ABSTRACT
Klebsiella infection is often the leading cause of morbidity and mortality. Resistance pattern of antimicrobial susceptibility to commonly prescribed drugs were studied in Klebsiella isolates from a hospital in Madinah, Saudi Arabia. Clinical samples were collected from 6840 patients and screened for Klebsiella species over a period of 14 months. The strains were identified using microbiological and biochemical tests while the antibiotic susceptibility was performed by disk diffusion assay. Of all the positive samples isolated, only 275 samples were identified as Klebsiella species. Of these 66% were from males indicating that females are less vulnerable. Maximum isolates (66 to 70%) were collected from sputum and wound swabs of males. About 90% species were isolated from wound, sputum and catheter tips swabs. Antimicrobial sensitivity was studied using seventeen different antibiotics. Results revealed that antibiotic Imipenem has the highest sensitivity of 99.5% while Ampicillin revealed 100% resistance. The prescription of Imipenem antibiotic is recommended for the treatment of Klebsiella infections. In case of resistance to Imipenem, other antibiotics mainly Ceftazidime, Aztreonam, Ciprofloxacin, Cefoxitin and Piperacilline may be recommended. In acute cases, use of combined antimicrobial therapy may be required. Results also indicate that the intensity of Klebsiella infections is higher during summers (46.5%) than in winters (27.2%), while autumn (13.9%) and spring seasons (12.5%) showed the least recorded percentage. Our study will help in persistent and continuous monitoring of antimicrobial susceptibility and supplement the already available data on prevalence of antimicrobial resistance patterns of Klebsiella.
Key words: Klebsiella, Enterobacteriaceae, antimicrobial susceptibility pattern, antibiotic resistance, multi-drug-resistance.
Antibiotic resistance is a major problem world-wide including Saudi Arabia (Yezli et al., 2014). Several factors are responsible for the emergence of multidrug resistance, the major being wide-spread use of broad spectrum antibiotics (Ventola, 2015). The resistant bacteria can be easily transmitted among patients and from healthcare workers to patients and vice versa (Chan, 2012; Aly and Balkhy, 2012). Besides, the resistance pattern to antimicrobial agents has changed dramatically. With time an increase in the rate of antimicrobial resistance has been observed in several species of Gram-negative bacteria (Mahmoud et al., 2016; Chastre, 2008). The resistance rates are higher in Intensive Care Units (ICUs). It is even more alarming to see that the antibiotics used to treat bacterial infections in humans are also used in animal industry (CDCP, 2013). There is hence a need for novel and more stringent antibiotic prescription guidelines to deal with this problem.
The Klebsiella species are non-motile rod shaped Gram-negative bacteria with a prominent capsule. These opportunistic human commensals belong to the family Enterobacteriaceae where Klebsiella pneumoniae and Klebsiella oxytoca, are two species that are responsible for the majority of infections (Nordamann et al., 2009; Algowaihia et al., 2016). These inherently opportunistic nosocomial pathogens are known to cause pneumonia specifically in chronic alcoholics. They also cause infections in urinary tracts, gastrointestinal tracts, surgical wound infections and blood (Kamal et al., 2017). Klebsiella species are often resistant to many antibiotics, including cephalosporins and aminoglycosides (Manikandan and Amsath, 2013).
K. pneumoniae has been identified as an important common pathogen for nosocomial pneumonia, septicaemia and wound infections (Jadhav et al., 2012). Epidemic and endemic nosocomial infections caused by Klebsiella species are leading causes of morbidity and mortality. In the United States of America, Klebsiella is the eighth most important infectious pathogens in hospitals (Manikandan and Amsath, 2013). In Saudi Arabia, the predominant organisms that cause UTIs are Gram negative bacteria which are highly resistant to commonly used oral antibiotics (Yezli et al., 2014).
Clinical isolates of K. pneumoniae are generally resistant to a wider range of antibiotics, and virtually always naturally resistant to ampicillin and amoxycillin. β-lactam antimicrobial agents are the most common treatment options for such infections (Jadhav et al., 2012). Extended-spectrum β-lactamases (ESBLs) are often found in the Enterobacteriaceae family of Gram-negative bacilli, particularly Klebsiella species, Escherichia coli and Proteus mirabilis (Al Johani et al., 2010). Klebsiella species that produce Klebsiella pneumoniae carbapenemase (KPC) are of serious concern as they have high-level resistance against most antibiotics. These KPC enzymes efficiently hydrolyze carbapenems, as well as other β-lactam antibiotics (Paterson et al., 2005). A study comparing ICU isolates from KSA and Kuwait showed that E. coli and Klebsiella species demonstrated multidrug resistance to monobactams, cephems, and aminoglycosides (Rotimi et al., 1998).
The risk of bacterial infection increases with long term antibiotic exposure, prolonged stay in ICUs, other severe illnesses, and instrumentation or catheterization (Jiao et al., 2015). Since there may be variations in antibiotic susceptibility depending on factors like gender, location, age, etc., it is essential to understand the antimicrobial susceptibility pattern of Klebsiella species so that relevant measures can be taken to control the rapid spread of multidrug resistance. Hence, a descriptive hospital based study was undertaken to isolate and identify Klebsiella species from various clinical samples using microbiological and biochemical methods and to study gender distribution of isolates and their antimicrobial sensitivity profile.
Sample collection
Different clinical samples such as sputum, wound swab, cerebrospinal fluid (CSF), tracheal aspirate (Tr. asp.), throat aspirate (Th. asp.), catheter Tip, pus, abdominal abscess (Abd. ab.), ear swab, peritoneal wound swab (Peri. w.s.), pleural fluid (Pler. fluid), App: Appendix (App.), bile, Urethra (Ur), and semen were collected from 6840 patients suspected of bacterial infection at King Fahd Hospital at Madinah, Saudi Arabia. Clinical samples were cultured to isolate the organisms. Demographic data such as sex of the patients was recorded prior to sample collection.
Cultivation and Identification
The clinical samples were collected according to Centers for Disease Control and Prevention Specimen Collection Guidelines (CDCP, 2013), aseptically inoculated on plates of Blood agar, Chocolate agar, Cystine-Lactose-Electrolyte-Deficient (CLED) agar and MacConkey agar (Oxoid Cambridge, UK) and incubated at 37°C for 24 h.
Identification was done based on morphological characteristics of the colonies including size, shape, colour, pigmentation and haemolytic nature.
Biochemical characteristics
Suspected Klebsiella colonies isolated were further identified through biochemical tests (Barrow and Felthan, 2003) using standard procedures and Phoenix automated microbiology 100 ID/AST system (Becton Dickinson Company, Sparks, Md.).
Antimicrobial susceptibility test
Susceptibility to antimicrobial agents was determined by using the disk diffusion method (Oqunshe, 2006), and Phoenix automated microbiology 100 ID/AST system (Becton Dickinson Company, Sparks, Md.). The following antimicrobial agents (obtained from BDH London, UK) were used: ampicillin (AP), augmentin (AUG), gentamycin (GM), cefoxitin (FOX), cephalothin (KF), cotrimoxazole (TS), amikacin (AK), ceftazidime (CAZ), aztreonam (AZT), piperacilline (PRL), imipenem (IMP), ciprofloxacin (CIP), cefpiramide (CPM), meropenem (Merop), tazobactam (Taz), colistin (Col), and nitrofurantoin (Nitro).
The inocula were prepared by growing the various Klebsiella strains on separate agar plates and colonies from the plates were transferred with a loop into 3 ml of normal saline. The density of these suspensions was adjusted to 0.5 McFarland standards. The surface of Muller-Hinton agar (Oxoid Cambridge, UK) plate was evenly inoculated with the organisms using a sterile swab. The swab was dipped into the suspension and pressed against the side of the test tube to remove excess fluid.
The wet swab was then used to inoculate the Muller-Hinton agar by evenly streaking across the surface. By means of a Disc Dispenser (Oxoid Cambridge, UK), the antibiotic discs were applied onto the surface of the inoculated agar and the plates were incubated overnight at 37°C. The diameter of zone of growth-inhibition observed was measured and compared to the chart provided by Clinical and Laboratory Standards Institute (CLSI, 2009).
Patients, especially in ICUs are at risk of acquiring infections, most of which are associated with the use of invasive devices such as catheters and mechanical ventilators (Shulman and Ost, 2005). The ever increasing crisis of antibiotic resistance has been attributed to the overuse and misuse of antibiotics which needs to be regulated under stringent guidelines (Ventola, 2015).
The antibiotic-resistant infections place a lot of burden on the economy of any country as health care costs rise (Golkar et al., 2014). The most serious Gram-negative infections occur in health care settings are most commonly caused by Enterobacteriaceae (mostly Klebsiella pneumoniae) (Ventola, 2015; Yezli et al., 2014). Untreatable or difficult-to-treat infections due to Carbapenem-resistant Enterobacteriaceae (CRE) bacteria are on the rise among patients in medical facilities. Each year around 1.5lakh, Enterobacteriaceae infections occur in the U.S. which is health care-associated and approximately 600 deaths result from infections caused by the two most common types of CRE, carbapenem-resistant Klebsiella species and carbapenem-resistant E. coli (CDCP, 2013).
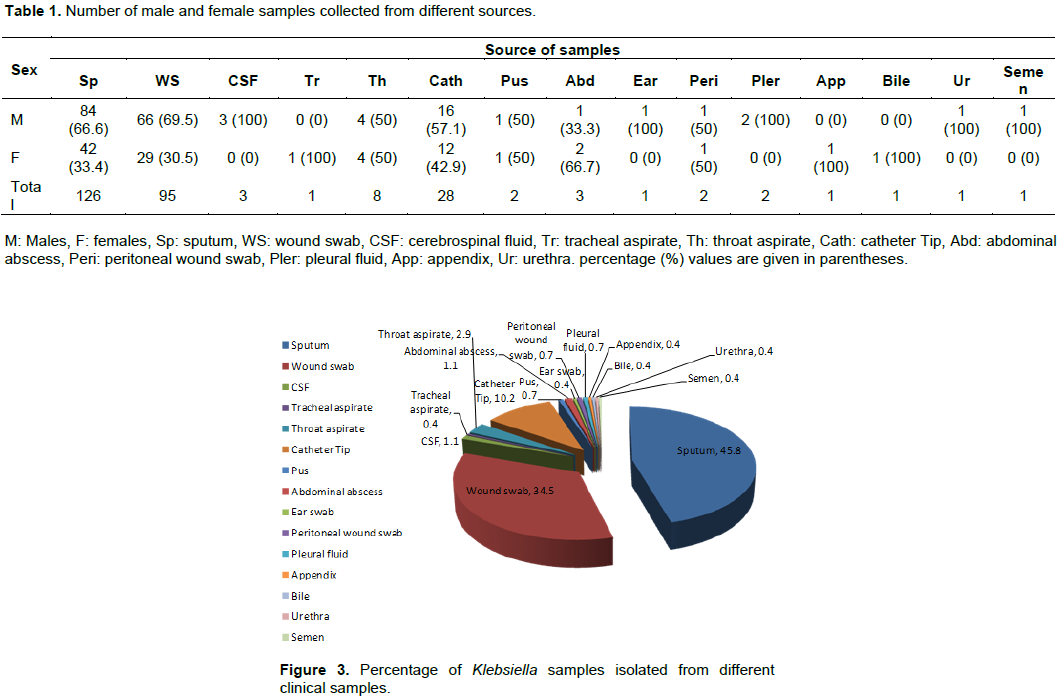
There are relatively few studies that deal with antibiotic use in ICUs in Saudi Arabia (Rotimi et al., 1998; Al Johani et al., 2010). In the present work, we study the antimicrobial resistance patterns of Klebsiella isolates from patients of a big hospital in Madinah, a city visited by lakhs of pilgrims every year. Exactly 6840 clinical samples were collected from patients suspected of bacterial infection in King Fahad Hospital during a period of 14 months. Samples were screened and in total 275 isolates were identified as positive for Klebsiella species, representing about 4% of all the positive samples (Figure 1). Some samples like urine, blood, ascetic fluid, nasal swabs, axilla and perineum showed complete absence of isolates. It was observed that of the positive Klebsiella isolates, 66% were from males while 34% from females (Figure 2) indicating that males show greater vulnerability for these infections. Klebsiella species were positive for nitrate reduction test; urease test, citrate utilization test, Voges-Proskauer test, lactose fermentation; and negative for H2S gas production, oxidase test, methyl-red test, indole test, and motility.
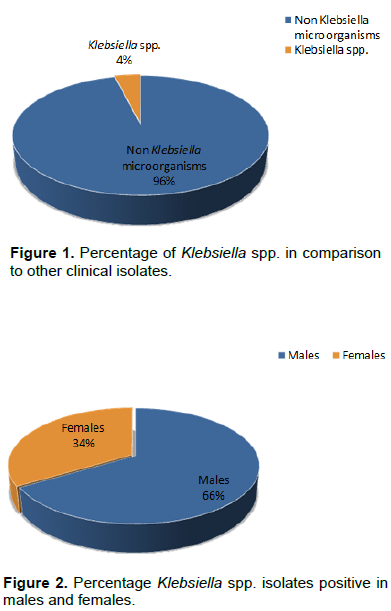
Table 1 gives an estimation of the number of male and female samples isolated from different sources. Majority of the Klebsiella species (66 to 70%) were isolated from the sputum and wound swabs of male patients. A higher isolation rate from sputum and wounds has been reported in earlier studies as well (Ali and Ali, 2014; Namratha et al., 2015). Of the 126 sputum samples that were positive for Klebsiella, 84 were obtained from males and 42 from females. In case of wound swabs, the male to female ratio was 66:29 indicating more than double cases in men. It has been reported earlier that adult males are more susceptible to infection with Klebsiella species than adult females (Janda and Abbott, 2006). A similar situation was seen with catheter tips where there were 16 male samples against 12 females. Moreover, in a country like Saudi Arabia, where males represent a larger workforce, they are exposed more to infections, pollution, road accidents and hence more bone fractures and surgeries. All the 3 samples obtained from the cerebrospinal fluid were from males. In case of throat aspirates, the cases were from 50% males and 50% females. The numbers of the remaining samples were not enough to draw any substantial conclusion. We obtained only 1 sample each from tracheal aspirate, ear, appendix, bile, urine and semen; 2 to 3 samples from pus, abdominal abscess, peritoneal wound swab and pleural fluid. Figure 3 shows the percentage of samples from various clinical sources that were positive for Klebsiella species. As mentioned earlier, the majority of isolates was from sputum and wound swabs, 45.8 and 34.5%, respectively followed by Catheter tips (10.2%). Very few strains were obtained from other clinical sources. The percentage of positive Klebsiella isolates from throat aspirates was 2.9% while from CSF and abdominal abscess it was 1.1%. Pus, peritoneal wound swabs And pleural fluid provided only 0.7% positive isolates. Tracheal aspirates, ear, appendix, bile, urethra, and semen were poor sources providing only 0.4% positive isolates each. The results here showed that the maximum number of Klebsiella species isolate were from sputum and wound swabs. They represented about 80% of all the clinical samples while the least number of isolates were from appendix, bile, urethra, tracheal aspirate, ear swabs and semen constituting a total of only 2.4%.
The positive samples were segregated on the basis of gender and the results have been summarized as Table 1. It was observed that in case of sputum and wound swab, around 66% and 69% samples, respectively were from males. Samples from catheter tips also included greater Klebsiella species from males (57%) and (42.9%) females. Another source throat aspirate revealed that male and female contribute equally, 50% each. The percentage of other samples was not enough to compare the male to female ratio (gender profile) and hence did not give reproducible results.
From the clinical samples considered here, results reveal that males are more vulnerable than females in acquiring Klebsiella infections which corroborates with previous reports (Shah et al., 2010; Namratha et al., 2015). As reported earlier (Janda and Abbott, 2006), this may be due to the fact that males are the major working class and being in the open, experience greater exposure to various infections.
Antimicrobial drug sensitivity was performed by disc diffusion assay using discs impregnated with seventeen antibiotics belonging to different families. These were ampicillin, augmentin, gentamycin, cefoxitin, cephalothin, cotrimoxazole, amikacin, ceftazidime, aztreonam, piperacilline, imipenem, ciprofloxacin, cefpiramide, meropenem, tazobactam, colistin, and nitrofurantoin. As shown in Table 2, Imipenem was the most effective antibiotic against Klebsiella species with 99.5% sensitivity followed by CAZ with 98.6% sensitivity for Klebsiella. The high sensitivity of Imipenem has been shown by others as well (Namratha et al., 2015; Gupta et al., 2012; Shiju et al., 2010). AZT was the third most sensitive antibiotic (96.6%) used in the present study showing high sensitivity against Klebsiella species. Amikacin is a fourth generation aminoglycoside which showed fairly good sensitivity, results being consistent with the previous studies (Ali and Ali, 2014; Namratha et al., 2015).

The percentage sensitivity with the remaining antibiotics in the present study was in this sequence: GM (87%) ˃ AK (86.6%) ˃ PRL (86.2%) ˃ AUG (66.1%) ˃ TS (62.7%) ˃ KF (49.1%). The antibiotics CPM, Merop, Taz, Col and Nitro showed 100% sensitivity but as the number of strains was very low, significant conclusions could not be made. Klebsiella species showed 100% resistance to Ampicillin. Previous studies have shown similar resistance pattern with this drug (Jadhav et al., 2012). The chromosomally encoded β-lactamases could be responsible for this intrinsic resistance (Namratha et al., 2015). Nevertheless, it was encouraging to see that, most of the remaining antibiotics were showing high sensitivity towards Klebsiella. This was not the case with Proteus species as reported in a previous study conducted by the same group (Bahashwan and Shafey, 2013). The Proteus strains showed high resistance to most of the antibiotics except Imipenem which showed around 91% sensitivity. Imipenem was shown to be the most effective antimicrobial drug against Gram-negative bacteria by others (El-Tahawy, 2000).
Understanding seasonal trends in the incidence of nosocomial infections will help in improving surveillance and evaluation of infection prevention guidelines. Several reports claim that bacterial infections always peak during summers and winters (Eber et al., 2011; Anderson et al., 2008). Table 3 depicts the percentage of Klebsiella infections during the four different seasons. It was observed that the percentage of infections was the highest during summers (46.4%) followed by winters (27.2%). During autumn and spring, the percentages are not too high being 13.9% during autumn and 12.5% during spring. Winters are known for the outbreaks of bacterial infections especially related to the respiratory systems (Anderson et al., 2008; Eber et al., 2011). The autumn season incidentally coincides with the major pilgrimage period when a large number of pilgrims visit Madinah during Haj. But interestingly, the percentage of Klebsiella infections was not very high in this period in comparison to summers and winters. This can be attributed to the efforts of the health authorities who take special care to control the outbreak of bacterial and other infections. We have reported a similar pattern in case of Proteus infection during the same period of study (Bahashwan and Shafey, 2013). Although Madinah, just like Makkah, expects a large population all year around, it is during the Haj season that there are dangers of an outbreak of epidemic.

To avoid epidemic like situation, special care is taken which could be the reason for such low percentage of infection during the pilgrimage season. The introduction and implementation of the World Health Organization (WHO) hand hygiene program by Saudi Arabia, and the conception of the Gulf Cooperation Council (GCC) Infection Control Program can be seen as a good initiative in reducing spread of resistant pathogens in healthcare units (Yezli et al., 2014).
Males are more vulnerable to Klebsiella infections than females. The most effective antibiotic with the highest sensitivity and lowest resistance was Imipenem which can hence be prescribed to patients with least reservations. All Klebsiella strains were resistant to Ampicillin indicating that this antibiotic should be prescribed with care.
The intensity of infections was highest during summers followed by winters. Lack of awareness, self-medication and misuse of antibiotics has aggravated multidrug resistance in microbes. There is an ardent need to formulate and adhere to new guidelines for drugs based on their sensitivity profiles.
By studying the antimicrobial resistance pattern of pathogens, we can formulate and implement better infection control policies. Developing nationwide, healthcare guidelines is essential nowadays due to increasing resistance patterns.
Furthermore, by developing a local antibiogram database we can improve the knowledge of antimicrobial resistance patterns in a particular area and will help in improving treatment strategies in Saudi Arabia. Compliance to infection prevention guidelines is essential to eliminate major outbreaks in the future.
The authors have not declared any conflict of interests.
REFERENCES
|
Al Johani S M, Akhter J, Balkhy H, El-Saed A, Younan M, Memish Z (2010). Prevalence of antimicrobial resistance among Gram-negative isolates in an adult intensive care unit at a tertiary care center in Saudi Arabia. Ann Saudi Med. 30(5):364-369.
Crossref
|
|
|
|
Algowaihia R, Ashgarb S, Siragc B, Shalama S, Nassirb A, Ahmed A (2016). Draft genome sequence of a multidrug-resistant Klebsiella pneumoniae strain isolated from King Abdullah Medical City, Makkah, Saudi Arabia. Genome Announc. 4(3):e00375-16.
|
|
|
|
|
Ali ARKA, Ali MAM (2014). Prevalence of extended spectrum β-lactamase producing Klebsiella pneumoniae in clinical isolates. Jundishapur J. Microbiol. 7(11):e17114.
|
|
|
|
|
Aly M, Balkhy HH (2012). The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob. Resis. Infect. Control 1:26.
Crossref
|
|
|
|
|
Anderson DJ, Hervé R, Chen LF, Spelman DW, Hung YJ, Andrew T, Huang AT, Sexton DJ, Raoult D (2008). Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J. Infect. Dis. 197(5):752-756.
Crossref
|
|
|
|
|
Bahashwan SA, El Shafey HM (2013). Antimicrobial resistance patterns of Proteus isolates from clinical specimens. Eur. Sci. J. 9(27):188-202.
|
|
|
|
|
Barrow GI, Felthan RKA (2003). Cowan and Steel's Manual for the Identification of Medical Bacteria. 3rd Ed. Cambridge University Press. Cambridge UK. pp. 351-353.
View.
|
|
|
|
|
Center for Disease Control and Prevention (CDCP) (2013). Office of Infectious Disease Antibiotic resistance threats in the United States.
View.
|
|
|
|
|
Chan M (2012). Antimicrobial resistance: no action today, no cure tomorrow, WHO World Health Day - 7 April 2012.
View
|
|
|
|
|
Chastre J (2008). Evolution problems with resistant pathogens. Clin Microbiol Infection. 3:3-14.
Crossref
|
|
|
|
|
Clinical and Laboratory Standards Institute (CLSI) (2009). (formerly the National Committee for Clinical Laboratory Standards or NCCLS) Performance standards for antimicrobial disk susceptibility tests, 12th ed. Approved standard M02-A10.Wayne, Pa: Clinical and Laboratory Standards Institute.
View.
|
|
|
|
|
Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN (2011). Seasonal and temperature-associated increases in Gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One. 6(9):e25298.
Crossref
|
|
|
|
|
El-Tahawy AT (2000). Bacteriology of diabetic foot. Saudi Med J. 21(4):344-347.
|
|
|
|
|
Golkar Z, Bagasra O, Pace DG (2014). Bacteriophage therapy: A potential solution for the antibiotic resistance crisis. J Infect. Dev. Ctries. 8(2):129-36.
Crossref
|
|
|
|
|
Gupta V, Kumarasamy K, Gulati N, Garg R, Krishnan P, Chander J (2012). Amp C β-lactamases in nosocomial isolates of Klebsiella pneumoniae from India. Indian J. Med. Res. 136(2):237-241.
|
|
|
|
|
Jadhav S, Misra R, Gandham N, Ujagare M, Ghosh P, Angadi K, Vyawahare C (2012). Increasing incidence of multidrug resistance Klebsiella pneumoniae infections in hospital and community settings. Inter. J. Microbiol. Res. 4(6):253-257.
Crossref
|
|
|
|
|
Janda, JM, Abbott SL (2006). The Genera Klebsiella and Raoultella. The Enterobacteria. Washington, USA: ASM Press. 2nd ed. pp. 115-129.
|
|
|
|
|
Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, Huang Y, Liu R (2015). Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: A retrospective study. Pathog. Glob. Health 109(2):68-74.
Crossref
|
|
|
|
|
Kamal F, Williams G, Akbar H, Khan MA, Kadaria D (2017). Klebsiella pneumoniae liver abscess: A case report and review of literature. Cureus. 9(1):e970.
Crossref
|
|
|
|
|
Mahmoud AM, Tarig MSA, Osama MS, Mariam MA (2016). Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac. J. Trop. Med. 9(8):771-776.
Crossref
|
|
|
|
|
Manikandan C, Amsath A (2013). Antibiotic susceptibility pattern of Klebsiella pneumoniae isolated from urine samples. Int. J. Curr. Microbiol. App. Sci. 2(8):330-337.
|
|
|
|
|
Namratha KG, Padiyath S, Subbannayya K, Dinesh PV, Hemachandra C (2015). Characterization and antibiogram of Klebsiella spp. isolated from clinical specimen in a rural teaching hospital. Sch. J. App. Med. Sci. 3(2E):878-883.
|
|
|
|
|
Nordamann P, Cuzon G, Naas T (2009). The real threat of Klebsiella pneumonia carbapenemase-producing bacteria. Lancet Infec. Dis. 9(4):228-236.
Crossref
|
|
|
|
|
Oqunshe AAO (2006). In vitro phenotypic antibiotic resistance in bacterial flora of some indigenous consumed herbal medications in Nigeria. J. Rural Trop. Public Health 5:9-15.
|
|
|
|
|
Paterson DL, Bonomo RA (2005). Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 18:657-686.
Crossref
|
|
|
|
|
Rotimi VO, Al-Sweih NA, Feteih J (1998). The prevalence and antibiotic susceptibility pattern of Gram negative bacterial isolates in two ICUs in Saudi Arabia and Kuwait. Diagn. Microbiol Infect Dis. 30:53-59.
Crossref
|
|
|
|
|
Shah RK, Singh YI, Sanjana R K, Chaudhary N, Saldanha D (2010). Study of extended spectrum beta-lactamases (ESBLs) producing Klebsiella species in various clinical specimens: A preliminary report. J. Coll. Med. Sci. Nepal. 6(3):19-23.
Crossref
|
|
|
|
|
Shiju MP, Yashavanth R, Narendra N (2010). Detection of extended spectrum beta lactamase production and multidrug resistance in clinical isolates of E. coli and K. pneumoniae in Mangalore. J. Clin. Diagn. Res. 6(4):2442-2445.
|
|
|
|
|
Shulman L, Ost D (2005). Managing infection in the critical care unit: how can infection control make the ICU safe? Crit. Care. Clin. 21(1):111-28.
Crossref
|
|
|
|
|
Ventola CL (2015). The antibiotic resistance crisis. Part 1: Causes and Threats. P & T. 40(4):277-283.
|
|
|
|
|
Yezli S, Shibl AM, Livermore DM and Memish ZA (2014). Prevalence and antimicrobial resistance among Gram-negative pathogens in Saudi Arabia. J. Chemother. 26(5):257-272.
Crossref
|
|



