ABSTRACT
Urinary tract comprises of kidney, ureter, urethra and genital organ and under normal circumstances these organs are sterile. Inflammation of these organ and parts may indicate the possibility of Urinary Tract Infection (UTI). It may be due to the colonization of wide range of bacteria either from normal microflora that is Escherichia coli or from other Gram negative or Gram positive bacteria. The present study was carried out to determine the prevalence of urinary tract infection in children and adolescents of hilly areas of Garhwal region of Uttarakhand, India. The sample collection was done from April 2013 to December 2013 from the Out Patient Department (OPD) and In Patient Department (IPD) of Hemvati Nandan Bahuguna Base Hospital, Srinagar Garhwal at an average height of 560 m (1837 ft) above sea level at foothills of Himalayas. Clean voided mid-stream urine samples were collected in sterile universal containers from 76 children of age group 0 to 10 years and 129 adolescents of age group 11 to 20 years. Bacterial counts greater than or equal to 1x105 CFU/mL in culture plates were taken as positive, which was an indication of UTI as introduced by Kass. Colony morphological characteristics were used for identification of bacterial isolation followed by Gram’s staining and biochemical tests. Our study showed a high prevalence of E. coli as the most dominant bacteria causing UTI in children and adolescents of hilly Garhwal region of Uttarakhand.
Keywords: Urinary tract infection (UTI), coagulase negative staphylococcus (CoNS), outdoor patient department (OPD), indoor patient department (IPD).
Urinary tract infection (UTI) is one of the commonest conditions encountered by medical practitioners (Najar et al., 2009) and women (Fry, 1969; Royal College, 1995; Car, 2006; Nicolle et al., 2006). It was estimated that 60% of all women reported usually have UTI at least once in their lifetime (Foxman et al., 2000; Foxman, 2002; Al-Badr and Al-Shaikh, 2013). Worldwide, about 150 million people (Brumbaugh and Mobley, 2012; Stamm and Norrby, 2001) are diagnosed with UTI each year, costing in excess of 6 billion dollars (Gonzalez and Schaeffer, 1999). Among both outpatients and inpatients, Escherichia coli is the most common isolate, accounting for 75 to 90% of uncomplicated UTI isolates (Junuzovic et al., 2014; Uzunović, 2009; Gupta et al., 2001). Staphylococcus saprophyticus, Klebsiella sp., Proteus sp., Enterococcus sp. and Enterobacter sp. are organisms less commonly isolated from outpatients (Hooton, 2012; Smith, 2002). It is the common practice of medical practitioner and health officer to prescribe broad spectrum antibiotics on the empirical basis before the final bacteriology that is, antibiotic sensitivity results become available. Therefore, it is essential to have area-specific monitoring studies to document the microorganisms causing UTI and their antimicrobial susceptibility is important for helping the selection of an effective empirical treatment (Smith and Coast, 2002). UTIs are often treated with different broad-spectrum antibiotics when commonest or narrow spectrum antibiotics activity may be appropriate because of concerns about infection with resistant organisms (Daoud and Afif, 2011). Fluoroquinolones are preferred as initial agents for empiric therapy of UTI in area where resistance is likely to be of concern (Schaeffer, 2002; Biswas et al., 2006). This is because they have high bacteriological and clinical cure rates, as well as low rates of resistance, among most common uropathogens (Goldstein, 2000; Gupta et al., 2002). Kulkarni et al. (2017) analyzed 1000 urine samples from a Tertiary Care Hospital of North Eastern Karnataka in which 395 cases were culture-positive for Escherichia coli. These isolates were also tested for antibiotic susceptibility by disk diffusion method. Majority of E. coli isolates are multi drug resistance (MDR) and show resistance to most commonly used antibiotic which was used to treat UTI.
The resistance pattern of community acquired UTI pathogens has not been studied extensively (Goldstein, 2000). The extensive uses of antimicrobial agents have invariably resulted in the development of antibiotic resistance, which, in recent years, has become a major problem worldwide (Kumar et al., 2006). The etiology of UTI and the antibiotic resistance of uropathogens have been changing over the past years, both in a community and nosocomial or hospital acquired infection (Manges et al., 2006; Kahan et al., 2006). However, there is need for study on etiology and resistance pattern of community acquired UTIs in India. This retrospective study was done to compare the frequency and drug resistance pattern in uropathogens isolated from patients with UTIs in Garhwal region, India. The diagnosis and treatment of UTI in children have been considered to be particularly important due to both short term and long term sequelae. UTI is one of the causes of serious bacterial illness in infants requiring hospital admission and has been associated with significant morbidity (Doern and Richardson, 2016). UTI has also been thought to associate with the development of renal scarring and later to renal failure, hypertension, and pre-eclampsia (Byington et al., 2003).
Study population
Urine samples were collected from a total of 205 patients in which 76 children and 129 adolescents between the ages of 0 to 20 years, 0 to10 years children and 11 to 20 years (adolescents) - during the period of April 2013–December 2013. All these persons were IPD and OPD patients visiting the H.N.B. Base Hospital, Srikot Ganganali, Srinagar Garhwal, Uttarakhand. For the collection of urine from patients the following exclusion criteria were used: 1. sample in non-sterile container; 2. samples of patients on antibiotics were excluded from the study.
Sterilization of media and materials
The media used for the experiments were nutrient agar (NA), MacConkey agar (MCA), blood agar (BA) and Muller Hinton agar. All medium were purchased from Hi Media laboratory Ltd. All glassware and media was sterilized by autoclaving at 121 lbs for 15 min before setting an experiment.
Urine sample collection
Clean catch mid-stream urine specimens were obtained from patients in sterile universal containers which were given to their parents/attendant for collections and transported to the laboratory immediately for urine analysis.
Isolation of uropathogens from urine samples
Ten-fold serial dilutions were made by transferring 1.0 ml of the sample in 9.0 ml of sterile normal saline (0.85% NaCl); 1 ml was then added into molten nutrient agar in Petri dishes and rotated gently to mix. The contents were allowed to set and the plates were then incubated at 37°C for 24 h. Bacterial colonies appearing on the plates after the incubation period were enumerated to determine urine samples with significant bacteriuria. A loop full of each urine sample was also streaked on MacConkey agar and blood agar plate for the isolation of the bacteria present in the urine. After incubation, plates with growth were selected, the colonies were isolated using an inoculating loop and subsequently sub-cultured on agar slants for use in further tests.
Bacterial identification
Bacterial isolates were identified and characterized on the basis of Grams stain followed by a microscopic examination, motility test and other conventional biochemical tests such as catalase, oxidase, IMViC, urease, H2S production, TSI etc. (Chandra et al., 2016).
Antibiotic sensitivity test
Antimicrobial sensitivity testing was done by using the agar disc diffusion method as described by Bauer et al. (1966). The turbidity of the bacterial suspension was compared with 0.5 MacFarland’s standard. The standardized bacterial suspension was then swab-inoculated onto Muller Hinton agar using sterile cotton swabs, and then left to dry for 10 min before placing the antimicrobial sensitivity discs. Antibiotics impregnated discs of 6 mm in diameter were used for the test. Discs containing the following antibacterial agents were placed onto the agar surface and incubated for 18 to 24 h. Thefollowing antibiotics were used for antibiotic sensitivity testing ampicillin, amoxicilin-clavulanic acid, amikacin, cefazolin, cefotaxime, cefuroxime, chloramphenicol, co-trimoxazole, oxacillin/cephoxitin, ciprofloxacin, erythromycin, gentamicin, netilmicin, penicillin, tetracycline, piperacillin tobramycin nitrofurantoin aztreonam, cefoperazone- sulbactum, cefepime, clindamycin, meropenem, rifampicin, ticarcillin-clavulanic acid, linezolid, teicoplanin, piperacillin- tazobactam polymixin-B, azithromycin, colistin and vancomycin. All antibiotic used for antibiotic sensitivity were purchased from HiMedia laboratory Ltd, India. After incubation, the diameter of the zone of inhibition was measured and compared with a zone diameter interpretative chart from HiMedia to determine the sensitivity/resistance of the isolates to the antibiotics. The procedure is intended for in vitro susceptibility testing of common rapidly growing and certain fastidious bacterial pathogens (Colle et al., 1996).
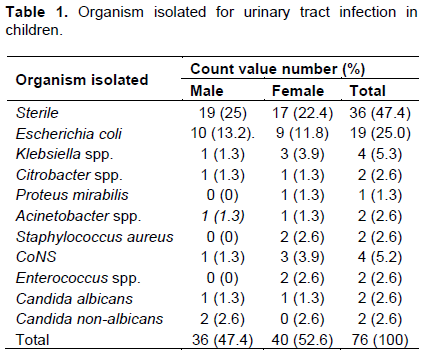
The results obtained from data analysis are represented in the following tables. Table 1 shows the microorganism responsible for causing UTI in children. For the children of age group 0 to 10 years, the most prevalent bacteria responsible for urinary tract infection is E. coli followed by Klebsiella sp. and coagulase negative Staphylococcus (CoNS).
It was evident from Table 1 that the organism highly responsible for urinary tract infections in children is E. coli; it is a causative organism of UTI in 13.2% male and 11.8% female children, which is 25% approximate of all the isolates. The other pathogens which were isolated in the urine sample in children population 0 to 10 year(s) age group, are Citrobacter sp. (2.6%), Candida albicans (2.6%), Candida non-albicans (2.6%), Acinetobacter sp. (2.6%) and Enterococcus sp. (2.6%).
The Klebsiella spp. is responsible in 5.3% children population (1.3% male and 3.9% female) for causing urinary tract infections, which is highest after E. coli; CoNS is responsible for causing urinary tract infections in 5.2% (1.3% male, 3.9% female) while the Citrobacter sp. is responsible for causing urinary tract infections only in 1.3 and 2.6% children population.
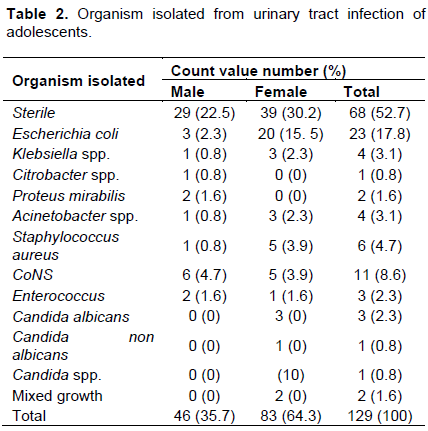
Table 2 shows the organism isolation in adolescent population that is, 11 to 20 years (age group). The main responsible organisms for causing the urinary tract infection among adolescents population was found to be E. coli. It was evident from the above table that the organism highly responsible for urinary tract infections is E. coli, meaning that it is the main pathogen for UTI in 2.3% male and 15.5% female adolescents, which is approximately 17.8%. The other bacteria isolated in the samples responsible for causing the urinary tract infections in the adolescent population are CoNS (8.6%), Staphylococcus aureus (4.7%), Klebsiella sp. (3.1%), Enterococcus (2.3%) and C. albicans (2.3%).
The second highly responsible organism for causing UTI in adolescent population was found to be CoNS which caused infection in 8.6% population and third prevalent organism for causing urinary tract infections was found to be S. aureus, which is responsible for affecting 4.7% population.
The antibiotic sensitivity pattern in children and adolescents in Garhwal region is shown in Table 3. On the basis of antibiogram, it was observed that ciprofloxacin (19, 25%), co-trimoxazole (17, 22%), cefazolin (17, 22%), cefuroxime (17, 22%), amoxicillin-clavulanic acid (16, 21%), cefotaxime (16, 21%), ampicillin (15, 20%), gentamycin (13, 17%), ticarcillin-clavulanic acid (10, 13%), cefepime (9, 12%), and aztreonam (5, 7%) antibiotics are highly resistant in case of children, who are of 0 to 10 years age group. The drug which was found effective against uropathogens isolated from children was nitrofurantoin (22, 28.9%), meropenem (18, 23.68%), piperacillin- tazobactam (18, 23.68%), cefoperazone-sulbactum (17, 22%), gentamicin (17, 22%), amikacin (16, 21%), aztreonam (10, 13.15%) and co-trimoxazole (9, 12%).

As far as adolescent population is concerned, the adolescent population showed high resistance for the drugs ciprofloxacin (26, 20.15%), co-trimoxazole (25, 19.37%), cefotaxime (24, 18.60%), amoxicillin-clavulanic acid (21, 16.27%), gentamycin (20, 15.50%), cefazolin (19, 14.72%), ampicillin (18,13.95%), ticarcillin-clavulanic acid (18, 13.95%), cefuroxime (17, 13.17%), aztreonam (15, 11.62%) and cefepime (10, 77.51%). For the sensitivity for the drugs against uropathogens in the case of adolescent group, it was found that the adolescent population showed their sensitivity for the drugs amikacin (33, 25.58%), nitrofurantoin (24, 18.60%), piperacillin- tazobactam (20, 15.50%), gentamicin (20, 15.50%), cefoperazone- sulbactum (19, 14.72%), amoxicillin-clavulanic acid (18, 13.95%), meropenem (18, 13.95%), ciprofloxacin (15, 11.62%), co-trimoxazole (12, 9.30%), and aztreonam (10, 7.75%).
It was also observed that there are some drugs for which the adolescent population has shown their resistance as well as sensitivity, meaning that there are some common drugs for which the population has showed their resistance and at the same time they also showed their sensitivity to the same drugs, applied against the urinary tract infection causing bacteria (Table 3).
The study was undertaken to determine the incidence of urinary tract infection in children and adolescents as well as to evaluate the bacterial agents involved in this UTI. Out of the 76 patients in children group that participated in this study, pathogens were isolated only in 40 (17 males and 23 females, 52.63%) urine samples while 36 (19 male and 17 females, 47.37%) urine samples were sterile and out of 129 patients in adolescent group that participated in this study, pathogens were isolated only from 61(17 males and 44 females, 47.28%) urine samples, while in 68 (29 male and 39 females, 52.71%) urine samples, no pathogen could be isolated.
A large number of microorganisms were isolated from female patients with high bacteria count. This study shows a higher incidence of urinary tract infection in females than males. In this study, 22.36% males and 30.26% of females had positive urine cultures in 0 to 10 years age group, while in 11 to 20 years age group, 17 males (13.17%) and 44 females (34.10%) had positive urine cultures. This is similar to those obtained by other studies. Anochie et al. (2001) reported a predominance of female patients in a study carried out to determine the influence of instruction on the method of urine collection and storage on the prevalence of urinary tract infection. Similar findings were reported by Olowu (1996). The higher incidence of urinary tract infections in females is a result of a variety of factors, such as the close proximity of the female urethral meatus to the anus (Lipsky, 1990). Short incomplete urethra and in coordinate voiding of urine in school girls often leads to infection of the urinary tract (Mond et al., 1970). Alterations in vaginal microflora also play a critical role in encouraging vaginal colonization with coliforms and this can lead to urinary tract infection (Hooton et al., 1997).
The pattern and frequency occurrence of the bacterial isolates found in this study are similar to those reported by other workers. Alausa and Onile (1984) reported in their study that E. coli were the most commonly isolated pathogen in significant bacteriuria. The findings of Bishara et al. (1997) in Israel agreed with this statement. They reported that E. coli were responsible for 52% of cases of urinary tract infection and Klebsiella spp. (14%) and Enterococcus spp. in 4%. A higher percentage of the organisms found in this study were isolated mainly from females. The pattern reported in this work is similar to that reported by Okafor et al. (1993) in which 20.7% of case of urinary tract infection was reported in males. The result of this study shows that 47% of the isolates were sensitive to amoxicillin, 33.3% to cotrimoxazole, 50% to nitrofurantoin 30.6% to colistin, 63.9% to gentamicin, 77.8% to ciprofloxacin and 97.1% to ofloxacin. Prevalence of high number of E .coli that is, 67% was also reported by Yilmaz et al. (2016) and resistance rates of E. coli to antimicrobial agents was for ampicillin was 66.9%, cefazolin, 30.9%, cefuroxime, 30.9%, ceftazidime, 14.9%, cefotaxime, 28%; cefepime, 12%; amoxicillin-clavulanic acid, 36.9%; trimethoprim-sulfamethoxazole (TMP-SXT), 20%; ciprofloxacin, 49.9%; amikacin, 0.3%; gentamycin, 24%; nitrofurantoin, 0.9%, and fosfomycin 4.3%. They reported no resistance to imipenem or meropenem and the frequency of ESBL-producing E. coli strains was 24%.
Sorlózano-Puerto et al. (2017) reported similar finding in which the most prevalent bacterial species was E. coli which account for 60.3% of isolated uropathogens, followed by E. faecalis (22.4%) and Klebsiella spp. (6.5%). The highest E. coli susceptibility rates (>90%) were for piperacillin-tazobactam, cefuroxime, cefotaxime, ceftazidime, imipenem, gentamicin, nitrofurantoin, and fosfomycin, and the lowest were for amoxicillin-clavulanic acid and cotrimoxazole. They also suggested that empiric treatment with amoxicillin-clavulanic acid, cotrimoxazole, cephalosporins, and gentamicin may be inadequate due to their limited activity against uropathogens. The antibiotic sensitivity test of this study shows that ciprofloxacin for 0 to 10 years (19, 25%) and 11 to 20 years ( 26, 20.15%) was the most resistant antibiotic in in vitro testing followed by co-trimoxazole for age 0 to 10 years (17, 22%) and age 11 to 20 years (25, 19.37%). A reduced sensitivity to nitrofurantoin was observed in this study as opposed to the findings of Goldraichi and Manfrori (2002), who reported a higher efficacy of the drug against E. coli in vitro. They reported sensitivity of E. coli to nitrofurantoin of 92, 95 and 94%, respectively over a three year period. Olowu and Oyetunji (2003) reported a 57.9% sensitivity of pathogens towards nitrofurantoin. The most sensitive antibiotic in this study was Amikacin in 11 to 20 years age group and nitrofurantoin for 0 to 10 years age group followed by nitrofurantoin for age 11 to 20 years and meropenem and piperacillin- tazobactam for age 0 to 10 years, respectively.
In conclusion, this study specifies the incidence of urinary tract infection in children and adolescents. It also highlighted the major bacterial agent involved in this condition. The pattern of isolates reported in this study is consistent with the usually reported pattern, with E. coli being the most common organism isolated in cases of urinary tract infection. This was followed by Klebsiella spp., P. mirabilis, E. faecalis and P. aeruginosa. This study shows a high level of resistance to ciprofloxacin (19, 25%), co-trimoxazole (17, 22%), cefazolin (17, 22%), cefuroxime (17, 22%), amoxicillin-clavulanic acid (16,21%), cefotaxime (16, 21%), ampicillin (15,20%), gentamicin (13, 17%), ticarcillin-clavulanic acid (10, 13%), cefepime (9, 12%), aztreonam (5, 7%) antibiotics in 0 to 10 years age group and ciprofloxacin (26, 20.15%), co-trimoxazole (25, 19.37%), cefotaxime (24, 18.60%), amoxicillin-clavulanic acid (21, 16.27%), gentamycin (20, 15.50%), cefazolin (19, 14.72%), ampicillin (18,13.95%), ticarcillin-clavulanic acid (18, 13.95%), cefuroxime (17, 13.17%), aztreonam (15, 11.62%), cefepime (10, 77.51%) in 11 to 20 years age group, as such, these antimicrobials may not be suitable for treating case of UTI in this locality.
However, a large proportion of the isolates were sensitive to nitrofurantoin (22, 28.9%), meropenem (18, 23.68%), piperacillin- tazobactam (18, 23.68%), cefoperazone-sulbactum (17, 22%), gentamycin (17, 22%), amikacin (16, 21%) and co-trimoxazole(9,12%) in 0 to 10 years age group and amikacin (33, 25.58%), nitrofurantoin (24, 18.60%), piperacillin-tazobactam (20, 5.50%), gentamicin (20, 15.50%), cefoperazone-sulbactam (19, 14.72%), amoxicillin-clavulanic acid (18, 13.95%), meropenem (18,13.95%), ciprofloxacin (15, 11.62%), co-trimoxazole (12, 9.30%), aztreonam (10, 7.75%) in 11 to 20 years age group, and should be considered as first line drugs for treating cases of urinary tract infection in this Garhwal region. Ciproflaxin and co-trimoxazole are however best avoided in both groups children as well as in adolescents.
Since urinary tract infection may be asymptomatic in most cases (as this study has shown), it is therefore suggested that routine screening of patients of Pyrexia of unknown origin (PUO) be done for urinary tract infection and the appropriate antimicrobials administered after antibiotic sensitivity tests have been carried out in order to prevent the cases becoming symptomatic later with resultant renal damage.
The authors have not declared any conflict of interests.
REFERENCES
|
Alausa KO, Onile BA (1984). The epidemiological pattern of bacterial septicaemia at the University College Hospital, Ibadan. Niger. Med. J.14:55-62.
|
|
|
|
Al-Badr A, Al-Shaikh G (2013). Recurrent urinary tract infections management in women: A review. Sultan Qaboos Univ. Med. J. 13(3):359-367.
Crossref
|
|
|
|
|
Anochie IC, Nkanginieme KEO, Eke FU (2001). The influence of instruction about the method of urine collections and storage on the prevalence of urinary tract infection. Niger. J. Paediatr. 28(2):39-42.
Crossref
|
|
|
|
|
Bauer AW, Kirby WM, Sherris JC, Turck M (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45(4):493-496.
|
|
|
|
|
Biswas D, Gupta P, Prasad R, Singh V, Arya M, Kumar A(2006). Choice of antibiotic for empirical therapy of acute cystitis in a setting of high antimicrobial resistance. Indian J. Med. Sci.60(2):53-58.
Crossref
|
|
|
|
|
Brumbaugh AR, Mobley HL (2012). Preventing urinary tract infection: progress toward an effective Escherichia coli vaccine. Expert. Rev. Vaccines 11(6):663-676.
Crossref
|
|
|
|
|
Byington CL, Rittichier KK, Bassett KE, Castillo H, Glasgow TS, Daly J, Pavia AT (2003). Serious Bacterial Infections in Febrile Infants Younger Than 90 Days of Age: The Importance of Ampicillin-Resistant Pathogens. Pediatrics 111(5):964-968.
Crossref
|
|
|
|
|
Car J (2006). Urinary tract infections in women: Diagnosis and management in primary care. BMJ 332:94-97.
Crossref
|
|
|
|
|
Chandra H, Srivastava J, Agarwal RK (2016). Fundamental Techniques in Microbiology. Publisher John Publisher Pvt. Ltd, New Delhi. ISBN 978-81-928544-1-0.
|
|
|
|
|
Daoud Z, Afif C (2011). Escherichia coli Isolated from Urinary Tract Infections of Lebanese Patients between 2000 and 2009: Epidemiology and Profiles of Resistance. Chemother. Res. Pract. Volume 2011, Article ID 218431, 6 pages.
|
|
|
|
|
Doern CD, Richardson SE (2016). Diagnosis of urinary tract infections in children. J. Clin. Microbiol. 54(9):2233-2242.
Crossref
|
|
|
|
|
Foxman B (2002). Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med.113(Suppl 1A):5-13S.
Crossref
|
|
|
|
|
Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD (2000). Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 10:509-515.
Crossref
|
|
|
|
|
Fry J (1969). Medicine in Three Societies Lancaster Medical and Technical Publishing Co. Ltd.
|
|
|
|
|
Goldraichi NP, Manfroi A (2003). Febrile urinary infection. Escherichia coli susceptibly to oral antimicrobials Paediatr. Nephrol. 17(3):173-176.
|
|
|
|
|
Goldstein FW (2000). Antibiotic susceptibility of bacterial strains isolated from patients with community-acquired urinary tract infections in France. Multicentre Study Group. Eur. J. Clin. Microbiol. Infect. Dis.19:112-117.
Crossref
|
|
|
|
|
Gonzalez CM, Schaeffer AJ (1999) Treatment of urinary tract infection: What`s old, what`s new, and what works. World J. Urol. 6:372-382.
Crossref
|
|
|
|
|
Gupta K, Hooten TM, Stamm WE (2001) Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med 135:41-50.
Crossref
|
|
|
|
|
Gupta V, Yadav A, Joshi RM (2002): Antibiotic resistance pattern in uropathogen. Indian J Med Microbiol. 20:96-98.
|
|
|
|
|
Hooton TM, Stamm WE (1997). Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 11(3):551-581.
Crossref
|
|
|
|
|
Hooton TM (2012). Uncomplicated urinary tract infection. N. Engl. J. Med. 366(11):1028-1037.
Crossref
|
|
|
|
|
Junuzovic D, Zunic L, Dervisefendic M, Skopljak A, Pasagic A, Masic I (2014). The toxic effect on leukocyte lineage of antimicrobial therapy in urinary and respiratory infections. Med. Arch. 68(3):167-169.
Crossref
|
|
|
|
|
Kahan NR, Chinitz DP, Waitman DA, Dushnitzky D, Kahan E (2006). Shapiro M:Empiric treatment of uncomplicated urinary tract infection with fluoroquinolones in older women in Israel: Another lost treatment option? Ann. Pharmacother. 40(12):2223-2227.
Crossref
|
|
|
|
|
Kulkarni SR, Peerapur BV, Sailesh KS (2017). Isolation and Antibiotic Susceptibility Pattern of Escherichia coli from Urinary Tract Infections in a Tertiary Care Hospital of North Eastern Karnataka. J. Nat. Sci. Biol. Med. 8(2):176-180.
Crossref
|
|
|
|
|
Kumar MS, Lakshmi V, Rajagopalan R (2006). Related Articles, Occurrence of extended spectrum beta-lactamases among Enterobacteriaceae spp. isolated at a tertiary care institute. Indian J. Med. Microbiol. 24(3):208-211.
|
|
|
|
|
Lipsky BA (1990). Urinary tract infection in men: Epidemiology, pathophysiology, diagnosis and treatment. Ann. Inter. Med. 110:138-150.
Crossref
|
|
|
|
|
Manges AR, Natarajan P, Solberg OD, Dietrich PS, Riley LW (2006). The changing prevalence of drug-resistant Escherichia coli clonal groups in a community: evidence for community outbreaks of urinary tract infections. Epidemiol. Infect. 134(2):425-431.
Crossref
|
|
|
|
|
Mond MC, Gruneberg RN, Smellie JM (1970). A study of childhood urinary tract infection in general practice. Br. Med. J. 1:602-605.
Crossref
|
|
|
|
|
Najar MS, Saldanha CL, Banday KA (2009). Approach to urinary tract infections. Indian J. Nephrol. 19(4):129-139.
Crossref
|
|
|
|
|
Nicolle L, Anderson PA, Conly J, Mainprize TC, Meuser J, Nickel JC, Senikas VM, Zhanel GG (2006). Uncomplicated urinary tract infection in women Current practice and the effect of antibiotic resistance on empiric treatment. Can. Fam. Physician 52(5):612-618.
|
|
|
|
|
Okafor HV, Okoro BA, Ibe BC (1993). Prevalence of asymptomatic bacteriuria among nursery school children. Niger. J. Paediatr. 20:84-88.
|
|
|
|
|
Olowu WA (1996). Symptomatic bacteriuria in children. Niger. Med Pract. 27:76-80.
|
|
|
|
|
Olowu WA, Oyetunji TG (2003). Nosocomial significant bacteriuria prevalence and pattern of bacterial pathogens among children hospitalized for non-infective urinary tract disorders. West Afr. J. Med. 22(1):72-75.
|
|
|
|
|
Royal College (1995). Royal College of General Practitioners Office of Population Consensus & Survey. Department of Health Morbidity Statistics from General Practice (1991–1992). London: HMSO.
|
|
|
|
|
Schaeffer AJ (2002). The expanding role of fluoroquinolones. Am. J. Med. 113(Suppl 1A):45S-54S.
Crossref
|
|
|
|
|
Smith RD, Coast J (2002). Antimicrobial resistance: a global response. Bull. World Health Organ. 80:126-133.
|
|
|
|
|
Sorlózano-Puerto A, Gómez-Luque JM, Luna-Del-Castillo JD, Navarro-Marí JM, Gutiérrez-Fernández (2017). Etiological and resistance profile of bacteria involved in urinary tract infections in young children. Biomed. Res. Int. 2017 Apr 11; 2017.
Crossref
|
|
|
|
|
Stamm WE, Norrby SR (2001). Urinary tract infections: disease panorama and challenges. J. Infect. Dis. 183(Suppl 1):S1-S4.
Crossref
|
|
|
|
|
Uzunović-Kamberović S (2009). Medicinska mikrobiologija. I izd ed. Zenica: Štamparija Fojnica. pp. 367-375.
|
|