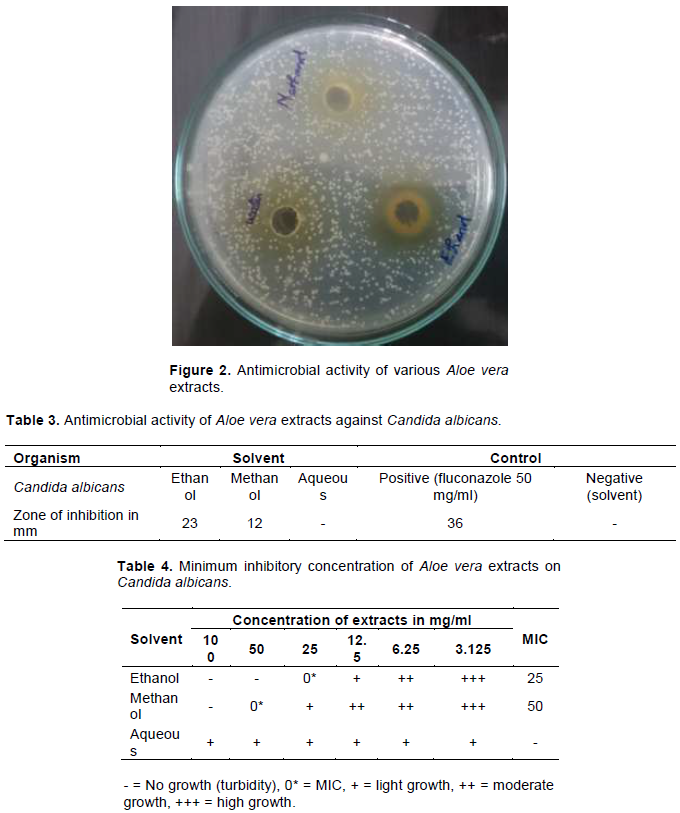
The present study on A. vera revealed the presence of medicinal active constituents. In the GC-MS analysis, 35 bioactive compounds were identified in the ethanol extract of A. vera based on the peak area, molecular weight and molecular formula. Of which 26 compounds were found to be biologically active. The prevailing compounds were hexadecanoic acid, ethyl ester (16.20%), octadecanoic acid, ethyl ester (13.62%), 13-hexyl-oxa-cyclotridec-10-en-2-one (1.94%), 9-octadecenoic acid (z)-, ethyl ester (7.10%), 1-nonadecene (6.19%) etc. These compounds were found to have anti-inflammatory (Langmead et al., 2004), anti-bacterial (Agarry et al., 2005), anti-fungal, anti-oxidant, immunomodulatory (Sun-A et al., 2010), analgesic (David, 1999) activity etc. Phytochemical compounds identified in plant material have been reported as having inhibitory action against C. albicans (Freeman and Beattle, 2008). Below the minimum inhibitory concentrations, growth of the microbes was observed ranging from light growth to high growth (Table 4).
The growth of C. albicans was inhibited by the ethanol and methanol extracts of A. vera with varying zones of inhibition while aqueous extract did not show any inhibitory activity. Ethanol extracts had a better minimum inhibitory concentration as compared to methanol and aqueous extract used in this investigation as reported by Nejad et al. (2014). The negative control using the corresponding solvent had no effect on the test organism. The positive control (Fluconazole) had wide effect against the organism. Fluconazole is preferred as positive control because it is a potent and selective inhibitor of fungal enzymes and also it does not have the hepatotoxicity as the imidazoles (Akpan and Morgan, 2002). A previous study conducted by Doddanna et al. (2013) demonstrated the antimicrobial effects of alcoholic extracts of tea leaves, onion leaves, onion bulb, A. vera, mint leaves and curry leaves against C. albicans and our results on ethanol extract was consistent with their results.
Oropharyngial candidiasis (OPC) is an opportunistic infection of oral cavity. Oral candidiasis can appear as erythematous patches or white, scrapable lesions. Oral cavity is colonized by C. albicans or other Candida species in 40-60% of healthy persons. Many factors contribute to the development of oropharyngeal candidiasis (OPC) including malnutrition, poor oral hygiene, dental malocclusion, and immunosuppression.
In immune compromised patients, Candida species can trigger a variety of disease manifestations ranging from localized mild oral lesion to a disseminated candidiasis (Berberi et al., 2015). Oral candidiasis caused by C. albicans is generally managed by treatment with fluconazole and amphotericin B. Prolonged or frequent treatment with these azole drugs results in the rise of drug resistant organism. Use of medicinal plants is considered as an adjunct antifungal therapeutic strategy nowadays. According to the World Health Organization (WHO), as many as 80% of the world’s people depend on traditional medicine for their primary healthcare needs. The development of indigenous medicines and the use of medicinal plants carry considerable economic benefits in the treatment of various diseases (Gunjan et al., 2013). Numerous studies have been carried out all over the world with A. vera and it has been reported that A. vera exhibits different levels of anti microbial property. The purified Aloe protein has been found to exhibit potent antifungal activity against Candida paraprilosis, Candida krusei and C. albicans (Das et al., 2011). Garnick et al (2008) evaluated a gel that combined allantoin, A. vera and silicon dioxide and its effects on aphthous ulcers of the oral cavity. A.vera tooth gel was as effective, and in some cases more effective than the commercial brands at controlling cavity-causing organisms (Namiranian and Serino, 2012). Sema and Suleyman (2009) reported that, a processed A. vera juice preparation showed inhibition of the growth of C. albicans.
This study has revealed the presence of many secondary metabolites in the leaves of A. vera due to which it showed antifungal activity against C. albicans. Thus, based on the results obtained in the present study, ethanol extracts of A. vera may be useful as a better alternative for the treatment of antimycotic resistant C. albicans with respect to oral candidiasis. It is suggested that more in vivo and clinical research works should be done in the future.
The authors have not declare any conflict of interest.
The authors thank the Principal and the Management of Karpagam University, Coimbatore, India for providing facilities to carry out the work. They also thank the Principal and the Management of PSG College of Arts and Science, Coimbatore, India for their consistent support and help during this study.
REFERENCES
|
Agarry OO, Olaleye MT, Bello-Michael CO (2005). Comparative antimicrobial activities of Aloe vera gel and leaf. African Journal of Biotechnology 4:1413-1414.
|
|
|
|
Akpan A, Morgan R (2002). Oral candidiasis. Postgraduate Medical Journal 78:455-459.
Crossref
|
|
|
|
|
Azmi MGD, Tamer AD (2014). What Makes Oral Candidiasis Recurrent Infection? A Clinical View. Journal of Mycology Article ID 758394.
|
|
|
|
|
Berberi A, Noujeim Z, Aoun G (2015). Epidemiology of Oropharyngeal Candidiasis in Human Immunodefi ciency Virus/Acquired Immune Deficiency Syndrome Patients and CD4+ CountsJournal of International Oral Health 7(2):1-4
|
|
|
|
|
Bernardes I, Felipe Rodrigues MP, Bacelli GK, Munin E, Alves LP, Costa MS (2012). Aloe vera extract reduces both growth and germ tube formation by Candida albicans. Mycoses 55:257-61
Crossref
|
|
|
|
|
Botelho A, NAP Nogueira, GM Bastos (2007). Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Brazilian Journal of Medical and Biological Research 40(3):349-356.
Crossref
|
|
|
|
|
CLSI (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd Edn., Clinical and Laboratory Standards Institute, Wayne.
|
|
|
|
|
Cowan MM (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews 12(4):564-582.
Crossref
|
|
|
|
|
Dalli AK, Saha. G, Chakraborty. U (2007). Characterization of Antimicrobial compounds from a common fern, Pterin biaurita. Indian Journal of Experimental Biology (5):285-290.
|
|
|
|
|
Das S, Mishra B, Gill K, Ashraf MS, Singh AK, Sinha M, Sharma S, Xess I, Dalal K, Singgh TP, Dey S (2011) Isolation and characterization of novel protein with anti-fungal and anti-inflammatory properties from Aloe vera leaf gel. International Journal of Biological Macromolecules 48:38-43.
Crossref
|
|
|
|
|
David U (1999). Aloe vera-Nature's Gift: Aloe vera in veterinary practice. Great Britain, Blackdown Publications.15.
|
|
|
|
|
Doddanna SJ, Shilpa Patel, Madhusudan AS, Ravindra SV (2013). Antimicrobial activity of plant extracts on Candida albicans: An in vitro study. Indian Journal of Dental Research 24(4).
Crossref
|
|
|
|
|
Ernst EJ, Roling EE, Petzold CR, Keele DJ, Klepser ME (2002). In vitro activity of micafungin (FK-463) against Candida spp.: Microdilution, timekill and post antifungal-effect studies. Antimicrobial Agents and Chemotherapy 46:3846-3853.
Crossref
|
|
|
|
|
Fadipe LA, Haruna K, Mohammed I (2014). Antibacterial Activity of 1,2-Benzenediccarboxylic Acid, Dioctyl Ester Isolated from the Ethyl Acetate Soluble Sub-portion of the unripe Fruits of Nauclea latifolia. International Journal of Pure & Applied Bioscience 2(1):223-230.
|
|
|
|
|
Fothergill AW (2011). Antifungal SusceptibilityTesting: Clinical Laboratory and Standards Institute (CLSI) Methods. In: Intraction of Yeast, Moulds and Antifungal Agents, Hall, G.S. (Ed.), Humana Press, New York, ISBN-10: 1597451347, pp.65-74.
|
|
|
|
|
Freeman BC, Beattle GA (2008). An Overview of Plant Defenses against Pathogens and Herbivores. The Plant Health Instructor. DOI: 10.1094/PHI-I-2008-0226-01.
Crossref
|
|
|
|
|
Garnick JJ, Singh B, Winkley G (2008). Effectiveness of a medicament containing silicon dioxide, aloe, and allantoin on aphthous ulcers. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology 86:550-556.
Crossref
|
|
|
|
|
Gunjan K, Md. Jalaluddin, Purnendu R, Rajat M, Dileep CL (2013). Emerging Trends of Herbal Care in Dentistry. Journal of Clinical and Diagnostic Research 7(8):1827-1829
|
|
|
|
|
Harbourne JB (1991). Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. London: Chapman and Hall.
|
|
|
|
|
Jurkić LM, Cepanec I, Pavelić SK, Pavelić K (2013). Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metabolism 10:2.
Crossref
|
|
|
|
|
Langmead L, Makins RJ, Rampton DS (2004). Anti-inflammatory effect of Aloe vera gel in human colorectcal mucosa in vitro. Aliment. Pharmacology & Therapeutics 19:521-527
Crossref
|
|
|
|
|
Mohammed MP, Aysha OS, Dhamotharan R (2014). Insilico identification of potent inhibitor from andrographis paniculata for esbls. International Journal of advances in Pharmacy, Biology and Chemistry 3(3).
|
|
|
|
|
Murugesan S, Vijayakumar R, Panneerselvam A (2011). Evaluation of Phytochemical Constituents from the Leaves of Memecylon umbellatum Burm.f. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2(4).
|
|
|
|
|
Muthumary J, Yogeswari S, Ramalakshmi S, Neelavathy R. 2012. Identification and Comparative Studies of Different Volatile Fractions from Monochaetia kansensis by GCMS. Global Journal of Pharmacology 6(2):65-71.
|
|
|
|
|
Namiranian H, Serino G (2012). The effect of a toothpaste containing Aloe vera on established gingivitis. Swedish Dental Journal 36(4):179-185.
|
|
|
|
|
Nandal U, Bhardwaj RL (2012). Aloe vera for human nutrition, health and cosmetic use -A review. International Journal of Plant Sciences 3(3):38-46.
|
|
|
|
|
NCCLS (1997). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards,Wayne, Pa.
|
|
|
|
|
Nejad BS, Mahsa R, Mamoudabadi AZ, Majid Z (2014). In Vitro Anti-Candida Activity of the Hydroalcoholic Extracts of Heracleum persicum Fruit against Phatogenic Candida Species. Jundishapur Journal of Microbiology 7(1):e8703.
|
|
|
|
|
Nejatzadeh-Barandozi F (2013). Antibacterial activities and antioxidant capacity of Aloe vera. Organic and Medicinal Chemistry Letters3:5.
Crossref
|
|
|
|
|
Richa W, Siraj DAAK, Gaurav S, Sabreena S (2014). Aloe vera: A Boon in Dentistry. International Journal of Pharmaceutical Sciences Review and Research 4(3):147-151.
|
|
|
|
|
Sathyaprabha G, Kumaravel S, Ruffina D, Praveenkumar P (2010). A Comparative study on Antioxidant, Proximate analysis, Antimicrobial activity and phytochemical analysis of Aloe vera and Cissus quadrangularis by GC-MS. Journal of Pharmacy Research 3(12):2970-2973.
|
|
|
|
|
Sema A, Suleyman AJ(2009). Investigation of in vitro antimicrobial activity of Aloe vera juice. Journal of Animal and Veterinary Advances. 8(1):99-102.
|
|
|
|
|
Sun-A I, Young-Ran L, Young-Hee L, Myung-Koo L, Young IP, Sungwon L, Kyungjae K, Chong-Kil L (2010). In Vivo Evidence of the Immunomodulatory Activity of Orally Administered Aloe vera Gel. Archives of Pharmacal Research 33(3):451-456.
Crossref
|
|
|
|
|
Wei-seng ho, Zayed MZ, Ahmad FB, Pang S (2014). Gc-ms analysis of phytochemical constituents in leaf extracts of neolamarckia cadamba (rubiaceae) from malaysia. International Journal of Pharmacy and Pharmaceutical Sciences 6(9):123-127
|
|
|
|
|
Wiegand I, Hilpert K, Hancock REW (2008). Agar and broth dilution methods to determine the Minimal Inhibitory Concentration (MIC) of antimicrobial substances. Nature Protocols 3:163-175.
Crossref
|
|
|
|
|
Yebpella GG, Adeyimi HMM, Hammuel C, Magomya AM, Agbaji AS, Okonkwo EM (2011). Phytochemical Screening and Comparative Study of Antimicrobial activity of Aloe vera various extracts. African Journal of Microbiology Research 5(10):1182-1187.
Crossref
|
|