ABSTRACT
The alarming increase in the non-albicans Candida group (NAC) as the etiologic agent of bloodstream infections has made it necessary for the factors associated with candidemia caused by NAC to be elucidated. A cross-sectional retrospective study was conducted which included analysis of microbiological reports, medical records and hospital infection notifications in two tertiary hospitals (Mato Grosso, Brazil) over 8 years (2006 to 2014). Of 144 observed episodes of candidemia, the NAC group represented 64.6%. The prevalence of candidemia caused by NAC was equal to 1.10 × 1,000 admissions, which was statistically different (p<0.001) from and greater than the prevalence of Candida albicans (CA). Hospitalization in the intensive care unit (PR = 1.83; p = 0.05), length of stay ≥42 days (PR = 0.62; p = 0.01) and the use of H2 blockers (PR = 1.75; p = 0.03) were significantly associated with death in patients with candidemia caused by NAC, regardless of gender, use of central venous catheter, treatment with amphotericin B and mechanical ventilation. The incidence of candidemia caused by NAC was 34% higher in men and 40% higher in patients who remained hospitalized for ≥42 days, regardless of prematurity, neutropenia, catheter, mechanical ventilation and age. After 42 days of hospitalization, the chances of survival were 67.3% among patients with candidemia caused by NAC and 56.4% among patients with candidemia caused by CA. This study suggests a different behavior between CA and NAC groups, which should be especially considered for choice of treatment regimen with antifungals.
Key words: Candidemia, nosocomial bloodstream infection, epidemiology, tertiary care centers.
The frequency of candidemias in tertiary care hospitals is increasing constantly in the US (Pfaller et al., 2012), Europe (Berdal et al., 2014) and Brazil (Nucci et al., 2013). Hospitalization in the intensive care unit (ICU) (Heimann et al., 2015), and the increased use of invasive medical technologies (Hirano et al., 2015) enables the occurrence of bloodstream infection (BSI) caused by Candida yeasts, contributing to the increase in mortality and morbidity rates (Glöckner and Karthaus, 2011; Cornely et al., 2015).
According to Yapar (2014), the incidence of candidemia (per 1,000 admissions) was equal to 0.20 in France, 0.38 in Italy, 0.32 in Sweden, 1.95 in Argentina, 0.33 in Chile, 1.96 in Colombia, 0.90 in Ecuador, Venezuela, 0.45 in Canada, 0.53 in Spain, 1.87 in the United Kingdom and 0.21 in Australia. In Brazil, it reduced from 2.49 (2003-2004) to 1.38 (2008-2010); however, in a study conducted in the Midwest Region of Brazil, it reached 1.80 between 2006 and 2011 (Hoffmann-Santos et al., 2013).
The proportion of the non-albicans Candida group (NAC) as an etiological agent of candidemia has been higher in developing countries than among those developed: 56.1% in the USA (Pfaller et al., 2012), 46.5% in Europe (Cornely et al., 2015), 55.8% in Italy (Caggiano et al., 2015), 57.6% in Japan (Morii et al., 2014), 24.1% in Norway (Berdal et al., 2014), 80.0% in India (Juyal et al., 2013); 72.9% in China (Wu et al., 2011; Nucci et al., 2013) and 61.5% in Brazil (Ng et al., 2015), 54.0% in Mexico (Corzo-Leon et al., 2014), 62.4% in Latin America and 59.5% in Brazil Midwest Region of Brazil (Hoffmann-Santos et al., 2013).
The risk factors for candidemia have been extensively studied worldwide, however, owing to the alarming increase of NAC as the etiologic agent of these infections (Colombo et al., 2007; Ortega et al, 2011; Mondelli et al., 2012; Guimarães et al., 2012; Diekema et al., 2012; Muñoz et al., 2016; Imran and Alshammry, 2016), it is also important that further exploration is performed regarding the factors associated with candidemia caused by NAC, because in Brazil, the C. parapsilosis complex has shown resistance to fluconazole and caused an outbreak in an intensive care unit (Pinhati et al., 2016).
Adult patients who developed candidemia by NAC remained hospitalized for longer than Candidaemia by C. albicans. In this age group, the average cost per hospitalization was about $14,000 dollars higher for patients with candidemia by NAC than among those who developed C. albicans candidemia (Moran et al., 2009).
Study design and data collection
An epidemiologic, observational, analytical, cross-sectional retrospective study using secondary data, such as microbiological reports (automated detection and species identification system), medical records and hospital infection notifications, was conducted. Because of this, the patients selected were not exposed to any risk. The information collected was stored in the Epi Info 7™ software (CDC, Atlanta, USA).
The sample consisted of cases that had been confirmed in the clinical and laboratory settings as BSI caused by Candida in patients in two university tertiary hospitals in the city of Cuiabá (Mato Grosso, Brazil), during the period from January 2006 to December 2014. Patients who had a second positive blood culture result in a Candida spp. in a time interval lasting ≤30 days or those who did not show clinical signs and symptoms of infection and lacking information for the selected variables or generic laboratory identification, were not included. Yeast identification were performed by the hospital microbiology laboratory using the VITEK 2 system (bioMérieux, Marcy-I’Étoile, France).
Patient characteristics and statistical analysis
The following variables were considered: age, gender, length of stay, death, use of antifungals, hospital sector, the presence of infection by Candida spp, prematurity, low birth weight, neutropenia (neutrophil ≤500 cells/mm3), fever axillary ≥ 38°C, previous surgery, the use of catheters, corticosteroids, H2 blockers, parenteral nutrition and mechanical ventilation.
For the age groups, the authors considered those aged less than or equal to 30 days as neonatal patients; those aged between 31 days and 17 years were considered paediatric patients; those aged between 18 and 59 years were considered adults, and those aged 60 years or above were considered elderly.
Through the Stata Statistical Software® version 12.0 (College Station, TX), the Pearson’s chi-square test was performed to check for association between categorical variables; two proportions test to check equality of prevalences; and the unpaired Student t-test to compare two independent means, or its nonparametric analogue, the Mann-Whitney test, when necessary, considering a p-value of <0.05 in the two-tailed test as significant. In order to estimate the strength of association, the prevalence ratio (PR) and the analysis of their respective confidence intervals (95% CI) were applied. This measure was preferred over the odds ratio, since it can overestimate the PR interfering with the inference of analysis. To determine the independent effect of explanatory variables on the dependent variable, Poisson regression with robust variance (Barros and Hirakata, 2003) was used to adjust the covariates, as it is the appropriate multiple model. The variables selected for this model had a p-value ≤ 0.20 in the univariate analysis, or biological plausibility.
Finally, considering the length of stay of each patient, death from all causes such as failure and discharge as censorship, the Kaplan-Meier curve was produced to visualize the distribution of the probability of survival.
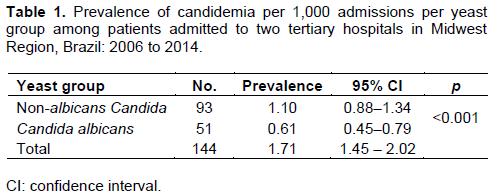
There were 144 observed episodes of candidemia, of which Candida parapsilosis complex was the main etiologic agent (n = 56; 38.8%), followed by Candida albicans (n = 51; 35.4), Candida tropicalis (n = 26; 18.1%), Candida glabrata complex (n = 7; 4.9%), Candida guilliermondii (n = 2; 1.4%), Candida haemulonii (n = 1; 0.7%) and Candida krusei (n = 1; 0.7%). The NAC group represented 64.6% of the total candidemias, a ratio 1.8 times the number of candidemias caused by C. albicans (CA).
The mean age was 29.9 years (95% CI = 25.3–34.5), and the median was 29 years. Men had a mean age of 27.8 years and women, 31.4 years, with no statistically significant difference. The average length of stay was equal to 52.2 days (95% CI = 45.0–59.5) with a median of 42 days. The average length of stay was 56.4 and 49.3 days for men and women, respectively, with no statistically significant difference.
There was no statistical difference between the average length of stay and the mean age of patients who developed candidemia caused by NAC or CA. The average age of patients who died and had candidemia caused by both NAC and CA was statistically similar (p = 0.79). In the group of patients with candidemia caused by NAC, those who died showed a statistically different mean age (p<0.01), which was higher than that of those who were discharged. In the group of patients with CA, this difference was not observed. Among those who had candidemia caused by NAC, the average length of stay was statistically different (p = 0.01) and lower for the patients who died, as compared to the average length of stay of discharged patients. In the group of patients with CA, this difference was also not observed.
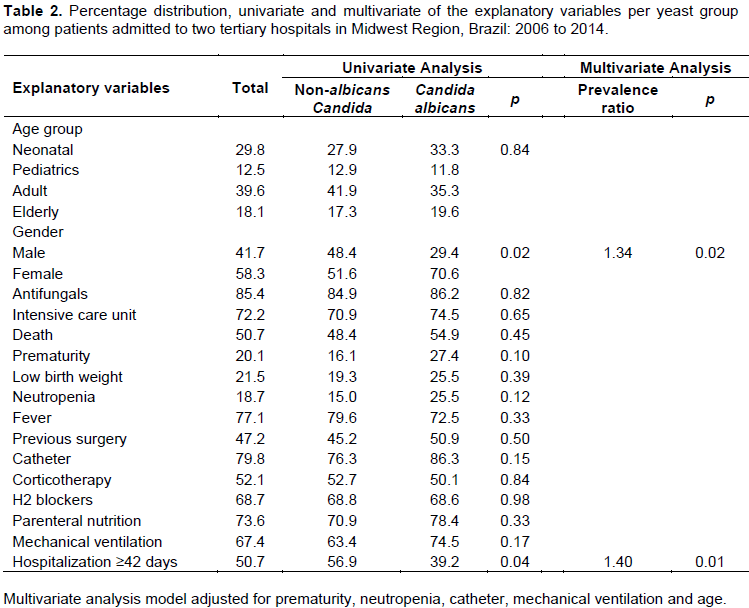
The prevalence of BSI caused by NAC was statistically different from and higher than the prevalence of candidemia caused by CA (Table 1). Univariate analysis showed a statistical association of candidemia caused by NAC with gender (PR = 1.64; 95% CI = 1.02–2.64; p = 0.03) and length of stay ≥42 days (PR = 1.45; 95% CI = 1.00–2.13; p = 0.04). The other univariate analyses and the respective percentages of the explanatory variables in multivariate analysis are shown in Table 2.
Of all patients with candidemia, 73 died (50.7%). Among patients who had candidemia caused by NAC (n = 93), 45 died (48.4%), while among those who had candidemia caused by CA (n = 51), 28 died (54.9%), without a statistically significant difference between groups.
Among patients who had candidemia caused by NAC, the univariate analysis showed the existence of a statistical association between death and the use of parenteral nutrition (p<0.01), admission to the ICU (p<0.01), mechanical ventilation (p<0.01), length of stay ≥42 days (p = 0.02), use of H2 blockers (p = 0.02), catheter use (p = 0.02), gender (p = 0.03) and fever (p = 0.03). In the multivariate analysis, admission to the ICU (PR = 1.83; 95% CI = 1.00–3.33; p = 0.05), length of stay ≥42 days (PR = 0.62; 95% CI = 0.42–0.91; p = 0.01), and the use of H2 blockers (PR = 1.75; 95% CI = 1.05–2.92; p = 0.03) were significantly associated with death in patients with candidemia caused by NAC, regardless of gender, and the use of catheter treatment, amphotericin B and mechanical ventilation.
Among patients who had candidemia caused by CA, the univariate analysis showed the existence of statistical association of death with parenteral nutrition (p<0.01) and gender (p = 0.05). In the multivariate analysis, only the use of antifungals (PR = 0.55; 95% CI = 0.34-0.88; p = 0.01) was statistically associated with death, with the protective effect in patients with candidemia caused by CA, regardless of gender, the use of parenteral nutrition, steroids, catheter, admission to ICU, low birth weight and mechanical ventilation.
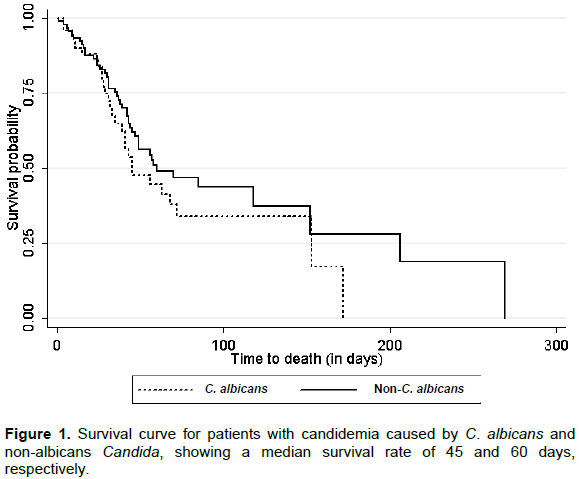
Figure 1 shows the comparison of survival curves between patients with candidemia caused by CA and NAC. The median survival probability (p.50) in patients with candidemia caused by NAC occurred with 59 days of hospitalization, while in the group with candidemia caused by CA, it occurred in 46 days. After 42 days of hospitalization, the chance of survival was 67.3% among patients with candidemia caused by NAC and 56.4% among patients with candidemia caused by CA.
Among patients diagnosed with candidemia caused by NAC, those who died showed statistically different and higher mean age (M = 39.2; 95% CI = 31.2–47.1) than patients who were discharged (M = 21.3; 95% CI = 14.1–28.5). This difference can be explained by the fact that the elderly who died had a higher mean age (M = 70; 95% CI = 67.0–72.9) than the elderly who were discharged (M = 67.2; 95% CI = 61.2–73.3). The depletion of clinical reserves of the patient with advanced age confronting fungemia is believed to be one of the reasons for this difference, since 75.0% of elderly patients with candidemia caused by NAC died.
The predictive nature of a risk factor does not mean that it is necessarily the cause of the disease, as a risk factor can indirectly predict an outcome through association with some other variable that is indeed a determinant of disease. Not being the cause of candidemia does not decrease the value of a risk factor as a way of predicting the likelihood of this outcome, but identifies it as a marker for increased probability of this condition, the absence of which may not decrease the risk of developing candidemia.
The epidemiological design of this study did not allow us to infer risk, but allowed us to determine the existence of factors associated with candidemia caused by NAC that, as a BSI agent, was 34% higher in men than in women and 40% higher in patients who remained hospitalized for ≥42 days. Although, not statistically significant on the multivariate analysis (p = 0.056), death was 33% lower among patients who had candidemia caused by NAC and were treated with amphotericin B.
One of the limitations of the epidemiological design of this study is that it did not allow inference of cause and effect for determining both the exposure and the outcome simultaneously, since a temporal relationship between them has not been established. In order to reduce the possibility of non-inclusion of cases that have presented their outcome before the study conducted, long time for data collection (8 years) was considered. The existence of different associated factors in candidemias caused by NAC and CA suggests a clinical behaviour that should be better evaluated by studies with other epidemiological designs.
The literatures has shown NAC as the main cause of candidemias (Wu et al., 2014; Lotfi et al., 2015; Ng et al., 2015; Caggiano et al., 2015), with special attention paid to C. parapsilosis complex among the three major etiologic agents, as was also observed in this study. The finding agree with the result of Imran and Alshammry (2016) showing that NAC isolated from blood samples of ICU patients in Iraq (Middle East) were: C. parapsilosis complex 20.34%, C. membrenifaciens 2.97% and 0.25% for Candida sake, while C. albicans was 12.7%. These Candida spp. were identified based on sequencing analysis of the whole ITS region and the ITS2 of the rDNA.
The incidence of death in patients with candidemia caused by NAC of this study was statistically different from (p<0.01) and higher than that published in the study by Wu et al. (2014), who presented similar epidemiological design and hospital setting (tertiary university hospital in China), in which 238 cases of candidemia were identified in 3 years of study (2009 to 2011). Although, the number of beds in the Chinese hospital is almost three times higher and the number of episodes of candidemia is almost double as compared to this study, with only diabetes mellitus (p<0.001) being observed as an independent variable in the multivariate analysis with the outcome candidemia caused by NAC.
Comparing the annual incidences of candidemia in hospitalized patients aged 65 to 84 years in a study conducted in Texas (Oud, 2016), with the prevalence of candidemia detected by this study for the same age group and year, there was a statistically significant similarity between 2006 and 2009. In 2010, the prevalence of candidemia in hospitalized patients aged 65 to 84 years of this study (0.91 per 10,000) were statistically different (p = 0.04) and lower than the incidence of candidemia for the same age group and year in the Texas study.
A multicenter study with 227 patients with candidemia (2008 to 2010) in China (Li et al., 2015) stratified the distribution of Candida yeasts isolated into two groups: acquired and not acquired in the ICU. There was no statistical association between the species and the hospital sector, which was also observed in hospitals evaluated in Cuiabá (Mato Grosso, Brazil). The absence of preference of Candida yeasts as the etiologic agent of candidemias in ICU demonstrated by this cross-sectional study was supported by the aforementioned cohort.
In the study conducted in China (Li et al., 2015), patients who acquired candidemia in the ICU had a higher mean age (p<0.05) than those who developed candidemia in other inpatient units. In the present study, however, the opposite behavior was observed, as the patients who remained hospitalized in the ICU had statistically different (p<0.001) and lower (24.3 years; 95% CI 18.9–29.7) mean age than patients who were not admitted to the ICU (44.6 years; 95% CI 37.6 to 51.6). This can be explained by the fact that the Chinese study included only patients older than 16 years, while in the present study, patients admitted to the neonatal ICU were also included.
The prevalence of candidemia (per 1,000 admissions) over 8 years (2006 to 2014) was statistically different from (p<0.05) and lower than that reported in a study conducted in Spain (Aguilar et al., 2015): 19.1 between 2012 and 2013; statistically similar to that observed in Italy (Barchiesi et al., 2016): 1.5 between 2010 and 2014; but statistically different (p<0.01) and higher than that found in France (Richet et al., 2002): 0.29 in 1995.
The Italian study (Barchiesi et al., 2016) showed that older age behaved as an independent variable statistically associated with higher risk of mortality. Of the elderly evaluated in this study (n = 26), 73.1% died, reinforcing advanced age as a predictive factor for high mortality in patients with candidemia, even when including aspects previously discussed.
Epidemiological evidence on the intravascular catheter indicates that its retention is statistically associated with increased risk of death (Fisher et al., 2016) and its removal is associated with an increased survival rate (Tortorano et al., 2002). In this study, death was 30% higher in patients using a central venous catheter (p = 0.02) and had candidemia caused by NAC. There was no association between the use of catheter and death among patients who had candidemia caused by CA.
A review article (Krcmery and Barnes, 2002) synthesized the specific risk factors for candidemia caused by NAC: colonization at two sites, venous catheter, APACHE score II, acute leukaemia, bone marrow transplantation, antifungal prophylaxis, neutron-penia, malignant disease of hematologic origin, surgery and kidney failure. In the present study, male gender and the length of stay ≥42 days behaved as independent variables associated with candidemia caused by NAC. These variables were not mentioned in the aforesaid article.
In the present study, the factors identified in the multivariate analysis as associated with candidemia caused by NAC distinguished from other studies that showed exposure to azole agents (OR = 3.36; p = 0.03) and surgery to implant artificial materials (OR = 37.5; p = 0.009) by Ding et al. (2015); hematological disorders (p<0.001), mean age (p = 0.02), presence of urinary catheter (p = 0.007) and neutropenia (p = 0.003) by Alp et al. (2015).
Factors associated with death in patients with candidemia identified in this study were different from the group of yeasts. In patients with candidemia caused by NAC, death was 83% higher among those who remained hospitalized in the ICU and 75% higher among those who used H2 blockers, but 38% lower among patients who remained hospitalized for ≥42 days. Among patients with candidemia caused by CA, death was 45% lower among those who used antifungals. The evidence seems to suggest the existence of epidemiological differences between candidemia caused by CA and NAC. This distinct behaviour must be taken into account when choosing antifungal therapy for the treatment of hospitalized patients, if possible, before the identification of species and their respective antifungal susceptibility profile (Ding et al., 2015).
In this scenario, it should be mentioned, the important study by Pfaller et al. (2014), in which the authors observed 2,147 cases of candidemia caused by NAC. The C. parapsilosis complex was the most common etiologic agent among the NAC group, as was also observed in this study. The fact that C. parapsilosis complex represents more than 60% of NAC species in this study and that this species has the characteristic of having one of the greatest chances of survival among the Candida yeasts that act as BSI agents (Morii et al., 2014; Cornely et al., 2015) may explain the less aggressive behaviour by the NAC group when compared with CA, as shown in the Kaplan-Meier curve.
According to Gonçalves et al. (2010), C. parapsilosis complex is the third most commom agent of candidaemia in hospitalized patients in Europe, EUA and Latin American. However, the epidemiology of BSI caused by complex is still not completely understood. Data from the Nationwide Sentinel Surveillance of Candidemia in Brazil (Colombo et al., 2006) identified C. parapsilosis complex with a third cause in eleven medical centers. In this study, the percentage of resistance of C. parapsilosis complex to the antifungal 5-Flucytosine was four times greater than the resistance of C. albicans to the same drug. In adult cases, C. parapsilosis complex was the third most commom agent of candidemia and in pediatric cases, was the second. In the study conducted in the Midwest Region of Brazil, C. parapsilosis complex was the first cause of candidaemia in adult and neonatal intensive care unit.
Isolates from C. parapsilosis complex in hospitals located in the Southeast of Brazil (Ziccardi et al., 2015) showed two molecular types: C. orthopsilosis and C. parapsilosis. The first species was two times most frequent in blood (C. orthopsilosis = 80.0%; C. parapsilosis sensu stricto = 41.9%), but presented smaller MIC mean for antifungal caspofungin (C. orthopsilosis = 0.139; C. parapsilosis sensu stricto = 0.465; p=0.03). About hydrolytic enzyme production, C. orthopsilosis showed larger mean of caseinolytic activity that the C. parapsilosis sensu stricto, with statistical difference (p<0.01), evidentiating its important virulent character. The study conducted in the Midwest Region of Brazil did not aim to perform molecular identification, but noted that the death in patients with C. parapsilosis complex was 46.4% (26/56), but less than C. albicans with 54.9% (28/51).
The important epidemiological differences between the behaviour of candidemias caused by NAC and CA, such as prevalence, associated factors, and the probability of survival, should be considered by the multidisciplinary team in the clinical management of hospitalized patients. Although, similar associated factors have been observed in studies conducted in different countries, these hospital settings have technological, socioeconomic, structural and microbiological conditions that require continuous monitoring of the hospital epidemiology profile of fungal infections in each health unit, and their particularities should be both valued and compared.
The authors declare that there is no conflict of interest.
REFERENCES
|
Aguilar G, Delgado C, Corrales I, Izquierdo A, Gracia E, Moreno T, Romero E, Ferrando C, Carbonell JA, Borrás R, Navarro D (2015). Epidemiology of invasive candidiasis in a surgical intensive care unit: an observational study. BMC Res. Notes 8:491.
Crossref
|
|
|
|
Alp S, Arikan-Akdagli S, Gulmez D, Ascioglu S, Uzun O, Akova M (2015). Epidemiology of candidaemia in a tertiary care university hospital: 10-year experience with 381 candidaemia episodes between 2001 and 2010. Mycoses 58:498-505.
Crossref
|
|
|
|
|
Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E (2016). Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection 44:205-2013.
Crossref
|
|
|
|
|
Barros AJD, Hirakata V (2003). Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 3:21.
Crossref
|
|
|
|
|
Berdal JE, Haagensen R, Ranheim T, Bjørnholt JV (2014). Nosocomial candidemia; risk factors and prognosis revisited; 11 years experience from a Norwegian secondary hospital. PLoS One 9:e103916.
Crossref
|
|
|
|
|
Caggiano G, Coretti C, Bartolomeo N, Lovero G, De Giglio O, Montagna MT (2015). Candida bloodstream infections in Italy: changing epidemiology during 16 years of surveillance. BioMed Res. Int. 2015:1-9.
Crossref
|
|
|
|
|
Colombo AL, Guimaraes T, Silva LR, de Almeida Monfardini LP, Cunha AK, Rady P, Alves T, Rosas RC (2007). Prospective observational study of candidemia in São Paulo, Brazil: Incidence rate, epidemiology, and predictors of mortality. Infect. Control Hosp. Epidemiol. 28:570-576.
Crossref
|
|
|
|
|
Colombo AL, Nucci M, et al. and for the Brazilian Network Candidemia Study (2006). Epidemiology of candidemia in Brazil: a Nationwide Sentinel Surveillance of Candidemia in Eleven Medical Centers. J. Clin. Microbiol. 44:2816-2823.
Crossref
|
|
|
|
|
Cornely OA, Gachot B, Akan H, Bassetti M, Uzun O, Kibbler C, Marchetti O, de Burghgraeve P, Ramadan S, Pylkkanen L, Ameye L (2015). Epidemiology and outcome of fungemia in a cancer cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin. Infect. Dis. 61:324-331.
Crossref
|
|
|
|
|
Diekema D, Arbefeville S, Boyken L, Kroeger J, Pfaller M (2012). The changing epidemiology of healthcare-associated candidemia over three decades. Diagn. Microbiol. Infect. Dis. 73:45-48.
Crossref
|
|
|
|
|
Ding X, Yan D, Sun W, Zeng Z, Su R, Su J (2015). Epidemiology and risk factors for nosocomial Non-Candida albicans candidemia in adult patients at a tertiary care hospital in North China. Med. Mycol. 53: 684-690.
Crossref
|
|
|
|
|
Fisher BT, Vendetti N, Bryan M, Prasad PA, Localio AR, Damianos A, Coffin SE, Bell LM, Walsh TJ, Gross R, Zaoutis TE (2016). Central venous catheter retention and mortality in children with candidemia: a retrospective cohort analysis. J. Pediatr. Infect. Dis. Soc. 5(4):403-408
Crossref
|
|
|
|
|
Glöckner A, Karthaus M (2011). Current aspects of invasive candidiasis and aspergillosis in adult intensive care patients. Mycoses 54:420-433.
Crossref
|
|
|
|
|
Gonçalves SS, Amorim CS, Nucci M, Padovan ACB, Briones MRS, Melo ASA, Colombo AL (2010). Prevalence rates and antifungal susceptibility profiles of the Candida parapsilosis species complex: results from a nationwide surveillance of candidaemia in Brazil. Clin. Microbiol. Infect. 16:885-887.
Crossref
|
|
|
|
|
Guimarães T, Nucci M, Mendonça JS, Martinez R, Brito LR, Silva N, Moretti ML, Salomao R, Colombo AL (2012). Epidemiology and predictors of a poor outcome in elderly patients with candidemia. Int. J. Infect. Dis. 16:e442-e447.
Crossref
|
|
|
|
|
Heimann SM, Cornely OA, Wisplinghoff H, Kochanek M, Stippel D, Padosch SA, Langebartels G, Reuter H, Reiner M, Vierzig A, Seifert H (2015). Candidemia in the intensive care unit: analysis of direct treatment costs and clinical outcome in patients treated with echinocandins or fluconazole. Eur. J. Clin. Microbiol. Infect. Dis. 34:331-338.
Crossref
|
|
|
|
|
Hirano R, Sakamoto Y, Kudo K, Ohnishi M (2015). Retrospective analysis of mortality and Candida isolates of 75 patients with candidemia: a single hospital experience. Infect. Drug Resist. 8:199-205.
Crossref
|
|
|
|
|
Hoffmann-Santos HD, Paula CR, Yamamoto AC, Tadano T, Hahn RC (2013). Six-year trend analysis of nosocomial candidemia and risk factors in two intensive care hospitals in Mato Grosso, Midwest Region of Brazil. Mycopathologia 176:409-415.
Crossref
|
|
|
|
|
Imran ZK, Alshammry ZW (2016). Molecular diagnosis of Candidemia of intensive care unite patients based on sequencing analysis of ITS regions. Int. J. PharmTech Res. 9(12):658-668.
|
|
|
|
|
Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N (2013). Emergence of non-albicans Candida species in neonatal candidemia. N. Am. J. Med. Sci. 5:541-545.
Crossref
|
|
|
|
|
Krcmery V, Barnes AJ (2002). Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260.
Crossref
|
|
|
|
|
Li C, Wang H, Yin M, Han H, Yue JF, Zhang F, Shan TC, Guo HP, Wu DW (2015). The differences in the epidemiology and predictors of death between candidemia acquired in intensive care units and other hospital settings. Intern. Med. 54:3009-3016.
Crossref
|
|
|
|
|
Lotfi N, Shokohi T, Nouranibaladezaei SZ, Nasrolahi Omran A, Kondori N (2015). High recovery rate of non-albicans Candida species isolated from burn patients with candidemia in Iran. Jundishapur J. Microbiol. 8:e22929.
Crossref
|
|
|
|
|
|
|
|
Mondelli AL, Niéro-Melo L, Bagagli E, Camargo CH, Bruder-Nascimento A, Sugizaki MF, Carneiro MV, Villas Boas PJ (2012). Candidemia in a Brazilian tertiary hospital: microbiological and clinical features over a six-year period. J. Venom. Anim. Toxins Incl. Trop. Dis. 18:244-252.
Crossref
|
|
|
|
|
Moran C, Grussemeyer CA, Spalding JR, Benjamin DK, Reed SD (2009). Candida albicans and non-albicans bloodstream infections in adult and pediatric patients: comparison of mortality and costs. Pediatr. Infect. Dis. J. 28:433-435.
Crossref
|
|
|
|
|
Morii D, Seki M, Binongo JN, Ban R, Kobayashi A, Sata M, Hashimoto S, Shimizu J, Morita S, Tomono K (2014). Distribuition of Candida species isolated from blood cultures in hospitals in Osaka, Japan. J. Infect. Chemother. 20:558-562.
Crossref
|
|
|
|
|
Mu-oz P, Vena A, Valerio M, Álvarez-Uría A, Guinea J, Escribano P, Bouza E (2016). Risk factors for late recurrent candidemia. A retrospective matched case-control study. Clin. Microbiol. Infect. 22:e11-20.
|
|
|
|
|
Ng KP, Kuan CS, Kaur H, Na SL, Atiya N, Velayuthan RD (2015). Candida species epidemiology 2000-2013: a laboratory-based report. Trop. Med. Int. Heath 20:1447-1453.
Crossref
|
|
|
|
|
Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI (2013). Epidemiology of Candidemia in Latin America: a laboratory-based survey. PLoS One 8:e59373.
Crossref
|
|
|
|
|
Ortega M, Marco F, Soriano A, Almela M, Martínez JA, López J, Pitart C, Mensa J (2011). Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. J. Hosp. Infect. 77:157-161.
Crossref
|
|
|
|
|
Oud L (2016). Secular trends in utilization of critical care services among candidemia-associated hospitalizations: a population-based cohort study. J. Clin. Med. Res. 8:40-43.
Crossref
|
|
|
|
|
Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE (2014). Epidemiology and outcomes of invasive candidiasis due to non-albicans species of candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS One 9:e101510.
Crossref
|
|
|
|
|
Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D (2012). Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004-2008. Diagn. Microbiol. Infect. Dis. 74:323-331.
Crossref
|
|
|
|
|
Pinhati HMS, Casulari LA, Souza ACR, Siqueira RA, Damasceno CMG, Colombo AL (2016). Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect. Dis. 16:433.
Crossref
|
|
|
|
|
Richet H, Roux P, Des Champs C, Esnault Y, Andremont A (2002). Candidemia in French hospitals: incidence rates and characteristics. Clin. Microbiol. Infect. 8: 405-412.
Crossref
|
|
|
|
|
Tortorano AM, Biraghi E, Astolfi AT, Ossi C, Tejada M, Farina C, Perin S, Bonaccorso C, Cavanna C, Raballo A, Grossi A (2002). European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51:297-304.
Crossref
|
|
|
|
|
Wu JQ, Zhu LP, Ou XT, Xu B, Hu XP, Wang X, Weng XH (2011). Epidemiology and risk factors for non-Candida albicans candidemia in non-neutropenic patients at a Chinese teaching hospital. Med. Mycol. 49:552-555.
|
|
|
|
|
Wu Z, Liu Y, Feng X, Liu Y, Wang S, Zhu X, Chen Q, Pan S (2014). Candidemia: incidence rates, type of species, and risk factors at a tertiary care academic hospital in China. Int. J. Infect. Dis. 22:4-8.
Crossref
|
|
|
|
|
Yapar N (2014). Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manage. 10:95-105.
Crossref
|
|
|
|
|
Ziccardi M, Souza LO, Gandra RM, Galdino AC, Baptista AR, Nunes AP, Ribeiro MA, Branquinha MH, Santos AL (2015). Candida parapsilosis (sensu lato) isolated from hospitals located in the Southeast of Brazil: Species distribution, antifungal susceptibility and virulence attributes. Int. J. Med. Microbiol. 305:848-859.
Crossref
|
|