ABSTRACT
Nosocomial infections are enhanced due to a flop in the infection control processes such as disinfection. The aim of this study was to assess long term effectiveness and the stability of disinfectants currently used within healthcare settings in Accra, Ghana against two indicator pathogens. Two locally produced and two imported disinfectants usually used in health care settings were obtained. The efficacy and long-term stability of the disinfectants was done using the in-use method, with identified microbial culture isolates of Pseudomonas aeruginosa and Staphylococcus aureus. Counts of both test organisms (Pseudomonas aeruginosa and Staphylococcus aureus) were over and above the 5 ≥ CFU recommended standard. All the disinfectants failed the test. However, contamination was observed to be higher in local disinfectants as compared to those imported. Furthermore, Enterobacter spp was isolated as contaminant from one imported disinfected and Proteus spp was also isolated from one local disinfectant. Interestingly, one of the imported disinfectants (Disinfectant I) showed more stability and was effective in the long term. All disinfectants did not pass the in-use test. However, disinfectants that are produced locally are more unstable and ineffective.
Key words: Infections, disinfectants, drug stability, hospital, pathogens, contamination.
Disinfectants are widely used in hospitals and various health care facilities for diverse applications, including topical as well as hard surface. Generally, they are important part of infection control practices and help in the prevention of hospital acquired infections (HAI) or nosocomial infections (Cadnum et al., 2017; Liu and Dickter, 2020; Rutala, 1951; Ling, 2020). HAI are one of the foremost infectious diseases that present an enormous economic effect globally (Barrasa-Villar et al., 2017; Kollef et al., 2021). HAI accounts for the hospitalization of about two (2) million people worldwide annually (Abbasi et al., 2010). Empirical evidence shows that the occurrence of HAI is more than two folds higher in the developing world (Nasiri et al., 2021; Nejad et al., 2011). The hospital environment serves as an essential reservoir for various infectious microorganisms. Thus, the prevention and control of HAI is a matter of grave concern and a key challenge to contend with (Liu and Dickter, 2020; Ling, 2020). This is because within the health care environment, inanimate objects are potential conduits for the transmission of infections microorganisms. However, disinfection provides the avenue in to help break the epidemiological sequence of infections (Liu and Dickter, 2020). Disinfection involves the application of chemical agents to remove microorganisms, except spores of bacteria (Liu and Dickter, 2020; Ling, 2020). The level of annihilation of microorganisms is largely dependent on their relative sensitivities to the chemical disinfection process. Usually high-level disinfection comprises the removal of all except huge quantities of bacteria spores, second, intermediate level disinfection provides for the annihilation of all microbial life excluding spores. Low-level disinfection will not dependably destroy mycobacteria or spores.
Multiple HAI outbreaks resulting from the use of a contaminated disinfectants are well documented (Kampf, 2018; Rosca et al., 2021). For example, in 1966, Mitchell and Hayward reported that seven different incidents of urinary tract infections observed in children following a cystoscopy procedure, were later traced to the contamination of chlorhexidine solution that was applied for the disinfection to bladder-irrigation reservoir. Similarly, Dulake and Kidd (Dulake and Kidd, 1966; Pitten et al., 2003) described the isolation of Alcaligenes foecalis from samples of urine obtained from thirty gynaecological patients. These patients underwent bladder drainage via indwelling catheter. The cause of the infection was subsequently traced to a jar that contained 0.1% chlorhexidine, which was used for spigots storage (Dulake and Kidd, 1966). Disinfectants could be contaminated due to extrinsic and intrinsic occurrences. Inappropriate manufacturing techniques or through transportation unusually accounts for intrinsic occurrences (intrinsic contamination). Extrinsic contamination on the other hand occurs during the use of the disinfectants within the healthcare environment Extrinsic contamination is further reflected in contaminated in stock of disinfectants, that are contaminated not replacing disinfectants after long usage, failure to wash disinfectant jars prior to refilling as well as refilling containers that are contaminated (Rosca et al., 2021).
However, on the market today there are varieties of products available that presents with moderate or even inadequate antimicrobial action (Pitten et al., 2003). Ideally disinfectants must possess ‘permanent’ antimicrobial activity without compromising the danger of engendering resistant microorganisms. Thus, disinfectants must not only be easy to use but safe, and effective against an extensive range of microbial pathogens, without leaving any toxic residues (Tipton et al., 2018; Simoes et al., 2010; Fraise, 1999). However, many medical facilities in developing nations are still actively using phenolic disinfectants, contrary to the developed nations where their use is being discouraged. Similarly, the use of gluteraldehydes has been ceased, due to toxicity and related issues (BSG Guidelines, 2003). However, in developing nations, they are still in active use. Additionally, some local companies have gotten into the production of disinfectants locally with very little or no regulation to guarantee quality.
Regrettably, most healthcare facilities such as hospitals in developing world, lack the needed resources to evaluate and monitor the efficacy of these new disinfectants against set microbiological standards before usage. The aim of this paper was to assess long term effectiveness and the stability of disinfectants currently used in healthcare settings in Accra, Ghana.
Disinfectants usually used in hospitals and other health care settings in Accra were randomly selected for this research work. Two locally produced and two imported disinfectants were obtained randomly from different places within Accra. The samples obtained were labelled and transported to the laboratory in their original packages. Contents of the various disinfectants were aseptically withdrawn from their respective containers for the antimicrobial study. They were then prepared according to manufacturer’s instructions. The composition and pH of the disinfectants used in the study are presented in Table 1. Data analysis was performed using GenStat statistical analysis soft software.
Microbial culture and inoculum preparation
Identified microbial culture isolates of Pseudomonas aeruginosa - ATCC 15442 and Staphylococcus aureus - ATCC 8043 were obtained for this study. A 24 h culture of the test isolates was prepared; Staphylococcus aureus isolate was prepared on a Baird Parker agar (Oxoid). Pseudomonas aeruginosa on the other hand was prepared on a Pseudomonas C-N agar (Oxoid). Each of the isolates was subsequently sub-cultured into 1% sterile peptone water and the turbidity concentration standardized to 0.5 McFarland standards corresponding to 108 cfu ml-1. Pure culture inoculum of both microbes was thoroughly mixed on a vortex mixer for 2 min after which 2.5 ml of each organism was pipetted aseptically into a sterile universal bottle. A mixed inoculum from the two microbes was also prepared and the turbidity standardized as above.
Disinfectant testing
The efficacy and long-term stability of the disinfectants obtained was done using the in-use method (Burdon and Whitby, 1967). The in-use test method was opted for because of its ease to use and requires no sophisticated or complicated equipment. Plastic screw-cupped tubes, each containing 9 ml of disinfectants, were used. The manufactures’ instructions were followed to the letter during the preparation of the disinfectant for the test. Tubes were prepared in triplicates (3 sets) for each disinfectant as follows: 1 for Pseudomonas aeruginosa, 1 for Staphylococcus aureus and 1 for a mixture of both microbes. Dirty conditions were stimulated by the addition of 1ml of blood to each test tube. 1 ml of each standard inoculum was then added to each disinfectant. The preparations were then then kept at room temperature in the laboratory for 4 days. 1 ml of the prepared sample was aseptically withdrawn from each disenchants after a proper shake to mix. It was then added to sterile peptone water (0.1%) that contained 3% between 80 (an activator). Ten drops form each diluted sample was subsequently placed on Nutrient Agar plates, and kept for seven days at 37°C in an incubator and also at room temperature. Contamination was indicated by the presence of 5 or more colonies on either plate.
The results in Table 2 present the sensitivity results of Pseudomonas aeruginosa to the four disinfectants that were tested. The results as shown in Table 2 indicate that at both room temperature and at 37°C incubation all the disinfectants (4) failed the test against Pseudomonas aeruginosa. A similar observation is in seen in Table 3, where Staphylococcus aureus was used as the test organism. However, Local products (disinfectant III and disinfectant IV) had higher counts (Too Numerous to Count- TNTC) of Staphylococcus aureus than imported products (disinfectant I and disinfectant II) which ranged from 92CFU-320CFU per plate. Sensitivity of a mixed inoculum of Staphylococcus aureus and Pseudomonas concentrations tested. However, locally produced disinfectants (disinfectant III and disinfectant IV) had higher microbial counts were compared to imported products (disinfectant II and disinfectant I). Interestingly, Enterobacter spp was isolated as a contaminant in disinfectant II, while Proteus spp was also isolated from disinfectant III.
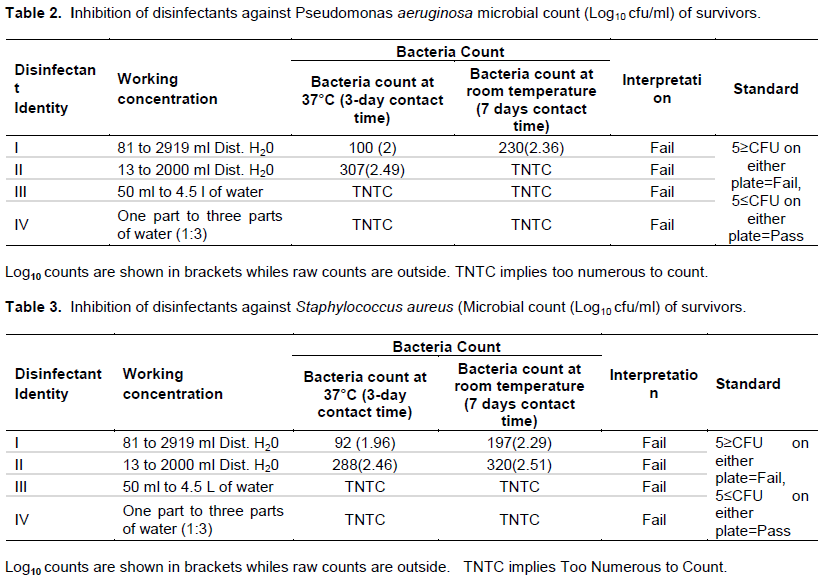
The results from this current study found all of disinfectants tested to be contaminated at the conditions and concentrations tested. Generally, locally produced disinfectants, both of which contain chlorine compounds were found to be more contaminated compared to disinfectants that were imported. The very high resistance of Staphylococcus aureus and Pseudomonas aeruginosa to the tested disinfectants (Table 4) is of great concern. Staphylococcus aureus and Pseudomonas aeruginosa are associated pneumonia, cystic fibrosis and chronic wound (Kawamura-Sato et al., 2010; Harrison, 2007). Originally, only a hostile relationship between both organisms was portrayed as the existence of one is associated with the absence of the other (Harrison, 2007). In wound infection for example, Staphylococcus aureus mostly inhabits the wound surface; whereas Pseudomonas aeruginosa is located in the deep layers (Kirketerp-Møller, 2008). However, in this study Staphylococcus aureus and Pseudomonas aeruginosa appeared to have acted in synergy presenting with high resistance and contamination (Table 4).
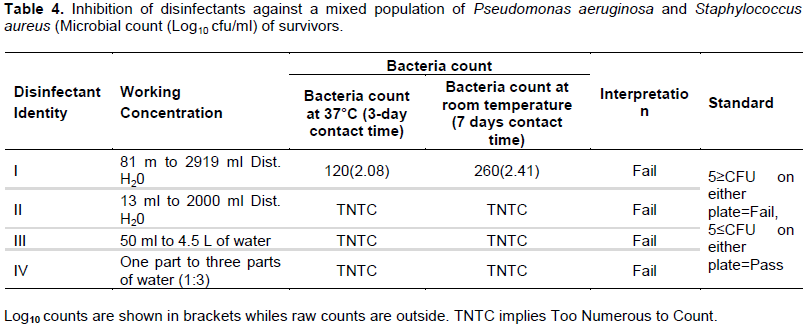
This study also found that disinfectant I and III were contaminated with Enterobacter spp and Proteus spp respectively. Proteus rods are well known opportunities microbial pathogen. They cause urinary tract infection under favorable conditions (Hasan et al., 2021; Hamilton and Kamm, 2018). They have also caused rheumatoid arthritis, meningitis in infants, and wound infection (Hasan et al., 2021). Enterobacter species on the other hand are known to cause a wide range of hospital acquired infections including but not limited to those that affect the urinary tract, the lungs, intraabdominal cavity (Toleti et al., 2015; Dautzenberg et al., 2018). E. sakazakii causes meningitis and neonatal sepsis (Elkhawaga et al., 2020). Thus, the contamination of disinfectants as observed in this study by the aforementioned pathogenic microorganisms is of huge significance. In the case of disinfectant II and disinfectant IV, no microorganisms aside the test bacteria were identified; which suggest these disinfectants were more active in the presence of organic matter than the other disinfectants.
Contamination of disinfectants has been linked to nosocomial infection and thus it is of a serious concern. Several studies have demonstrated this. For example, contaminated solution of chlorhexidine was reported by Mitchell and Hayward (1966) to have caused urinary tract infections in children after a cystoscopy procedure.
Contaminated disinfectants could arise due to a number of reasons including but not limited contaminated disinfectant stock, prolonged usage of disinfectants without changing, improper washing and cleaning of jars before the next refilling and refill of containers that are contaminated (Kirketerp-Møller, 2008). In this present study, the contamination observed in the disinfectants could be as a result of overgrowth of test organisms and residual microorganisms as a result of the inactivation of some of the disinfectants tested. This may be accounted for when the inoculum was left until the fourth day in the disinfectant. Furthermore, the blood that was added to stimulate dirty condition may have also aided the subsequent in activation of the active ingredients in the disinfectants. In an earlier study, Lewis and Arens (1995) indicated all manner of organic matter presented in the form of fecal, pus, blood or lubricant material may have an adverse interference with the antimicrobial activity and potency of disinfectants. This may occur in two ways first, through a chemical reaction between disinfectant and the organic material, thus less active ingredient in the disinfectant to attack the microbes. Second, the organic material may shield the microbes from attack by forming a physical barrier (Lewis and Arens, 1995; Sasaki and Imazato, 2020).
CONCLUSION AND RECOMMENDATIONS
In conclusion, all though, all disinfectants did not pass the in-use test, disinfectants that are produce locally are more unstable and ineffective. However, the key purpose of disinfectant usage is premised on their ability to efficiently and effectively curtail the spread of pathogenic microorganisms transmitting by direct or indirect contact within the healthcare environment. This has dire implication for infection control measures. This is because health workers and patients seeking health services in healthcare settings may acquire infection through contaminated and ineffective disinfectants. Efforts ought to be made towards the regular testing of disinfectants that are used in the hospital to monitor and ensure its efficacy.
The efficacy and long-term stability of the disinfectants was done using only, two identified microbial culture isolates (Pseudomonas aeruginosa and Staphylococcus aureus). Thus, recommendations cannot be generalized to all bacteria. However, this effect is mitigated since Pseudomonas aeruginosa (gram negative) and Staphylococcus aureus (gram positive), represents the two main classes of bacteria that is, gram-negative and gram-positive bacteria.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbasi FM, Ahmad H, Perveen F, Inamullah, Sajid M, Brar DS (2010). Assesment of genomic relationship between Oryza sativa and Oryza australiensis. African Journal of Mathematics and Computer Science Research 9(12):1312-1316.
Crossref
|
|
|
|
Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca-Doñate R, Moliner-Lahoz J (2017). Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clinical Infectious Diseases 65:644-52.
Crossref
|
|
|
|
|
BSG Guidelines: Guidelines for Decontamination of Equipment for Gastrointestinal endoscopy, 2003 at
View
|
|
|
|
|
Burdon D, Whitby JL (1967). Contamination of hospital disinfectants with Pseudomonas species. British Medical Journal 2(5545):153.
Crossref
|
|
|
|
|
Cadnum JL, Shaikh AA, Piedrahita CT, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ (2017). Effectiveness of disinfectants against Candida auris and other Candida species. Infection Control and Hospital Epidemiology 38(10):1240-1243.
Crossref
|
|
|
|
|
Dautzenberg MJ, Bayjanov JR, Leverstein-van Hall MA, Muller AE, Gelinck LB, Jansen CL, Leyten EM, Ruys T, Scharringa J, van der Starre RE, Fluit AC (2018). Dynamics of colistin and tobramycin resistance among Enterobacter cloacae during prolonged use of selective decontamination of the digestive tract. Antimicrobial Resistance and Infection Control 7(1):1-9.
Crossref
|
|
|
|
|
Dulake C, Kidd E (1966). Contaminated irrigating fluid. The Lancet 287(7444):980.
Crossref
|
|
|
|
|
Elkhawaga AA, Hetta HF, Osman NS, Hosni A, El-Mokhtar MA (2020). Emergence of Cronobacter sakazakii in cases of neonatal sepsis in upper Egypt: first report in North Africa. Frontiers in Microbiology 11:215.
Crossref
|
|
|
|
|
Fraise AP (1999). Choosing disinfectants. Journal of Hospital Infection 43(4):255-264.
Crossref
|
|
|
|
|
Hamilton AL, Kamm MA, Ng SC, Morrison M (2018). Proteus spp. asputative gastrointestinal pathogens. Clinical Microbiology Reviews 31(3):e00085-17.
Crossref
|
|
|
|
|
Harrison F (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153(4):917-923.
Crossref
|
|
|
|
|
Hasan TH, Alasedi KK, Jaloob AA (2021). Proteus Mirabilis Virulence Factors. International Journal of Pharmaceutical Research 13(1).
Crossref
|
|
|
|
|
Kampf G (2018). Efficacy of ethanol against viruses in hand disinfection. Journal of Hospital Infection 98(4):331-338.
Crossref
|
|
|
|
|
Kawamura-Sato K, Wachino JI, Kondo T, Ito H, Arakawa Y (2010). Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. Journal of antimicrobial chemotherapy 65(9):1975-1983.
Crossref
|
|
|
|
|
Kirketerp-Møller K, Jensen PØ, Fazli M, Madsen KG, Pedersen J, Moser C, Tolker-Nielsen T, Høiby N, Givskov M, Bjarnsholt T (2008). Distribution, organization, and ecology of bacteria in chronic wounds. Journal of clinical microbiology 46(8):2717-2722.
Crossref
|
|
|
|
|
Kollef MH, Torres A, Shorr AF, Martin-Loeches I, Micek ST (2021). Nosocomial Infection. Critical care medicine 49(2):169-187.
Crossref
|
|
|
|
|
Lewis DL, Arens M (1995). Resistance of microorganisms to disinfection in dental and medical devices. Nature Medicine 1(9):956-958.
Crossref
|
|
|
|
|
Ling HW (2020). What do We Need to Know to Prevent and Control Nosocomial Infections Completely? International Journal of Collaborative Research on Internal Medicine and Public Health 12(4):1021-1021.
|
|
|
|
|
Liu JY, Dickter JK (2020). Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointestinal Endoscopy Clinics 30(4):637-652.
Crossref
|
|
|
|
|
Mitchell RG, Hayward AC (1966). Postoperative urinary-tract infections caused by contaminated irrigating fluid. The Lancet 287(7441):793-795.
Crossref
|
|
|
|
|
Nasiri N, Gholipour S, Akbari H, Koolivand A, Abtahi H, Didehdar M, Rezaei A, Mirzaei N (2021). Contamination of obsterics and gynecology hospital air by bacterial and fungal aerosols associated with nosocomial infections. Journal of Environmental Health Science and Engineering 19(1):663-670.
Crossref
|
|
|
|
|
Nejad SB, Allegranzi B, Syed SB, Ellis B, Pittet D (2011). Health-care-associated infection in Africa: a systematic review. Bulletin of the World Health Organization 89:757-765.
Crossref
|
|
|
|
|
Pitten FA, Werner HP, Kramer A (2003). A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. Journal of Hospital Infection 55(2):108-115.
Crossref
|
|
|
|
|
Rosca I, Ursu EL, Fifere A (2021). A Microbiological Epilogue-Nosocomial Infections. New Trends in Macromolecular and Supramolecular Chemistry for Biological Applications, pp. 179-189. Springer, Cham.
Crossref
|
|
|
|
|
Rutala WA (1951). Guidelines Committee: Draft Apic guideline for selection and use of disinfectants. American journal of infection control 17(1):24A-38A.
Crossref
|
|
|
|
|
Sasaki JI, Imazato S (2020). Autoclave sterilization of dental handpieces: A literature review. Journal of Prosthodontic Research 64(3):239-242.
Crossref
|
|
|
|
|
Simoes M, Simoes LC, Vieira MJ (2010). A review of current and emergent biofilm control strategies. LWT-Food Science and Technology 43(4):573-583.
Crossref
|
|
|
|
|
Tipton KA, Chin CY, Farokhyfar M, Weiss DS, Rather PN (2018). Role of capsule in resistance to disinfectants, host antimicrobials and desiccation in Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy 62(12):e01188-18.
Crossref
|
|
|
|
|
Toleti S, Manivasagan M, Prudhve S, Raavi T, Shaik N, Myneni RB (2015). Candida and Enterobacter co-infection in a case of periampullary carcinoma with obstructive jaundice. International Journal of Research in Medical Sciences 3(7).
Crossref
|
|