ABSTRACT
Uvaria chamae is a plant used traditionally in treatment of wounds and other ailments. This work was conducted on qualitative assessment of crude extracts of U. chamae against wound isolated strains of Pseudomonas aeruginosa and Proteus mirabilis, determining the resistance and plasmid profiles of the isolates. Standard techniques were employed in crude extraction of roots and leaves of the plant followed by phytochemical screening. The test isolates were obtained from University of Uyo Medical Centre and re-screened using standard microbiological techniques. Antibacterial activities of the extracts on the isolates were assessed using agar-well diffusion technique. Plasmids were analyzed using gel electrophoresis. U. chamae contains tannis, alkaloids and other bioactive components. Antibacterial activities showed that ethanolic roots extracts possessed greater inhibitory effects on test isolates than any other extract forms with zones of inhibitions (Z.I) that ranged from 18±2.0 to 21±1.2 mm and resistances with no zone of inhibitions (N.I) observed with P. aeruginosa-1, P. mirabilis -1 and P. mirabilis-3 at 50 mg/ml concentration. Some test isolates were resistances to other extract forms and standard antibiotics. Some harbored plasmids ranged from 564 bps -23.13 kbp. Whereas resistance patterns of some isolates were not reverted, resistances of these bacteria to various antimicrobial agents are of public health implications.
Key words: Antibacterial activities, Uvaria chamae, wound isolates, resistance, plasmids.
Plants have been a source of medicine in the past centuries and today, plant-based formulation continues to play an important role in health care of greater number of people worldwide (Karachi, 2006). They are used in various complementary, traditional and alternate systems of treatment of human diseases (Sher, 2009). Scientists and the general public recognize the value of plants as a source of new or complimentary medicinal products. In some Africa countries, there has been an increasing concern to source for locally available drugs and alternative medicine to treat ailments. This is due to the fact that many synthetic and routine drugs are inaccessible and increase loss of effectiveness and potency due to increased multi-drugs resistance organisms to these agents (WHO, 2004; Wang et al., 2010). Other factors are inability by many to afford conventional chemotherapeutic agents, pay hospital bills as well as economic recession with increased poverty in some African’s countries including Nigeria. The aforementioned situations have rapidly increased the rate of scientific researches into the use, effects and constituents of medicinal plants especially in Nigeria (Ross et al., 2001). Hence many studies have been generated on the phytochemistry and medicinal potency of a number of plants commonly found in Nigeria. For instance, studies by Andy et al. (2008) on Heinsia crinata, Udoh et al. (2011) on Centella asiatica, Udoh et al. (2012) on Lansiathera africana, Udoh et al. (2017) and Udoh et al. (2018) who worked and reported on antimicrobial potencies of Ocimum gratissimum and Uapaca Staudtii plants respectively.
Uvaria chamae (P. Beauv) is one the plants commonly found Nigeria. It belongs to the family Annonaceae (Omale et al., 2013; Olumese et al., 2016) and is found growing naturally in the savannah and rain forest regions of Africa and tropical areas of the world. It is an evergreen plant that can grow to height of between 3.6 - 4.5 m (Olumese et al., 2016). U. chamae is commonly called “finger root” or “bush banana” in English. The plant is also known by the Efik people as “Nkarika Ikot”, “Oko aja” or “Eruju” by the Yorubas, “Kas Kaifi” by the Hausas, IIgala people called it “Awuloko” or “Ayiloko” and “Akotompo” by the Fula-fainte of Ghana (Omale et al., 2013; Olumese et al., 2016).
U. chamae (P. Beauv) is used in trado-medical practices to cure various ailments as it is a plant with both medicinal and nutritional values. Every part of the plant has several local uses (Iwu, 1993; Okokon et al., 2006; Omale et al., 2013). Many researchers reported the use of this plant’ parts in the local treatments. For instance, the root infusion and roots-bark have a widely spread reputation in native treatments. When pounded, it can be used in treatment of nose bleeding, deep wound treatment, sepsis, heart disease (bronchi, lungs), inflammation, blood in urine, pile, fever and, malaria (Adams and Moss, 1999; Etukudo, 2003; Okokon et al., 2006; Omajali et al., 2011). The stem ashes serve as salt substitution in food. The tender leaves are eaten as vegetables. The leaf juice is applied to wounds, sores ulcers, and cuts while the leaf in fusion prepared as lotion is used to treat injuries, swellings, ophthalmic and conjunctivitis (Iwu, 1993; Oluremi et al., 2010; Okwuosa et al., 2012). The fruits have aromatic flavor and is popularly used in beverage industry to add flavor to drinks and food. Moreover, the root yields yellow dye widely used in textile industries to dye fabrics and cosmetics (Igoli et al., 2005).
Wound infection is one of the clinical problems faced by physicians worldwide. Wound can be infected by a wide variety of microorganisms ranging from bacteria, fungus and parasites (Bowler et al., 2001; Yah et al., 2004). Among the bacteria that are frequently recovered from the wounds are Pseudomonas aeruginosa and Proteus mirabilis (Yah et al., 2004; Gus Gunzalez et al., 2006). According to Ejikeugwu et al. (2013), there are in fact some reports on the existence of multidrug resistance microorganisms and some extended spectrum activities among these clinically important bacterial pathogens, and according to Yah et al. (2007), is one of the ways bacteria confer resistance to the antimicrobials by acquisition of plasmids. Hence they develop resistance to available antibiotics on acquiring these important mechanisms. It is pertinent to search for alternative antimicrobial agents, as some plants prove to function in this aspect and also assess the resistance of these clinical isolates to the plant’s crude extracts. U. chamae (P. Beauv) have been used locally to treat wound cases and is claimed to be very effective while P. aeruginosa and P. mirabilis are bacteria frequently isolated from the wounds which could sometimes develop resistance for routine antibiotics. Therefore, studies were conducted on qualitative assessment of U. chamae extracts against wound isolated strains of P. aeruginosa and P. mirabilis and the resistance and plasmid profiles of these bacterial isolates were also determined.
Plant collection and identification
Fresh root and leaves of the plant were collected from Ikono Local Government Area of Akwa Ibom State, Nigeria. The plant part were identified as U. chamae (P. Beau) at the Department of Botany and Ecological Studies University of Uyo, Uyo, Akwa Ibom State, Nigeria by a Taxonomist and was taken to Pharmacognosy Laboratory, University of Uyo for crude extracts and phytochemical analysis. The study was conducted from June 2015 to October, 2015.
Source of test isolates
Six (6) isolates of P. aeruginosa and P. mirabilis bacteria isolated from wound samples were collected from University of Uyo Medical Centre Uyo, Akwa Ibom State, Nigera, re-cultured, and re-isolated using standard microbiological techniques. Re-characterizations and re-identification were done according to Holt et al. (1994) and
Cheesbrough (2000). The isolated strains were labeled P. aeruginosa (Pa-1), P. aeruginosa (Pa-2), P. aeruginosa (Pa-3) P. mirabilis (Pm-1), P. mirabilis (Pm-2) and P. mirabilis (Pm-3) respectively. They were maintained on Nutrient agar (Oxoid, USA) slants at 4°C prior to use.
Sample preparation: Extraction procedures and preparation extract concentration
The fresh root and leaves samples of U. chamae were prepared using Obi and Onuaha (2000), and Mukhtar and Tukur (2000) methods. The samples were shade-dried for one week (Andy et al., 2008; Ladpo et al., 2010), thereafter the dried root and leaves were separately ground into fine powder using a mortar (Taura et al., 2014). The crude extracts of the root and leaves were extracted using standard procedures. The ethanolic extraction of U. chamae was carried out by soaking 500 g of the dried powdered root and leaves of U. chamae into separate sterile containers containing 1000 ml of 95% ethanol. The containers were covered and allowed to stand for 72 h under regular shaking condition at room temperature to allow maximum extraction of the bioactive components. The root and leaves extract were then filtered using Whatman filter paper (No. 1) and the filtrate were evaporated to dryness using a rotatory evaporator. The residue were retained as crude extracts in reagent bottles and maintained in the refrigerator until they were used. Similarly, for the aqueous extraction, 500 g of the dried powdered U. chamae leaves and root were separately weighed out and dispersed into separate sterile containers containng 1000 ml of water. The root and leaves solution were covered and shaken and were allowed to stand for about 72 h at ambient room temperature for maximum extraction, and then the infusion were filtered using Whatman filter paper (No. 1). The filtrates were evaporated to dryness using a rotatory evaporator. The residue were retained as crude extracts in reagent bottles and maintained in the refrigerator until they were used.
The concentration of the root and leaves extracts of U. chamae were prepared following the methods of Esinemore et al. (1998) and Akujobi et al. (2004) with some modifications. The crude extracts of 200, 150, 100 and 50 mg were respectively diluted with 1 ml of 20% dimethyl sulfoxide (DMSO) for ethanol and aqueous extracts respectively to obtain 200, 150, 100 and 50 mg/ml concentrations for the respective extracts. The reconstituted extract concentrations were stored at 15°C until required for use.
Phytochemical screening
Phytochemical screening tests were conducted on the respective root and leaves extracts using standard methods of Trease and Evans (1994) and Sofowora (2008), which were adopted and employed by Ekong and Udoh (2015a), and Udoh et al. (2017). The tests were; alkaloids test, saponin test, tannis test, test for cardiac glycosides, flavonoids determination and cyanogenic glycosides.
Antibacterial assay of U. chamae extracts
Ability of the U. chamae leaves and roots extracts to inhibit growth of the wound isolated strains of P. aeruginosa and P. mirabilis was determined using the agar-disc diffusion method (Ogbulie et al., 2004). Sterile filter paper discs of 6 mm in diameter were soaked in equal volumes of varying concentrations of extracts (50, 100, 150 and 200 mg/ml) and left for 2 h undisturbed. The 0.1 ml of 18 h peptone water culture of each of the test bacterial isolates was spread on the sterile Mueller-Hinton Agar (Difco Laboratories, Detroit, Mich) plates. The disc were picked with sterile forceps and placed at different areas on the surface of each plate inoculated with these isolates. Control experiments using routine antibiotic susceptibility test discs were also performed on the isolates by means of Kirby-Bauer disc diffusion method using the guidelines provided by Clinical Laboratory Standard Institute (CLSI, 2005). The antibiotics were: Ciprofloxacin, Tetracycline, Cephaloxin, Gentamicin, Amoxillin, and Nitrofurantoin. This was done by impregnating the disc on each of the culture plate. The sensitivity tests on both the crude extracts and standard antibiotics were performed in triplicates. The plates were incubated at 37°C for 24 h. Antimicrobial activity of the extracts on the test organisms was determined by measuring the zones of inhibition in Milliliter (mm) diameter of the respective disc. Clear zones of inhibition indicated the susceptibility of the organism to the extracts while absence of such zones showed no inhibitory effect of extracts on the test organism. The Z.I values > 15 mm = Sensitive, 12 – 15 mm = moderately sensitive and < 12 mm = Resistant.
Plasmid profiling using agar gel electrophoresis analysis
The plasmid profiling of the bacterial isolates was carried out using gel electrophoresis analysis. The method as described by Ehrenfeld and Clowell (1987), and Akinjogunla and Enabulele (2010), were adopted with slight modification to determine the plasmid profile of P. aeruginosa and P. mirabilis isolates. The test was also carried out to find out if the resistance pattern exhibited by the test isolates were plasmid mediated or not and to assess the molecular weight of the bacterial plasmids DNA that conferred resistance on these isolates to the crude extracts and the antibiotics (Lipps, 2008).
Plasmid curing
The curing of the resistant plasmids of the isolates was performed using sub-inhibitory concentration of 0.10 mg/ml of acridine orange as described by Sheikh et al. (2003), and Akortha and Filgons (2009) with sight modification. The purpose was to determine if the resistance of P. aeruginosa and P. mirabilis isolates to standard antibiotics and the crude extracts of U. chamae were plasmid or chromosomal mediated.
Statistical analysis
The values of zones of inhibitions (Z.I) recorded were calculated from the means of three measurements of zones of inhibitions on the triplicate cultures and their standard deviation of the mean was also calculated (Mean±SD).
The phytochemical analysis of both the ethanolic and aqueous crude extracts of U. chamae roots and leaves revealed that the plant contains some phytochemical constituents such as tannis, alkaloids, flavonoids, cardiac glycoside, and cyanogenic glycoside as bioactive components. These substances were found in varied concentrations depending on extract forms. Some components were abundantly present (+++) in ethanolic crude forms especially root extracts as compared to the crude leaves extracts in which some of these bioactive substances were found in trace (+) forms. For instance, in ethanolic root extract and ethanolic leaves extract, tannins, flavonoids and cardiac glycoside were abundantly present (+++). However, it is noteworthy that in aqueous root extracts and aqueous leaves extracts these substances were found in trace (+) forms or in moderate amount (++) (Table 1).
The antimicrobial potency of ethanolc root extracts of U. chamae on test isolates was assessed. P. aeruginosa -1 was resistant to ethanolic root extract with Z.I of 11±0.81 mm at 200 mg/ml, 10±0.48 mm at 150 mg/ml but N.I at 100 and 50 mg/ml respectively. Aqueous root extract of U. chamae had Z.I of 10±0.1 mm at 200 mg/ml, 9±0.62 mm at 150 mg/ml, and N.I was recorded for 100 and 50 mg/ml respectively for P. aeruginosa -1. P. aeruginosa -2 had a wider Z.I of 21±1.2 mm from ethanolic root extract at 200 mg/ml, but for aqueous root extract, resistance was observed with P. aeruginosa -2 as Z.I of 11±0.58 mm was recorded. P. aeruginosa -3 was resistant to the ethanol root extract with Z.I ranging from 10±1.2 - 11±0.83 mm at 50 and 100 mg/ml but moderately sensitive at 200 mg/ml concentration with Z.I of 15±0.47 mm, while for aqueous root extract, resistance were recorded. Moreover, P. mirabilis -1 was observed to be resistant to ethanolic root extract at 50 and 100 mg/ml but moderately sensitive at 150 mg/ml with Z.I of 15±2.0 and 16±1.75 mm at 200 mg/ml, while for aqueous root extract, Z.I ranged from N.I -13±0.57 mm. Outstandingly, for ethanol root extract, P. mirabilis -2 progressively showed sensitivity with increased concentration of the extract. The Z.I recorded ranged from 11±1.15 - 20±0.67 mm at 50 – 200 mg/ml, respectively whereas for aqueous root extract, Z.I ranged from 8±0.6 -13±0.57 mm. P. mirabilis -3 had resistance for ethanolic root extract at 50, 100 and 150 mg/ml but moderately sensitive at 200 mg/ml with Z.I of 15±1.67 mm respectively while for aqueous root extract, it was resistant as N.I and smaller Z.I were recorded. Assessing individual crude extract form at different concentrations, ethanolic roots extracts had greater inhibitory effects with zones of inhibitions (Z.I ranged from 18±2.0 mm -21±1.2 mm) on P. aeruginosa and P. mirabilis bacteria used in the study than aqueous root extracts (Z.I ranged from 8±0.1 - 15±2.0 mm) respectively (Table 2).
The antimicrobial potency of ethanolc leaves extracts on test isolates was also assessed. P. aeruginosa -1 was resistant to ethanolic leaves extracts with N.I at 50 and 100 mg/ml, respectively but Z.I of 8±0.2 mm at 150 mg/ml and 9±1.0 mm at 200 mg/ml. Smaller Z.I values or N.I were also observed with P. aeruginosa -1 isolate when subjected to crude aqueous leaves extracts. Inhibitory effect of ethanolc leaves extracts on P. aeruginosa-2 increased with increased concentration Z.I of 8±0.6, 10±0.1, 12±1.2 and 13±0.57 mm at 50, 100, 150 and 200 mg/ml, respectively whereas for aqueous leaves extracts, P. aeruginosa-2 was resistant to the extract form as N.I, 8±0.68 mm, 8±0.5 and 9±0.56 mm were recorded with used concentrations. P. aeruginosa-3 had resistance against ethanolc leaves extracts as Z.I of 8±0.53 mm was recorded at 50 mg/ml, but at 100, 150 and 200 mg/ml and Z.I of 9±0.25, 9±0.047 and 10±0.5 mm respectively while for aqueous leaves extracts, P.aeruginosa-3 was resistance as smaller Z.I were recorded. P. mirabilis -1 was resistance to ethanolic and aqueous leaves extracts at all the concentrations used in the study as N.I and smaller Z.I were recorded. P mirabilis -2, was moderately sensitivity to ethanolic leaves extracts at 150 and 200 mg/ml with Z.I of 12±0.87 and 13±0.61 mm, respectively but resistance was observed with aqueous root extracts as Pm-2 had smaller Z.I values of 8±0.46 mm at 200 mg/ml. P mirabilis -3 showed moderate sensitivity to ethanolic leaves extract at 200 mg/ml with Z.I of 13±0.52 mm but resistance at other concentrations used in the study as N.I while for aqueous leaves extracts Pm-3 was resistance to the extract with N.I recorded at 50 and 100 mg/ml with smaller Z.I recorded at other concentrations. The potency of each form of leaves extract at different concentrations showed ethanolic leaves extracts had at least some inhibitory effects on test isolates (Z.I ranged from 7±1.0 - 13±0.61 mm) than the aqueous leaves extracts with zones of inhibitions (Z.I ranged from 7±0.2 - 11±0.2 mm) respectively (Table 3).
Some test isolates that exhibited multiple resistances to both the ethanolic and aqueous root and leaves extracts also showed resistances to some standard antibiotics used as control. P. aeruginosa -1 was resistant to Cephaloxin, Nitrofuratoin and Amoxillin, moderately sensitive to Tetracycline and Gentamicin but highly sensitive to Ciprofloxacin with Z.I values of 18±0.76 mm. P. aeruginosa-2 was highly sensitive to all the standard antibiotics with wider zone of Z.I of 25±0.67 mm recorded for Cephaloxin but P. aeruginosa-3 was resistant to Cephaloxin, Gentamicin and Nitrofuratoin but highly sensitive to Cephaloxin with wider zones of Z.I of 31±0.88 mm. P. mirabilis –1 formed resistance to Ciprofloxacin, Nitrofuratoin, Tetracycline and Amoxillin but high sensitivity was recorded with Gentamicin with wider zones of Z.I of 30±0.36 mm. P. mirabilis-2 was resistant to all the standard antibiotics used in the study except Ciprofloxacin where it had the Z.I of 35±0.54 mm. P. mirabilis–3 exhibited resistance to Ciprofloxacin, Cephaloxin, Gentamicin, Nitrofuratoin and Amoxillin but sensitive to Tetracycline with Z.I of 19±0.63 mm (Table 4).
The result of plasmid profiling of the strains of P. aeruginosa and P. mirabilis showed that some test isolates that exhibited multiple resistances to both the ethanolic and aqueous forms of both root and leaves extracts along with the control antibiotics used in the study was due to the fact that they harbored plasmids of different molecular weights ranging from 564 bps - 23.13 kbp. The multiple bands were clearly observed with P. aeruginosa-1, P. aeruginosa -3, P. mirabilis -1, and P. mirabilis -3 with low molecular weight of 564 bp, and 2.03 kbp to a high molecular weight of 23.13 kbp (Figure 1).
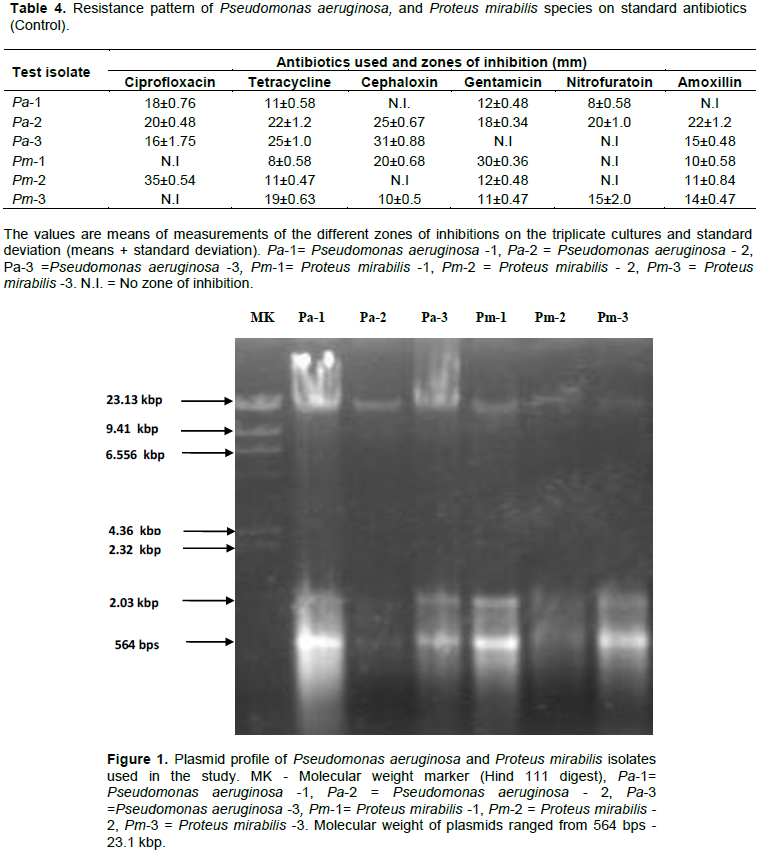
Resistance patterns of some isolates were reverted after curing plasmids with acridine orange while others still retained the plasmids. This was noted when some of the test organisms were found with no zone of inhibition after plasmid curing. Outstandingly was P. aeruginosa -1, where N.I and smaller Z.I in both the ethanolic and aqueous forms of the extracts at 50, 100, and 150 mg/ml concentration were recorded, whereas increased Z.I were observed from P. aeruginosa -2, P. aeruginosa -3, P. mirabilis -1, and P. mirabilis -2 at different extract forms, that might have possibly lost their plasmids after curing (Tables 5 and 6).
Furthermore, results obtained from antibiogram on test isolates after plasmid curing showed that the resistance patterns of some test isolates were reverted for standard antibiotics as some became sensitive while some isolates still harbored the plasmids. P. aeruginosa -1 with smaller Z.I was recorded for Cephaloxin and N.I was recorded against Amoxillin while increased Z.I was observed from P. aeruginosa -2 in nearly all the antibiotics with the wider Z.I of 35±1.2 mm recorded from Cephaloxin. The P. aeruginosa -3 and P. mirabilis -2 still had resistance against Nitrofuratoin with N.I, P. mirabilis -1 had smaller Z.I for Ciprofloxacin, Tetracycline, Nitrofuratoin and P. mirabilis -2 maintained resistance against Nitrofuratoin with N.I, and P. mirabilis -3 isolates which initially had N.I were found with smaller Z.I and increased Z.I for Tetracycline (Table 7).
The results obtained from this study showed that the plants U. chamae possess some active substances as bioactive constituents. This result confirm reports of Omajali et al. (2011) and Olumese et al. (2016), who reported that the plant has several active ingredients. Moreover, Okokon et al. (2006) reported the antimalarial activities of U. chamae while Omale et al. (2013) reported that the plants bioactive constituents have ability to neutralize snake venom in rats. Some researchers have discovered that antibacterial properties are usually associated with these bioactive constituents (Ameh, 2010; Udoh et al., 2017). These substances have their different functions and are known to work using different mechanisms. For instance, alkaloids present in plant are known to function as anesthetic, spasmolytic and anti-cholinergic agent (Iroabuchi, 2008). According to Batisa et al. (2004) and Burile et al. (2009), alkaloids have clinical importance and possess anticancer, antibacterial and anti-asthmatic activities. Tannins are known to inhibit the synthesis of cell proteins of bacteria by forming complexes that are irreversible with proline rich protein in bacteria. Tannins was reported to be responsible for the haemostatic activity where they arrest bleeding from damaged or injured vessels by precipitating protein to form vascular plugs (Okwu and Iroabuchi, 2004). Flavoniods which is also found in U. chamae in a very high concentration especially in ethanolic extracts is reported to function well in some biological aspects such as the protection against allergy, platelet aggregation, microbes invasion, ulcer, hepatoxin, viruses and tumors (Batisa et al., 2004; Burile et al., 2009). Nascimento et al. (2000) reported that flavonoid works well in human system in that it reduces the risk of estrogen-induced cancer by interfering with the enzymes that produce estrogen. Moreover, some researchers observed and reported that flavonoids are also found to form complexes with the extracellular soluble proteins leading to disruption of microbial cell membranes (Tsuchiya et al., 1996), while cardiac glycosides is useful in heart pumping (Godfraind, 1984; Ekong and Udoh, 2015b). The secondary metabolites or bioactive components present in this medicinal plant could be responsible for the therapeutic activity attributed to U. chamae (Monon et al., 2015). These natural substances are widely distributed in U. chamae and contribute greatly to their beneficial health effects. No wonder their natural antioxidant and antibacterial roles have attracted more interest in the plant’ use in the prevention and treatment of inflammatory problem, cancer, and cardiovascular diseases (Vârban et al., 2009; Monon et al., 2015).
The results obtained in this study showed that the ethanolic and water extracts of U. chamae have some inhibitory effects on some wound isolated strains of P. aeruginosa and P. mirabilis as they inhibited the growth of some test isolates at varied concentrations. This indicates that the plant possess some active substances that can inhibit the growth of these microorganisms. These results support the traditional use by herbalists in the treatment of various ailments such as urinary tract infection, treatment of wounds just to mention but a few. It was also observed in the study that much of the active substances were found in the ethanolic extracts than the aqueous form and the lower inhibitory effects of the aqueous extracts could be that probably this ethanolic solvent extracted its active constituents more than the aqueous form and water was not potent enough to extract much of the bioactive substances from U. chamae leaves and roots. Thus, the results indicated that ethanol is a better extraction solvent for extraction of U. chamae plant active principles than the aqueous. This corroborates the reports of Obi and Onuoha (2000), and Ogueke et al. (2006). Other researchers like Ogbulie et al. (2007), Udoh et al. (2011), and Monon et al. (2015) also reported that ethanol is a better extraction solvent for most plants bioactive substances of medicinal importance. No wonder as observed in some areas in Nigeria, some trado-medical practitioners always employ or recommend the use of ethanol (local gin) for the maceration and extraction of plants for native treatments, with exception on where the patients request otherwise.
Moreover, the effectiveness of the antimicrobial potency was observed to be more active in the ethanolic root extracts than ethanolic leaves extracts of U. chamae This was evident when P. aeruginosa -2 and strain of P. mirabilis -2 had wider zones of inhibitions when they were subjected to ethanolic root extracts than to aqueous root extracts, thus showing that the pathogens were highly susceptible to the ethanolic root extracts than the other form. The results indicated that in general, the ethanolic root extracts had greater inhibitory effects on the isolates than the leaves extract forms. This agrees with Monon et al. (2015), who reported that the root extract of U. chamae has high antibacterial efficacy.
The present study also showed that some wound isolated strains of P. aeruginosa and P. mirabilis species used in the study were resistant to both the crude extracts of U. chamae and standard antibiotics but this does not portray that these antimicrobials have no antibacterial potency but rather, these strains of P. aeruginosa and P. mirabilis were able to acquire resistance genes that are commonly found on plasmids. In the study, the strains of P. aeruginosa and P. mirabilis species harbored resistant plasmids. Akinnibosun and Adeola (2015) also reported from their works some P. aeruginosa and P. mirabilis that acquired plasmids and formed resistance to multiple antibiotics used in their study.
The plasmids isolated from the test organisms varied widely with regard to size and molecular weight. Some isolates harbored low molecular weights, while some were high, and were even seen with multiple plasmids bands. The molecular weight of the plasmid varies depending on the size of the plasmids. Reports by Yah et al. (2007) in their work reported that the sizes of the plasmids from Proteus species varied. Norma et al. (2004) reported that most organisms acquire their antibiotic resistance genes through transposons, chromosomes or from other plasmids, and that it is necessary to elaborate and enhance ways of controlling these phenomena. Many other researchers such as Ruth et al. (2011), Mordi and Momoh (2009), and Ismaeil and Kadhim (2017), in their work reported that Proteus species developed a wide range of resistance to several antibiotics. Asad, and Amna (2004) likewise reported from their work plasmid-mediated multiple antibiotic resistance in Proteus isolates from their work. Enabulele et al. (2006), Yah et al. (2007) and Auwaerter (2008) reported that Proteus species vary in their susceptibility pattern due to transfer of plasmid resistant genes among the gram negative organisms. Murry et al. (1998), Mukhtar and Tukur (2000) and Taura et al. (2014) likewise observed and ascertained that P. aeruginosa is inherently resistant to many antibiotics and can have the possibility of mutating to even more resistant strain during treatment. Moreover, according to Piddock (2006), these genes conferring antibiotic resistance are commonly found on elements known as integrons and transposons, which facilitate movement between different replicons such as between the bacterial chromosome and a plasmid. Moreover, Nikaido (2009) reported that R-plasmids found in bacteria often contain several resistance genes and these genes are steadily maintained in the host strains of bacteria in a stable manner and are transferred efficiently to other neighboring drug-susceptible bacterial cells which later become resistant to multiple antimicrobial agents. Remarkably, many such genes are often present on a single R- plasmid, so that multidrug resistance can be transferred to a susceptible bacterium in a single conjugation event. Hence, it is wise that treatment of infections caused by these isolates should be guided by adequate laboratory sensitivity test result.
It was further observed in this study that after plasmid curing, some isolates were susceptible to the crude extract and the routine antibiotics with increased zones of inhibition. This corroborates with report by Akinnibosun and Adeola (2015), who in their work recorded increased zones of inhibition after plasmid curing of the test isolates. However, in the study, some test isolates were still resistant to both crude extracts of U. chamae and standard antibiotics. This probably may be that some of them harbored resistant genes that could not be eliminated by acridine orange and possibly, the resistance observed in this work could be both plasmid and chromosomal mediated. This agrees with Bush et al. (1995), who stated that, the production of plasmid or chromosomal encoded b-lactamase enzyme is the most common mechanism of resistance in Gram-negative bacteria causing clinical significant infections. Piddocks (2006) affirmed that genes encoding efflux pumps resistance can be found on the chromosome and these genes can chromosomally encode multidrug resistance (MDR) efflux pumps. The reason why the intrinsic mechanism for multidrug resistance could be chromosomal is that it probably may be hard to remove by acridine orange as observed in this work.
The fact that crude extracts of both ethanolic and aqueous forms of U. chamae showed some antimicrobial activities against most of the test organisms is a major breakthrough in appreciating the medicinal potential of this plant especially in the management of wound infection. U. chamae could be exploited for the isolation of active principles for drug formulation with proper dose given for treatments of various infectious diseases caused by these test isolates. However, the resistance and plasmid profiles of these wound isolated strains of P. aeruginosa and P. mirabilis used in the study for both the crude extracts of U. chamae and standard antibiotics is of a great concern as the isolates exhibited multidrug resistance patterns that are mediated by both plasmids and chromosomes. Therefore, assessment and caution should be given during prescription and administration of any antimicrobial agents for proper and effective treatment of cuts, wounds and other ailments caused by these organisms.
The authors have not declared any conflict of interests.
The authors are grateful to Mr Etefia who brought the plant; other staff of Pharmacognosy Laboratory, University of Uyo, Akwa Ibom State; staff of Microbiology Laboratory, University of Uyo, Akwa Ibom State, Nigeria; along with staff of Biotechnology and Biochemistry Units, Nigerian Institute for Medical Research (NIMR), Yaba, Lagos State, Nigeria where extraction of plasmids was carried out for their assistance and cooperation.
REFERENCES
|
Adams MR, Moss MO (1999). "Food Microbiology Cambridge". The Royal Society of Chemistry, pp. 471-476.
|
|
|
|
Akinjogunla OJ. Enabulele IO (2010). Virulence Factors, Plasmid Profiling and Curing analysis of Multi-drug Resistant Staphylococcus aureus and Coagulase negative Staphylococcus spp. isolated from Patients with acute otitis media. Journal of American Sciences 6(11):1022-1033.
|
|
|
|
Akinnibosun FI Adeola MO (2015). Quality Assessment and Proximate Analysis of Amaranthus hybridus, Celosia argentea and Talinum triangulare obtained from open Markets in Benin City, Nigeria. Journal of Applied Science and Environmental Management 19(4):727-734.
Crossref
|
|
|
|
Akortha EE, Filgona J (2009). Transfer of Gentamicin resistance genes among Enterobacteriaceae isolated from the outpatients with urinary tract infections attending 3 hospitals in Mubi, Adamawa State. Science Research and Essay 4(8):745-752.
|
|
|
|
Akujobi C., Anyanwu B., Onyeze N, Ibekwe, V.I. (2004) Antibacterial Activities and Preliminary Phytochemical Screening of Four Medicinal Plants". Journal of Applied Science 7(3):4328-4338.
|
|
|
|
Ameh GI. (2010) Evaluation of the phytochemical composition and antimicrobial properties of crude methanolic extract of leaves of ocimum gratissimum. Journal of Natural Sciences, Engineering and Technology 9(1):147-152
|
|
|
|
Andy IE, Eja, WE, Mboto CI (2008). An evaluation of the antimicrobial potency of Lansianthera africana (BEAUV) and Heinsia crinata (G.Taylor) on Escherichia coli, Salmonella typhi, Staphylococcus aureus and Candica albicans. Malaysian Journal of Microbiology 4(1):25-29.
|
|
|
|
Asad UK, Amna M (2004). Plasmid-mediated multiple antibiotic resistance in Proteus mirabilis isolated from patients with urinary infection. Medical Science Monitor 10(1):598-602.
|
|
|
|
Auwaerter P (2008). Antibiotic guide Johns Hopkins ABX (antibiotic) Guide, Baltimore MD.
|
|
|
|
Batisa M, De Almeida A, De Pietro-Magri L, Toma W, Calvo T, Villegas W, Souza T (2004).Gastric and anti-ulcer activity of Syngonanthus arthroticus(Bill). Pharmaceutical Bulletin 27(3):328-332.
Crossref
|
|
|
|
Bowler P, Duerden B, Armstrong D (2001). Wound microbiology and associated approaches to wound management. Clinical Microbiological Review 14(2): 244-269.
Crossref
|
|
|
|
Burile E, Bonamomi G, Antignam V, Zolfughari B, Sajjadi SE, Scala F, Lanzolti V (2009). Saponins for Allium Minutiflorum with antifungal activity. Phytochemistry 68(5):596-603.
Crossref
|
|
|
|
Bush K, Jacopy GA, Medieiros AA (1995). "A Functional Classification Scheme of b- Lactamase and its Correlation with Molecular Structure". T. Antimicrobiological. Agent Chemotherapeutics 39:1211- 1233.
Crossref
|
|
|
|
Cheesbrough M (2000). District Laboratory Practice in Tropical Countries( Part2), Cambridge University Press, UK. pp. 134-137.
|
|
|
|
Clinical Laboratory Standard Institute (CLSI) (2009). Performance Standards for Antimicrobial Susceptibility Testing; Nineteenth Informational Supplement. CLSI document M100-S19. CLSI, Wayne PA.
|
|
|
|
Ehrenfeld EE, Clewell DD (1987). Transfer functions of Streptococcus faecalis plasmid pAD1: Organization of plasmid DNA encoding response to sex pheromone. Jounal of Bacteriology 169:3461-3473.
Crossref
|
|
|
|
Ejikeugwu C, Ugwu M, Iroha I, Gugu T, Duru C, Eze P, Esimone C (2013) Detection and antimicrobial susceptibility of some gram negative bacteria producing carbapenemases and extended spectrum β-Lactamases. International Journal of Microbiolological and Immunological Research 2(6):064-069.
|
|
|
|
Ekong US, Udoh DI (2015a). Phytochemical composition and comparative assessment of antibacterial activity of Honey against clinical bacterial isolates by Agar diffusion techniques. World Journal of Applied Sciences and Technology 7(1):16-23)
|
|
|
|
Ekong US, Udoh DI (2015b). Phytochemistry and comparative analysis of the antitussive potential of Allium sativum and Garcinia kola against clinical isolates of respiratory tract infections. World Journal of Applied Sciences and Technology 7(2):140- 148.
|
|
|
|
Enabulele IO, Yah SC, Yusuf EO, Eghafona NO (2006). Emerging quinolones resistant transfer genes among gram-negative bacteria,isolated from faeces of HIV/AIDS patients attending some Clinics and Hospitals in the City of Benin, Edo State, Nigeria. Online Journal of. Health and Allied Science 3:3.
|
|
|
|
Esimone CO, Adiukwu MU, Okonta JM (1998). Preliminary Antibacterial Screening of Ethanolic Extracts from Uvaria chamae (P.Beav). Journal of Pharmaceutical Research and Development 32(2):99-102.
|
|
|
|
Etukudo I (2003). "Ethnobotany Conventional and Traditional Uses of Plants", The Verdict Press P 191.
|
|
|
|
Godfraind T (1984). Mechanism of action cardiac glycosides. European Heart Journal 5:301-308.
Crossref
|
|
|
|
Gunzalez G, Bronze MS, Boyd DR (2018). Proteus Infections. Infectious Diseases. Medscape.
View
|
|
|
|
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994). Bergeys manual of determinative bacteriology(9th ed.) The Williams and Wilkins Company Baltimore, Maryland, USA pp. 660-980.
|
|
|
|
Igoli JO, Ogaji TAM, Tor-Anyiin Igoli NP (2005). Traditional Medicine Practice Amongst the Igede People of Nigeria. Part II. African. Journal of. Traditional Complementary and Alternative. Medicines 2(2):134-152.
Crossref
|
|
|
|
Iroabuchi F (2008). "Phytochemical Constituent" of Uvaria chamae (P.Beav) M. Sc Thesis, Micheal Okpara University of Agriculture. Umudike, Nigeria, pp. 3-16.
|
|
|
|
Ismaeil MI, Kadhim AS (2017). Antimicrobial resistance patterns and extended spectrum beta-lactamases producing by Proteus mirabilis isolated from different sources. Al-Mustansiriyah Journal of Science 28(1):42- 54.
Crossref
|
|
|
|
Iwu EC (1993). Ethnobotanical importance of some Nigerian plants. Journal of Ethnomedicine 55:893-906.
|
|
|
|
Karachi (2006). Importance of Medicinal Plants. Highlighted.
|
|
|
|
Lipps G Plasmids (2008) Current research and food trends. Caiser Academic press USA, pp. 10-35.
|
|
|
|
Monon K, Abdoulaye T, Karamoko O, Adama C (2015). Phytochemical Composition, Antioxidant and Antibacterial Activities of Root of Uvaria chamae P. Beauv. (Annonaceae) Used in Treatment of Dysentery in North of Côte d'Ivoire. International Journal of Pharmacognosy and Phytochemical Research 7(6):1047-1053.
|
|
|
|
Mordi RM, Momoh MI (2009). Incidence of Proteus species in wound infections and their sensitivity pattern in the University of BeninTeaching Hospital. African Journal of Biotechnolology 8(5):725-730.
|
|
|
|
Mukhtar MD, Tukur A (2000). Antibacterial activity of aqueous and ethanolic extract of Pistia xlratiotes. L. Journal of the Nigerian Society for Experimental Biology 1(1):51-59.
|
|
|
|
Murry PR, Rosenthal KS, Koba-geshi GS, Ptaller MA (1998). Medical microbiology. 3rd Edition. Published by lLibrary of Congress Cataloging. pp.175-183.
|
|
|
|
Nascimento GGF, Lacatelli J, Freitas PC, Sile GL (2000). Antibacterial Activity of Plants Extracts and Phytochemicals on Antibiotic-Resistant Bacteria. Brazilian Journal of Microbiology 31(4): 886-891.
Crossref
|
|
|
|
Norma SL, Anita T, Dailia PR, Eliane, Bianca RQ, Ernesto H (2004). Antimicrobial resistance and R-plasmid in Salmonella spp. from swine and abatoir5 environment. Pesquisa Veterinária Brasileira - Brazilian Journal of Veterinary Research 24(2):57-60.
Crossref
|
|
|
|
Nikaido H (2009). Multidrug Resistance in Bacteria. Annual Review of Biochemistry 78:119-146.
Crossref
|
|
|
|
Obi VI, Onuaha C (2000). "Extraction and characterization method of plants and plants products in biological and agricultural technique". (Ogbulie JN and Ojiako OJ (Eds) ) Webmedia Publication, Owerri. pp. 271-286.
|
|
|
|
Ogbulie JN, Ogueke CC, Okorondu S (2004). Antibacterial Properties of A. Corditola, M. Flurum, U. Chamae, B. Pinnatum, C. albidem and A. Cilata on some hospital isolates. Nigerian Journal of Microbiolology 18 (1-2):249-255.
|
|
|
|
Ogbulie JN, Ogueke CC, Nwanebu FC (2007). Antibacterial properties of Uvaria chamae, Congromena latifolium, Garcinia kola, Vemonia amygdalina and Afromomium melegueta. African Journal of Biotechnology 6(13):1549-1553.
|
|
|
|
Ogueke CC, Ogbulie JN, Njoku HO (2006). Antimicrobial properties and preliminary phytochemical analysis of ethanolic extracts of Alstonia bonnie. Nigerian Journal of Microbiolology 20(2):896-899.
|
|
|
|
Okokon E, Ita, BN, Udokpoh AE (2006). The in-vivo antimalarial activities of Uvaria chamae and Hippocratea Africana. Annals of Tropical Medicine and Parasitology 100(7):585-590.
Crossref
|
|
|
|
Okwu DE, Iroabuchi F (2004). Phytochemical analysis and antimicrobial activity screening of aqueous and ethanolic root extracts of Uvaria chamae BEAV and C ferriginea DC. Journal of Chemical Society of Nigeria 29(2):112-114
|
|
|
|
Okwuosa OM, Chukwura EI, Chukwuma GO, Okwuosa CN, Enweani IB, Agbakoba NR, Chukwuma CM, Manafa PO, Umedum CU (2012). Phytochemical and antifungal activities of Uvaria chamae leaves and roots, Spondias mombin leaves and bark and Combretum racemosum leaves. African Journal of Medicine and Medical Sciences 41:99-103.
|
|
|
|
Oluremi BB, Osungunna MO, Omafuma OO (2010). Comparative Assessment of Antibacterial Activity of Uvaria chamae Parts. African Journal of Microbiological Research 4(13):1391-1394.
|
|
|
|
Olumese FE, Onoagbeb IO, Eze GI, Omoruyi FO (2016). Safety assessment of Uvaria chamae root extract: acute and subchronic toxicity studies Journal of African Association of Physiological Sciences 4(1):53-60.
|
|
|
|
Omajali JB, Hussaini JS, Omale J (2011). Cytotoxicity and anti-inflammatory studies on Uvaria chamae. Journal of Pharmacology and Toxicology 2(7):1 - 9.
|
|
|
|
Omale J, Ebiloma UG, Idoko GO (2013). Uvaria chamae (Annonaceae) Plant Extract Neutralizes Some Biological Effects of Naja nigricollis Snake Venom in Rats. British Journal of Pharmacology and Toxicology 4(2):41-50.
Crossref
|
|
|
|
Piddock LJV (2006). Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clinical Microbiological Review 19(2):382-402.
Crossref
|
|
|
|
Ruth AA, Damian CO, Romanus II, Charles OE (2011). Antimicrobial resistance status and prevalence rates of extended spectrum beta-lactamase producers isolated from a mixed human population. Bosnian Journal of Basic Medical Sciences 11(2):91-96.
Crossref
|
|
|
|
Sheikh AR, Afsheen A, Sadia K, Abdu W (2003). Plasmid borne antibiotic resistance factors among indigenous Klebsiella. Pakistan Journal of Microbiology 35(2):243-248.
|
|
|
|
Sher A (2009). Antimicrobial activity of natural products from medicinal plants. Gomal Journal of Medical Science 7:72-78.
|
|
|
|
Soforowa EA (2008). Medicinal Plants and Traditional Medicine in Africa. (3rd Edition) Spectrum Books Ltd, Nigeria pp. 181-207.
|
|
|
|
Trease GE, Evans WC (1994). Textbook of Pharmacognosy (12th edn.) Balliese Tindall andCompany Publisher, London pp. 276-383.
|
|
|
|
Taura DW, Lawan S, Gumel SM, Umar, Sadisu UF (2014). AntiBacterial Activity of Ethanolic Extract of Zingiber Officinale and Pipper Nigrum against Some Clinical Isolates. Communications in Applied Sciences 2(1):52-64
|
|
|
|
Tsuchiya H, Sato M, Miyazaki T, Fujiwara, Tanigaki O, Tanaka T, Iinuma M (1996).Comparative study on the antibacterial activity of phytochemical flavanones against methicillin resistant Staphylococcus aureus. Journal of Ethnopharmacology 50:27-34
Crossref
|
|
|
|
Udoh DI, Udokpoh AE, Udofia SO (2011). Antimicrobial properties of the dark Variety of Lasianthera africana (Obubit Editan) Leaves' Extracts on Some Clinical Isolates. Journal of Medical Laboratory 20(2):1-9.
|
|
|
|
Udoh DI, Asamudo NU, Danladi-Ngyan Bala DN, Otung E (2012). Inhibitory Effect of Varying Concentrations of Leaves' Extracts of Centella asiatica (Gotu Kola) on Some Microorganisms of Medical Importance. International Journal of Chemical, Environmental and Pharmaceutical Research 3(2):142-148.
|
|
|
|
Udoh DI, Otu-Bassey IB, Umoh IE (2017). Evaluation of the Phytochemical and Antibacterial Properties of Crude Extracts of Ocimum gratissimum (Scent Leaves) on some Clinical Isolates. World Journal of Biomedical Research 4(2):32-3
|
|
|
|
Udoh DI, Johnson E, Asuquo S (2018). Phytochemical And Antibacterial Properties Of Different Forms Of crude Stem Bark Extract Of Uapaca Staudtii . CIBTech Journal of Microbiology 7(3):1-10.
|
|
|
|
Vârban DI, Duda M, Vârban R, Muntean S (2009). Research Concerning the Organic Technology for Satureja Hortensis L. Culture Bulletin UASVM Agriculture 66(2):225- 229.
|
|
|
|
Wang J, Li J, Jiang W (2010). Antifungal activities of neem (Azadirachta indica) seed kernel extracts on postharvest diseases in fruits. African Journal Microbiological Research 4:1100-1104.
|
|
|
|
World Health Organization WHO (2004). Drug Discovery and Drug Development 2004. Available online at who.int (accessed 11th September, 2013).
|
|
|
|
Yah SC, Eghafona NO, Oranusi S, Abouo AM (2007). Widespread plasmid resistance genes among Proteus species in diabetic wounds of patients in the Ahmadu Bello University Teaching Hospital (ABUTH) Zaria African Journal of Biotechnology 6 (15):1757-1762.
Crossref
|
|
|
|
Yah SC, Enabulele IO, Eghafona NO (2004). Bacteriological studies on infected Kerosene burn wounds in Benin City, Nigeria. Journal of Biomedical Investment 2(1): 4-9.
Crossref
|