Full Length Research Paper
ABSTRACT
The discharge of untreated wastes containing heavy metals into the environment is a challenge to living cells, and in the search for treatment method, biosorption has been an economical and easy technique for heavy metals removal. This study was carried out to determine the biosorption capacity of bacterial isolates from electronic wastes soil. The isolates from e-waste soil were screened and selected for heavy metals such as Chromium (Cr), Lead (Pb), Copper (Cu), and Cobalt (Co), using agar plate method incorporated with 10 ppm of analogous solution of the test heavy metals with each isolate spotted on the agar surface and incubated for 4 days, revealed that Bacillus cereus S13 had the highest biosorption efficiency (highest zone of clearance) of 98% for Pb and Cu, while the least adsorbed metals were Pb (93.5%), Co (93.7%), and Cr (93.9%) by B. cereus S25. The biosorption potential of the selected bacteria was measured with atomic absorption spectrophotometer (AAS). The spectrophotometric analysis of heavy metals biosorption by isolates showed that B. cereus S13 efficiently removed 97.4% Cr and 95.9% Pb, while B. cereus S36 adsorbed 95.5% Pb and Cr at 20 ppm. B. cereus S27 biosorption capacity increased with increase in concentration of heavy metals used except for Pb (96.9%) where larger percentages were removed from the solution at lower concentration. Conclusively this study affirmed that B. cereus strains from electronic waste remediated heavy metals in aqueous solution and therefore, could be promising adsorbent of heavy metals particularly chromium, lead, and cobalt.
Key words: Biosorption, heavy metals, bacteria, potential.
INTRODUCTION
Heavy metals are natural elements with atomic numbers greater than 20, characterized by a relatively high density (at least 5 gcm-3), with a health-impactful toxicity even at low concentrations on living organisms such as plants, animals and microbes (Murthy et al., 2012). They are characteristically existing components found in changing variation in the environments and are part of human daily activities, they are also found in important structures and in a range of other artificial mixes. The activities of human such as wood burning, mining, fertilizer application to the soil, combustion of coal, smelting, incineration and landfill disposal of wastes containing heavy metals have greatly
impacted their biochemical cycles (Srivastava et al., 2017; Ali et al., 2019). Scientist have searched and are still on the lookout for the cheapest source of removal of these metals from wastes. However, presently biosorption is a very cheap and effective means of removing heavy metals in liquid wastes, and being a non-living method of treating wastes contaminated with heavy metals using dead microbial cells, has proven to be effective over bioaccumulation (active uptake) via the feasibility study conducted on the large scale application of microorganisms in active uptake of heavy metals from liquid wastes, where the latter required continuous addition of nutrients in to the biomass medium thereby raising the biological oxygen demand (BOD) or chemical oxygen demand (COD) of the liquid wastes. Also not limited to this factor, is the difficulty in maintaining a healthy microbial population due to metal toxicity as well as the potential recovery of intracellularly absorbed metals are also narrow since these metals can form complexes with other metabolites in solution (Briffa et al., 2020). Diverse species of bacteria are present in the soil some of which have been used to rid-off heavy metals from liquid effluent. Bacterial species which have been isolated from the soil and reported to have been used for heavy metals biosorption are Pseudomonas, Micrococcus, Escherichia, Streptococcus, Enterobacter, Staphylococcus etc. (Murthy et al., 2012). Thus, this study aimed at isolating bacteria from e-waste polluted soil and using the isolates to adsorb heavy metals in aqueous solution.
MATERIALS AND METHODS
Collection of electronic waste soil
The Electronic waste soil was collected from an electronic dumping site at Apete Akufo area, Ibadan, Oyo State Nigeria. The soil was collected at four different locations on the site. Into a sterile polythene bags and were immediately transported to the microbiology laboratory of Oyo State College of Agriculture and Technology Igboora, for further microbiological analysis.
Isolation of bacterial from E-waste soil
Ten grams (10 g) of the soil sample was weighed in to 9 ml of sterile water in a conical flask and mixed together on an electric shaker. Serial dilution was performed on the soil solution by pipetting 1 ml of soil solution into 9 ml of the diluent (sterile water) in a test tube with gentle mixing. Desired serial dilutions were carried out on the soil solution and were plated on the nutrient agar plate in triplicated. The inoculated plates were incubated at 25-28°C for 48 h. Pure isolates of the bacteria from soil sample was obtained and isolates were stored on agar slant for further study (Kumar et al., 2010).
Screening of bacteria isolates for biosorption of heavy metals
The method of Kumar et al. (2010) was used with modification to screen heavy metals biosorption capacity of the bacterial isolates. The bacterial isolate was standardized using 0.5 McFarland standard. Sterile molten nutrient agar containing 1 ml of 10 ppm of each heavy metals (Co, Cr, Cu, and Pb) prepared in 100 ml standard flask was poured on plates and each standard isolate was inoculated on agar surface by swabbing and in replicates. Plates were incubated at 28 ± 2°C for 3 days. Colonies surrounded by a cleared zone were selected for further study. Bacterial isolates were selected for biosorption of the heavy metals.
Phenotypic identification of bacterial isolates with biosorption potential from e-waste soil
The preliminary phenotypic identity of the bacterial isolates with biosorption potential was done through the subjection of the isolates to various biochemical test such as catalase, oxidase, grams staining, spores staining, sugar utilization etc. as shown in Table 2. The isolates were identified using Bergey’s manual of bacteriological identification.
Molecular characterization of bacterial isolates
Extraction of the DNA
The method of Patra et al. (2010) was used for extraction with slight modification. The DNA was extracted from each bacterium by growing the culture in a 10 ml volume of broth medium. The cultures were grown for 5 days in a shaking incubator (80 rpm) at 25°C and 2 ml of each culture was centrifuged in a sterile micro centrifuge at 13,000 rpm. The supernatants were discarded and the pellet was transferred to Mo Bio Ultra Clean Soil DNA Kit (Laboratories Inc, CA, USA) and DNA extracted according to the manufacturer’s instructions. The extracted DNA was electrophoresed on 1% agarose gel in TAE buffer and visualized under UV (Gel Doc, Bio-Rad Laboratories, USA) to check for integrity. The DNA was stored at 20°C until further analysis.
Amplification of the DNA
A Mini Cycler (MJ Research, Inc., Watertown, MA) heated lid thermos cycler was used to amplify DNA; 25-ll reactions were prepared by adding 8 pmol of each primer (0.8 ll of primer mixture), 0.5 ll of DNA sample, 12.5 ll Master Mix from Promega, and pure sterile water to 25 ll. All amplification reactions were hot started at 95°C for 3 min. The polymerase chain reaction (PCR) protocol used with short universal primers was: 94°C 90 s and 33 cycles, a final extension step at 72°C for 3 min, 4°C. When Golden Mixtures (G1–G13) were used, PCR parameters were the same as above except for annealing temperature which was set at 5°C for 1 min (Patra et al., 2010).
Purification of the DNA
Agarose Gels Containing Ethidium Bromide (0.1 Lg Per Ml) were used throughout the study at concentrations of 1.2. 1.6% (w/v). LKB power supply (Biochrom, Cambridge, England) and UV Trans-illuminator (Dinco and Rhenium Industrial Ltd.,), 100-bp ladder was used as molecular weight markers. Gels were photographed using a digital camera (Casio Exilim, Tokyo, Japan) at 3-8 mega pixels with sepia or black and white filter (Sameer et al., 2010).
Sequencing of the amplified DNA
The 16SrRNA gene sequence of the bacterial strain was determined through lysis of the cells. The 16SrRNA fragments obtained was amplified by PCR using the universal primer forward 5’-AGATTT-GATCATG GCTCGA-3’ and the reverse 5’-GGCTACC-TTGTTACGACTT-3’ (position 1510-1492). The sequences of the amplified 16 rDNA fragments amplified was analysed using gene bank and compared with national centre for biotechnology information (NCBI) (Igiri et al., 2018).
Biosorption of the selected heavy metals
Five millimetres of sterile nutrient agar broth containing 1 ml of each standard 10 ppm and 15 ppm of each heavy metal were prepared separately in MacCartney bottles. 1 ml of each standard isolate was inoculated into each broth medium and inoculated bottles were incubated for 5 days at room temperature with constant shaking. After, centrifugation was carried out at 1792G for 25 min. The supernatant was digested using nitric acid of heavy metal solution sample. The concentration of metal was determined by absorption spectrophotometry (UV–Vis 752, UK) (Ahemad and Kibret, 2013). The percentage of biosorption was be determined with the formula; (%) biosorption= initial metal concentration − final metal concentration*100/initial metal concentration.
RESULTS AND DISCUSSION
The increase in industrialization has brought about a daunting increase in the discharge of heavy metals and other pollutants to the surrounding environment particularly soil and water resources. Diverse microorganisms are found in metal polluted environment, and some are said to have adapted and able to tolerate the toxic condition of heavy metals due to stress induced in solution. These microbes could be used to decontaminate the environment from heavy metals through various processes such as adsorption, oxidation and reduction, bio accumulation, methylation and demethylation (Briffa et al., 2020). The bacterial isolates were subjected to biosorption of the same concentration of heavy metals (10 ppm) under the same environmental conditions in order to know their sorption capacity. All the strains of bacteria as shown in Table 1, were able to adsorb heavy metals at different capacity (except for lead (Pb) where only S25 had the least adsorption percentage of 93.5%) which may be due to strains inherent properties such as nature of the metal binding sites and cell wall. The bacterial isolates S13 and S27 which were later identified as B. cereus strains, removed 96.7% of chromium from aqueous solution while S13 adsorbed 98% cupper more than the Pseudomonas aeruginosa used by Oyewole et al. (2019) to remove the same concentration of the metals in aqueous solution; an implication that the sorption efficiency of B. cereus than P. aeruginosa, may be due to its cell wall components and being gram positive bacterium have more peptidoglycan layers than P. aeruginosa (gram negative bacterium). Peptidoglycan layers are negatively charged by the presence of hydroxyl, amino and phosphate ions on their surfaces which are capable of binding positive metals ions in aqueous solution for removal (Kumar et al., 2010).
The biochemical identification of bacterial isolates with biosorption potential for heavy metals is shown in Table 2.
Isolates S36, S13, S25 and S27 were gram positive bacteria, motile, spore formers, catalase and citrate positive. Sugar utilization revealed that all the isolates were positive for glucose and galactose. S13, S25 and S27 were negative for gelatin hydrolysis while S25 demonstrated variable reactions for growth in potassium cyanide and oxidase reaction. All the isolates were indole and methyl red negative. However, S36, S13 and S25 were lactose negative. The probable bacteria identified in this study were various types of B. cereus.
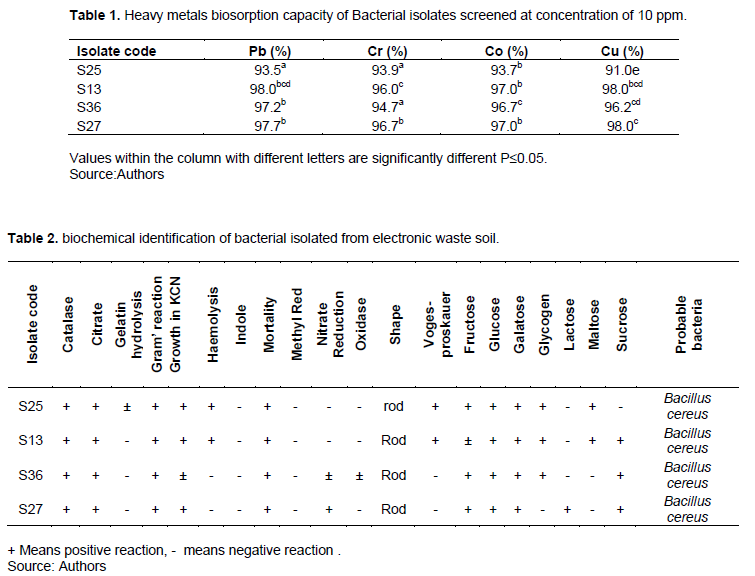
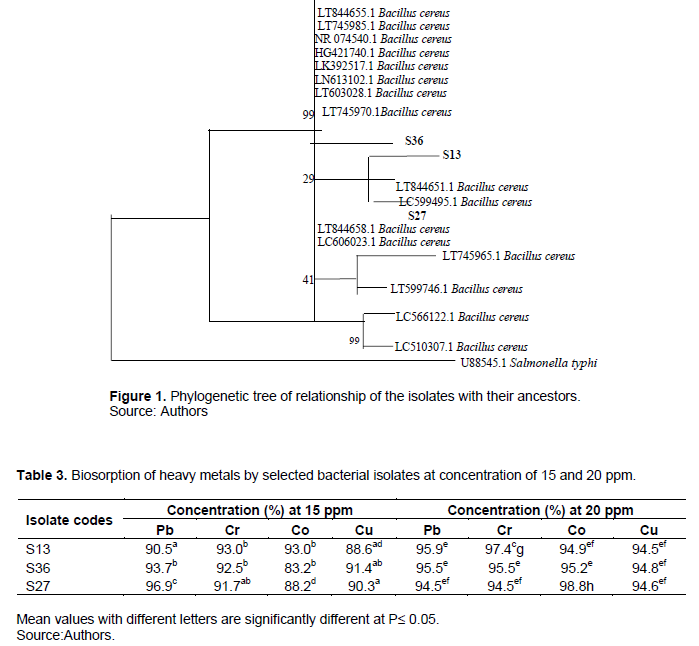
The phylogenetic tree of ancestral relationship of the bacterial isolates with potential for heavy metals biosorption using 16SrRNA relationship between nucleotide sequences is showing Figure 1. The phylogenetic tree was compiled based on the alignment of partial 16SrRNA sequence. The bacterial isolates S13, S36 and S27 were observed as B. cereus but of different strain. Table 3 showed the Atomic Absorption Spectrophotometric (AAS) analysis of heavy metals adsorbed by bacterial isolates from electronic waste soil. The three strains of B. cereus (S13, S36, and S27) selected for used in this study demonstrated comparable removal of heavy metals in aqueous solution. At the concentration of 20 ppm of the heavy metals in aqueous solution, S27 largely removed cobalt and lead (98.8 and 96.9%) followed by S13 and S36 which adsorbed 95.9 and 95.5% lead (Pb) respectively, this may be due to the difference in resistant mechanism of each strain to heavy metals. However, B. cereus S13 was able to effectively remove almost all the heavy metals in aqueous solution in contrast with the remaining B. cereus S36 and S27 because the percentage of heavy metals removal increased with increase in concentration. This may be the result of increase in the affinity of the cell-metal binding sites for the metal ions since microbes under an extreme environmental condition have high surface area to volume ratio which provides them large interaction with matters in the environment. This result disagrees with the findings of Murthy et al. (2012) where a decrease in percentage removal of Pb resulted from increased concentration of the metals. The phylogenetic relationship of the isolates with their ancestors is shown in Figure 1. S36, S13 and S27 were seen to be 99% closely related to their parent which was B. cereus.
CONCLUSION
Biosorption of heavy metals (Chromium, Lead, Copper and Cobalt) were conducted using bacterial isolates from electronic waste soil. The results obtained in this experimental work showed that the isolates were able to adsorb various concentrations of heavy metals. An implication that strains of bacteria used are effective adsorbent for removal of these heavy metals from electronic waste soil. The results of this work revealed that the bacterial isolates B. cereus used was able to remove the heavy metals significantly from the aqueous solution. Therefore, it is recommended that further research should be focused on the use of these isolates to remediate these metals from other wastes and also from electronic wastes at higher concentration with regards to variable factors such as pH, time of exposure of adsorbent to the heavy metals solution in order to further ascertain their potency.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Ahemad M, Kibret M (2013). Recent trends in microbial biosorption of heavy metals; a review. Biochemistry and Molecular Biology 1(1):19-26. |
|
|
Ali H, Khan E, Ilahi I (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry 14 p. |
|
|
Briffa J, Sinagra E, Blundell R (2020). Heavy metals pollution in the environment and their toxicological effects on human. Heliyon 6(9):4691. |
|
|
Igiri BE, Okoduwa SI, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018). Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tanney wastewater: A Review. Journal of Toxicology 16 p. |
|
|
Kumar A, Bisht BS, Joshi VD (2010). Biosorption of heavy metals by four acclimated microbial species Bacillus spp., Pseudomonas spp. and Aspergillus niger. Journal of Biological and Environmental Science 4(12):97-108. |
|
|
Kumar R Sharma AK, Singh P, Dhir B, Mehta D (2014). Potential of some fungal and bacterial species in bioremediation of heavy metals. Journal of Nuclear Physics, Material Sciences, Radiation and Applications 1(2):213-223. |
|
|
Murthy S, Bali G, Sarangi SK (2012). Biosorption of Lead by Bacillus cereus isolated from industrial effluents. British Biotechnology Journal 2(2):73. |
|
|
Oyewole OA, Zobeashia SS, Oladoja EO, Raji RO, Odiniya EE, Musa AM (2019). Biosorption of heavy metals polluted soil using bacteria and fungi isolated from soil. Applied Sciences 1(8):1-8. |
|
|
Patra RC, Malik S, Beer M, Megharaj M, Naidu R (2010). Molecular characterization of chromium (IV) reducing potential in gram positive bacteria isolated from contaminated sites. Soil Biology and Biochemistry 10(42):1857-1863. |
|
|
Sameer V, Naga DCH, Srinu BG, Ravi TY (2011). Role of biosorption in Environmental clean-up. Journal of Microbial and Biochemical Technology 1:1-8. |
|
|
Srivastava V, Sarkar A, Singh S, Singh P, De Araujo AS, Singh RP (2017). Agroecological Response of Heavy Metals Pollution with special Emphasis on soil Health and Plant Performances. Frontier in Environmental Science 5:64. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0