ABSTRACT
This study aimed at isolating and characterizing antibiotic producing actinomycetes from a specific geographic location Alba’qa, Jordan, with a known soil character, and study the effect of the environmental factors on their antimicrobial activity, in order to relate the actinomycetes flora to the environmental characteristics. Soils of the Alba’qa region are of uniform composition and contain antimicrobial producing microorganisms. Although the initial aim was to isolate organisms of the genera Actinomyces, other organisms such as Aspergillus and other antibiotic producing microorganisms “mostly bacteria” were also isolated. Actinomycetes showed antimicrobial effect on all tested bacteria “Escherichia coli SQ21, Staphylococcus aureus SQ31, Streptococcus pyogenes SQ33, Serratia marcescens SQ22, Staphyloccocus epidermidis SQ32 and Bacillus spp. SQ41” depending on the media they were cultured on and the temperature they were incubated at. It was established that the optimum antibacterial activity is shown when cultivated on GYE medium at 30°C. This made a beneficial first step to find treatments for the diseases caused by the pathogenic bacteria.
Key words: Actinomycetes, antibiotics, environmental factors, soil.
Actinomycetes are a broad group of gram positive bacteria that form thread-like filaments in the soil. A few are normal inhabitants of the mouth and several species are pathogenic (Actor, 2012). They are responsible for the distinctive scent of freshly exposed moist soil. Antibiotics are the best known products of actinomycetes. Over 5,000 antibiotics have been identified from the cultures of Gram positive and Gram-negative organisms, and filamentous fungi, but only about 100 antibiotics have been commercially used to treat human, animal and plant diseases. The genus, Streptomyces, is responsible for the formation of more than 60% known antibiotics while a further 15% are made by a number of related Actinomycetes, Micromonospora, Actinomadura, Streptoverticillium and Thermoactinomycetes (Waksman, 1954). Actinomycetes centered mainly on their ability to form antibiotics. Beginning with the discovery of actinomycin in 1940, the interest in the antibiotics produced by actinomycetes has been wondrous. Srtreptothricin, streptomycin, nystatin, chloramphenicol, tetracycline, erythromycin, the chain of the discoveries are endless (Mahajan and Balachandran, 2012).
Actinomycetes can be grown on the common bacteriological media used in the laboratory, such us nutrient agar, trypticase soy agar and blood agar (Nanjwade et al., 2010b). The actinomycetes have universal occurrence and play an active part in the cycle of nature. The morphology of actinomycetes growing on agar (the presence or absence of spores on the substrate mycelium, the formation of zoospores in specialized spore vesicles or sporangia), their spores arrangement and the features of their colonies can provide useful and rapid clues to their identity (Oskay et al., 2004).
Actinomycetes presence in soil is affected by the geographical location and the prevailing abiotic factors (soil temperature, soil type, depth, soil pH, organic matter content, cultivation, aeration and moisture content). Therefore, we believe that when attempting to isolate actinomycetes it is important to specify the soil type and related atmospheric conditions at the time of sampling. In the past years, focus on natural product discovery from actinomycetes shifted from the extensively investigated soil-dwelling isolates towards underexplored habitats of rare actinomycetes from unusual ecosystem. This strategy has an impact on the discovery platform for novel compounds with promising bioactivities despite the fact that a large amount of the reservoir of habitats still awaits exploration (Hug et al., 2018). Our interest was focused on screening the soil samples for actinomycetes with antibiotic production potential from Alba’qa area, Jordan, in addition to study the effect of the environmental factors on their antimicrobial activity.
Soil characterization
Alba’qa Region in Jordan was the geographic location of the soil samples. They were obtained from previous site studies applying the site characterization GLOBE protocol; the coordinates were determined to be 32.03234° north (Tables 1 and 2) and 35.90517° East at an elevation of 920 m. All readings were taken with a Garmin GPS12.
Isolation of antimicrobial substances producing microorganisms from soil
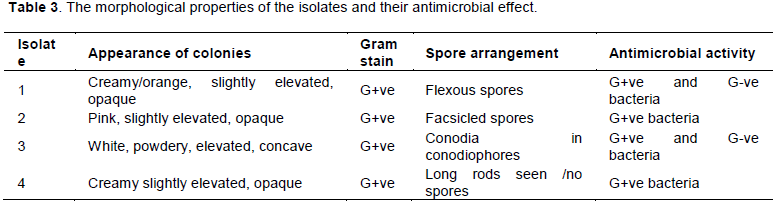
Actinomyces and other microorganisms such as Aspergillus and Gram positive bacteria” were isolated from soil samples collected from Alba’qa, Jordan (depending on their morphologically and cultural properties of the isolates shown in Table 3). For the isolation, 1 g of rhizosphere soil sample was dispensed into 10 ml of sterile deionized water. The soil suspension was then shaken on a rotary shaker (Sanyo Gallenhamp PLC, Leicester, LE 3 2uz, UK) at 180 rpm and 27°C for 30 min. Ten-fold dilutions were made in sterile saline solution and 100 µl aliquots were spread with a sterile glass rod over the surface of nutrient agar plates (per liter of distilled water) (peptone 5 g, sodium chloride 5 g, yeast extract 1.5 g, beef extract 1.5 g and agar 15 g) (HiMedia Laboratories Pvt. Limited, Bombay, India). Six plates were used per dilution and dried in a laminar flow-cabinet for 60 min before incubation at 27°C in the dark for 48-72 h. Bacterial colonies were sub-cultured and transferred onto nutrient agar plates. Single colonies were isolated and screened for antimicrobial activity using the agar streak method (El-Banna and Winkelman, 1998).
Bacterial strains
Escherichia coli SQ21, Staphylococcus aureus SQ31, Streptococcus pyogenes SQ33, Serratia marcescens SQ22, Staphylococcus epidermidis SQ32 and Bacillus spp. SQ41 were used as test microorganisms.
Culture media
Nutrient agar (NA), trypticase soya agar (TSA.), glycerol yeast extract (GYE), nutrient broth (NB) and GYE (5 ml of glycerol, 2 g of yeast extract, 1 g of dipotassium phosphate, 15 g agar, 1000 ml water).
Agar streak method
The microbial sensitivity of the soil isolates was analyzed by agar streak method. Each of the isolate was streaked as a straight line on medium and incubated at 27°C for 6 days (144 h). After the 6th day, different strains of microorganisms were streaked at right angle, but not touching each other, and then incubated at 37°C for 24 h in the case of bacteria. If the organism is susceptible to the antibiotic produced by actinomycetes, then it will not grow near the actinomycetes (Nanjwade et al., 2010a). The zone of inhibition against each test organism was noted. The isolated actinomycetes were screened against test microorganisms (Haque et al., 1996). Based on their antimicrobial properties, isolates were chosen for the further biochemical characterization.
Morphological and cultural characterization
The effect of the type of media on the antimicrobial activity of the isolated strains
Streaks of six bacterial species were inoculated on the plates of 6 days old cultures of isolates 1, 2, 3 and 4 incubated at 30°C for 24 h.
The effect of the temperature
The effect of the incubated temperature on the antimicrobial activity of the strain 1, 2, 3 and 4 incubated at different temperatures for 24 h.
Effect of isolate culture age
Isolates of different age were used to determine the effect of the culture on the potency of their antibiotic productivity.
Soil characterization
The results of the soil samples which were collected from Alba’qa, Jordan are shown in Tables 1 and 2.
Isolation of antibiotic producing organisms
During the initial isolation attempts, four morphologically different isolates were obtained (Table 3), showing significant antimicrobial activity (Figure 1). Variables that may affect the efficiency of antibiotic production were narrowed down to three factors that were examined separately. These were the type of medium, the incubation temperature and the age of isolate.
The effect of the type of media on the antimicrobial activity of the isolated strains
Streaks of six bacterial species were inoculated on the plates of 6 days old cultures of isolates 1, 2, 3 and 4 as shown in Tables 4, 5, 6 and 7 incubated at 30°C for 24 h. Figures 2, 3 and 4 showed the agar streaks test of strain 3 on different types of media.
The effect of the temperature
The effect of the incubated temperature on the antimicrobial activity of strains 1, 2, 3 and 4 incubated at different temperatures for 24 h are shown in Table 8 and Figures 5, 6, 7 and 8. The effect of the incubated temperature on the antimicrobial activity of the isolated strains 1, 2, 3 and 4 incubated at 37°C, for 24 h are shown in Tables 8 and 9, and Figures 9, 10, 11 and 12.
Effect of isolate culture age
The age of the culture seemed to have no effect on the potency of its antibiotic product.
The soil samples characterization in Table 1 showed no obvious difference between the 3 types of soil which were taken from the 3 different areas of the Alba’qa, Jordan. So even from different locations that are distant from each other in the region, they were almost the same type which had no significance in the isolation process. The presence of relatively large populations of actinomycetes in the soil samples of Alba’qa region indicates that it is a suitable ecosystem that promotes the isolation of actinomycetes during screening programmes.
Actinomycetes have been, for decades, one of the most important sources for the discovery of new antibiotics; an important number of drugs and analogs have been successfully introduced into the market and are still used today in clinical practice (Genilloud, 2017). In the course of screening for antimicrobial substances producing actinomycetes, four antibiotic-producing isolates (isolate 1, 2, 3 and 4) were recorded from soil samples taken in Alba’qa, Jordan. The isolate 1 had an antimicrobial effect on Streptococcus pyogenes SQ33, Escherichia coli SQ21 and Staphyloccocus epidermidis SQ32. Isolate 2 had an antimicrobial effect on Serratia marcescens SQ22 and S. epidermidis SQ32. Isolate 3 had an antimicrobial effect on Bacillus spp SQ41, Staphylococcus aureus SQ31, E. coli SQ21, Streptococcus pyogenes SQ33 and S. epidermidis SQ32. While isolate 4 had an antimicrobial effect on Bacillus spp SQ41, Streptococcus pyogenes SQ33 and S. epidermidis SQ32. A-4 isolated by Nanjwade et al. (2010a) showed broad spectrum of activity against both Gram-positive and Gram-negative organisms as well as antifungal activity. Three isolates (Ab18, Ab28 and Ab43) have shown high antagonistic activity against resistant pathogens, during the primary screening (Bizuye et al., 2013). As stated earlier, actinomycetes have provided many important bioactive compounds of high commercial value and continue to be routinely screened for new bioactive substances (Waksman, 1954). The results demonstrate that the type of culture medium, and incubation temperature have a significant effect on the antimicrobial agent production capability of antibiotic producing organisms. The preferred medium in this experiment was GYE, may be due to its enriched nature, and the preferred temperature being 30°C. This is in correlation with prevailing climate at the site of isolation, since the average maximum temperature at the site is approximately 30°C. In order to achieve maximum antibiotic production, experiments were conducted by James et al. (1991) and Nanjwade et al. (2010b to optimize the various parameters such as carbon source, nitrogen source, temperature, pH, DO2, and micronutrients etc. Isolating and screening action myceters from such areas in optimum conditions may contribute to the discovery of new antibiotics (Bizuye et al., 2013).
The findings from this investigation reveal that strains Actinomyces 1 and 2, Aspergillus and Gram positive bacterial rods were isolated from soils of Alba’qa region, Jordan. Actinomycetes showed antimicrobial effect on all the 6 bacteria used “E. coli SQ21, S. aureus SQ31, S. pyogenes SQ33, S. marcescens SQ22, S. epidermidis SQ32 and Bacillus species SQ41” depending on the media they were incubated on and the temperature they were incubated at. And it was established that the optimum antibacterial activity is shown when cultivated on GYE medium at 30°C.
The authors have not declared any conflict of interests.
REFERENCES
|
Actor JK (2012). Elseviers Integrated Review Immunology and Microbiology (Second Edition).
|
|
|
|
Bizuye A, Moges F, Andualem B (2013). Isolation and screening of antibiotic producing actinomycetes from soils of Gondar town, North west Ethiopia. Asian Pacific Journal of Tropical Disease 2:375-381.
Crossref
|
|
|
|
|
El-Banna N, Winkelmann G (1998). Pyrrolnitrin from Burkholderia cepacia: antibiotic activity against fungi and novel activities against streptomycetes. Journal of Applied Microbiology 85:69-76.
Crossref
|
|
|
|
|
Genilloud O (2017). Actinomycetes: still a source of novel antibiotics. Natural Product Reports 34:1233-1243.
Crossref
|
|
|
|
|
Haque S, Sen S, Pal S (1996). Antimicrobial spectra and toxicity of antibiotics From Streptomyces antibioticus sr 15-4. Indian Journal of Medical Microbiology 36:113-114.
|
|
|
|
|
Hug J, Bader D, Remskai M, Cirnski, Mueller R (2018). Concepts and methods to access novel antibiotics from actinomycetes. Antibiotics 7(2):44.
Crossref
|
|
|
|
|
James P, Wards C, Dawson M (1991). The effects of temperature, pH and growth rate on secondary metabolism in Streptomyces thermoviolaceus grown in a chemostat. Journal of General Microbiology 137:1715-1720.
Crossref
|
|
|
|
|
Mahajan G, Balachandran L (2012). Antibacterial agents from actinomycetes – A review. Frontiers in Bioscience E4(1):240-253.
Crossref
|
|
|
|
|
Nanjwade B, Chandrashekhara S, Goudanavar P, Shamarez A, Manvi F (2010a). Production of antibiotics from soil-isolated actinomycetes and evaluation of their antimicrobial activities. Tropical Journal of Pharmaceutical Research 9:373-377
Crossref
|
|
|
|
|
Nanjwade B, Chandrashekhara S, Shamarez A, Goudanavar P, Manvi F. (2010b). Isolation and morphological characterization of antibiotic producing actinomycetes. Tropical Journal of Pharmaceutical Research 9:231-236.
|
|
|
|
|
Oskay M, Usame T, Cem A. (2004). Antibacterial activity of some actinomycetes isolated from farming soils of Turkey. African Journal of Biotechnology 3:441-446.
Crossref
|
|
|
|
|
Waksman S (1954). The Actinomycetes. 1st edition, Watham, MASS, USA pp. 185-191.
|
|