ABSTRACT
Recent research has focused on natural plant products as alternative for disease control in both developed and developing countries. Medicinal plants can be a possible source for new potent antimicrobial agents to which pathogenic strains are not resistant. The present study was carried out to determine the in vitro antimicrobial activity of 14 plant species namely; Allium sativum, Aloe vera, Bryophyllum pinnatum, Cassia ocidentalis, Citrus sinensis, Euphorbia hirta, Mangifera indica, Myristica fragrans, Ocimium gratissimum, Piper guineese, Psidium guajava, Spermacoce verticilata, Vernonia amygdalina and Zingiber officinale and 3 antibiotic drugs namely; ampicillin, ciprofloxacin and streptomycin on Escherichia coli O157:H7 isolated from human clinical sample. The extracts of the plant species were prepared by cold percolation method using ethanol and water as solvents. Phytochemical analyses of the extracts of the different plant species were determined using standard methods. Agar well diffusion method was used to evaluate the antimicrobial sensitivity test of the plant extracts and that of antibiotic drugs at different concentrations ranging from 31.25 to 500 mg/ml. The minimum inhibitory concentration (MIC) of the antimicrobial agents against Escherichia coli O157:H7 was also conducted. Phytochemical analyses of the plant species revealed the presence of bioactivity principle such as alkaloids, balsam, cardiac glucoside, flavonoids, phenols, resins, saponins, tannins, terpenes and steroids. The results showed that all the antimicrobial agents exhibited inhibitory effects against the growth of the bacterial isolate at various degrees. Among the plant species employed in the study, the ethanolic and water extracts of P. guajava showed the highest inhibitory effect against the bacterium with growth inhibition mean zone diameters of 29.9 and 26.0 mm respectively at 500 mg/ml. Following P. guagava in order of inhibitory effect against E. coli O157:H7 are ethanolic extracts A. sativum, Z. officinale, V. amygdalina and M. indica with mean zones of inhibition of 21.2, 20.8, 20.3 and 19.9 mm respectively at 500 mg/ml. The results also revealed that of the three antibiotic drugs used in the study, ciprofloxacin exhibited the highest inhibitory effect against the organism with zone of inhibition of 38.6 mm, followed by streptomycin 30.2 mm, while ampicilin had the least 22.3 mm. The MIC results reveal that some of the plant species showed similar inhibitory effect against the bacterium, while the MIC results of the rest of the plants varied from one another. The in vitro study of the antimicrobial activity of the extracts of the various plant species and that of the antibiotic drugs against E. coli O157:H7 has demonstrated that certain folk medicine can be as effective as modern medicine in combating pathogenic microorganisms.
Key words: In vitro, antimicrobial activity, plant species and antibiotic drugs, Escherichia coli O157:H7.
Many works have been done which aim at knowing the different antimicrobial and phytochemical constituents of medicinal plants and using them for the treatment of microbial infections (both topical and systemic applications) as possible alternatives to chemically synthetic drugs to which many infectious microorganisms have become resistant (Akinpelu and Onakoya, 2006). Edeoga et al. (2005) reported that the pace of development of new antimicrobial drugs has slowed down; while the prevalence of resistance (especially multiple resistances) has increased astronomically. The increase in number of antibiotic resistant bacteria is no longer matched by expansion in the arsenal of agents available to treat infections. Literature reports and ethnobotanical records suggest that plants are the sleeping giant of pharmaceutical industry and they may provide natural source of antimicrobial drugs that will provide novel or lead compounds that may be employed in controlling some infections globally (Akinpelu and Onakoya, 2006; Cragg and Newmam, 2013; Gairola et al., 2014; Gordon et al., 2013; David et al., 2015).
The medicinal value of plants lies in archetypal plant constituents that produce a definite physiological action on the human body. The most important of these bioactive compounds of plants are alkaloids, cardiac glycosides, flavonoids, tannins, saponin, phenolic compounds, steroids and terpenes (Sofowora, 1993; Edeoga et al., 2005; Ojo et al., 2014; Omonkhua et al., 2015). Many phytochemical compounds have been shown to be bioactive, that is they exhibit remarkable biological activity in other living organism (Harborne et al., 1975; Hartog et al., 1993; Jin-Hyung et al., 2011). Many workers have demonstrated the antidiarrhoeal and antimicrobial activities of phytochemical compounds such as tannins (Mukherjee et al., 1998), flavonoids (Galvez et al., 1993; Ekuadzi et al., 2014), alkaloids (Gricilda and Molly, 2001), saponins, sterols and terpenes (Otshudi et al., 2000) containing plant extracts. The phytochemical research based on ethno-pharmacological information is generally considered as effective approach in the discovery of new antimicrobial agents from higher plants ((Erodgrul, 2002; Kloucek et al., 2005; Veeramuthu et al., 2006). In general, most of the plants used in folk medicine have not been screened for their antimicrobial activity (Kubmarawa et al., 2007).
Escherichia coli O157:H7 is an emerging cause of food borne illness such as haemorrhagic colitis (bloody diarrhoea), haemolytic-uremic syndrome (HUS) (leads to kidney failure) and thrombotic thrombocytopenic purpure (TTP) (leads to cardiac and neurological manifestations) (Doyle and Padhye; 1989; Center for Disease Control and Prevention (CDC), 2003). It was first recognized as a cause of illness in 1982 during an outbreak of severe bloody diarrhoea. The outbreak was traced to contaminated hamburgers (Riley et al., 1983). Since then, there have been many reports throughout the world describing the severe disease associated with this organism (Karch, 1996). This strain of E. coli produces powerful toxins (Belongia et al., 1993). It grows slowly at refrigeration temperature and can survive under acid environment (Thomas et al., 1995). No specific therapy has been proved in patients with E. coli O157:H7. Some studies have suggested that HUS is likely to develop in patients treated with antibiotics (Pavia et al., 1990).
As early as 1980’s Karch et al. (1986) performed an elegant in vitro experiment in which they added trimethoprim-sulfamethoxazole to cultures of E. coli O157:H7 and found that these drugs increased the release of Shiga toxin by the bacterium. These findings have since been extended to other enterohaemorrhagic strains of E. coli and other antibiotics. The findings have also raised the possibility that antibiotic treatment of E. coli O157:H7 infections might actually increase the risk of the haemolytic uraemic syndrome (CDC, 2003). Wong et al. (2000) provided data that validate this concern. Specifically, children who received antibiotics (trimethoprim-sulfamethazole or β-lactams) for diarrhoea caused by E. coli O157:H7 had a significant higher risk of the haemolytic-uraemic syndrome than those who did not receive antibiotics. The association was strong and independent of confounding variables, such as objective indices of the severity of illness. In 1996 an outbreak of infection with these organisms in Japan was associated with the consumption of white-radish sprouts (Wachsmuth et al., 1997). Subsequent analysis of risk factors for the haemolytic-uraemic syndrome revealed that antibiotics prevented the disease. In a Japanese study, the majority of patients received antibiotics, so the effect of no treatment could not be compared with that of treatment (Wong et al., 2000; Proulx et al., 1992).
Even though E. coli O157:H7 is widely considered sensitive to multiple classes of antibiotics (Griffin and Tauxe, 1991), strains showing multiple resistance to streptomycin, tetracycline and sulphisoxazole have been described in the USA (Swerdlow et al., 1992; Kim et al., 1994). Mawak and Ashamu (2006) also reported that out of the 8 antibiotics tested against E. coli O157:H7, only 4 namely; ampicillin, ciprofloxacin, gentamycin and to ease stomach cramps and stomach acidity in newborns (Sofowora, 1993) and gastrointestinal disorders (Hugo and Russel, 1997). ofloxacin inhibited the growth of the organism. The emerging resistance of E. coli O157:H7 and other enterohemorrhagic E. coli (EHEC) to antibiotics may have both epidemiologic and clinical implications. Resistant strains might have a selective advantage over other fecal flora of cattle to which antimicrobial agents are administered in feed or for therapeutic purposes, thereby increasing the frequency with which EHEC can be found in food of bovine origin (Dupont et al., 1996). Many factors influence the transmission of a gastrointestinal infection with E. coli O157:H7 to the haemolytic-uraemic syndrome (Lothar, 2000). The data of Wong et al. (2000) support the theory that antibiotics have an important role in this progression.
According to Lothar (2000) much has been learned since the publication of the 1983 report linking the haemolytic uraemic syndrome to gastrointestinal infection with E. coli O157:H7, but much work still needs to be done to devise specific therapies to halt this progression. One way to prevent antibiotics resistance of pathogenic species is by using new compounds that are not based on existing synthetic antimicrobial agents (Rogas et al., 2006). Medicinal plants might represent an alternative treatment in both severe and non-severe cases of infectious diseases and can also be a possible source for new potent antibiotics to which pathogenic strains are not resistant (Rogas et al., 2006).
Plants under study
Fourteen plant species used to determine antimicrobial activity against E. coli O157:H7 isolate are described as follows:
1. Garlic (Allium sativum) belongs to the family Liliaceae and is commonly known as garlic (David, 1997). The use of garlic in history goes back to thousands of years for treatment of numerous conditions throughout the world; garlic has generally been used for the treatment of diarrhoea, dysentery and many other ill health conditions (Murray, 1995). Garlic can be used as an antimicrobial agent, for immune enhancement and for cancer prevention (Rees et al., 1993; Lawson, 1998). Garlic has also been known to be capable of a broad spectrum antibiotic activity in inhibiting the growth of both gram positive and gram negative bacteria (Ross et al., 2001). In vitro, garlic powder induced inhibitory effect on the growth of E. coli O157:H7 with MIC value 10000 mg/liter (Eman and Hoda, 2008).
2. Indian aloe (Aloe vera) is a member of the family Liliaceae. It is related to onion, garlic and asparagus and has been noted to possess keratolic action (Hutchison et al., 2004). This is the action of removing damaged skin, and replacing it with new cells. It also allows the free flow of blood through the veins and arteries, clearing them of small blood clots (Erique, 1988). The same author reported that this plant has been proved to stop the destructive action of many bacteria such as Salmonella and Staphylococcus that produce pus. It also combats E. coli, Streptococcus faecalis as well as being effective against yeast (Candida albicans).
3. Life plant (Bryophyllum pinnatum) belongs to the family Crassulaceae. The crushed leaves or juice expressed from them are warmed as a poultice with shea-butter or palm oil and rubbed on abscesses or other inflammatory condition. It is also used for the treatment of arthritis and also used as anti-diarrhoeal plants (Iwu, 1993).
4. Stinking weed (Cassia occidentalis) belongs to the family Leguminosae. It is cosmopolitan in distribution (Akobundu, 1998). The plant has an unpleasant odour; however it is desirable because of its medicinal virtues. The documented properties and actions of this plant include: Antibacterial, antifungal, antiparasitic, antiseptic and insecticidal (George and Roger, 1998).
5. Sweet orange (Citrus sinensis) belongs to family Rutaceae and originated in tropical and sub-tropical Southeast Asia (Bangbose, 1980). The plants are large shrubs or small trees with spiny shoots. It is commonly known as sweet orange. The leaves, stems and bark of C. sinensis have high medicinal value and are used in treating viral, protozoan and bacterial infections (Sofowora, 1993).
6. Asthma herb (Euphorbia hirta) belongs to the family Euphorbiaceae. It is a creeping plant with very small leaves and branched stems. The plant is popularly known as Australian asthma herb. It is used to treat asthma and respiratory tract inflammation. It is also used
7. Mango (Mangifera indica) belongs to the family Anacardiaceae, which consists of about sixty genera and six hundred species, which are mainly tropical trees and shrubs (Trease and Evans, 1989). It was also observed that aqueous leaf extract of M. indica inhibited the growth of E. coli and some other pathogenic bacteria (Akinpelu and Onakoya, 2006).
8. Nutmeg (Myristica fragrans) belongs to the family Myristicaceae. Nutmeg is a common household remedy for diarrhoea (Shidore et al., 1985). It has been also reported that nutmeg may be of value in the treatment of refractory diarrhoea in some patients (Shafran et al., 1996).
9. Bush tea (Ocimum gratissimum) belongs to the family lamiaceae. It is a stout aromatic herb with its flower arranged in loose racemes (Trease and Evans, 1989). Apart from its flavouring purpose, its use as a medicinal plant is well documented. It is a folk remedy for many diseases such as fever, diarrhoea, dysentery, stomach ache, headache and cough (Tella, 1986).
10. Brown pepper (Piper guineense) belongs to the family Piperaceae. It is a native to South-Western India. It is cultivated in tropical regions around the world, and praised as a spice and a medicine since ancient times (Garland, 1984). They are used to treat nausea, stomach ache or lack of appetite. It is antiseptic and antibacterial and is effective in reducing fever (Duke, 1985).
11. Guava (Psidium guajava) belongs to the family Myrtaceae (Gill, 1988). The plant is used in folk medicine to treat fever, diarrhoea and as tonic in psychiatry (Iwu, 1993). The methanolic extracts of P. guajava were also shown to possess antibacterial effect on Bacillus subtilis, Stapylococcus aureus, E. coli and Pseudomonas aeruginosa (Abdelrahim, 2002). Clinical studies on plant drugs from leaves of P. guajava on some volunteers with gastrointestinal ailments were found to be effective (Olajide et al., 1999). Bark and leaf extracts of P. guajava is also used for diarrhoea, stomach ache and diabetes (Tanaka et al., 1992). In several studies, guava showed significant antibacterial activity against such common diarrhoea-causing bacteria as S. aureus, Shigella dysentriae, Salmonella typhi, Escherichia coli and P. aeuroginosa (Lozoya et al., 2002). Human clinical trials have also indicated the effectiveness of guava in treating diarrhoea in adults and infants (Tona, 1999; Lin, 2002).
12. Button weed (Spermacoce verticillata) belongs to family Rubiaceace. It is commonly referred to as shrubby false button weed (Burkill, 2000). It is used in treating acute diarrhoea and other gastrointestinal tract infections by the oral use of the leaf extracts (Burkill, 2000).
13. Bitter leaf (Vernonia amygdalina) commonly known as bitter leaf belongs to family Vernoniaceae. The water extracts serves as tonic drink for the prevention of certain illness (Kokwaro, 2000). The bitter taste is due to anti-nutritional factors such as alkaloids, saponins, tannins and glycosides (Tanaka et al., 1992). It possesses antimicrobial activities against organisms such as S. dysenteriae, S. aureus, Streptococcus pyogenes and E. coli (Nwokedi et al., 2003).
14. Ginger (Zingiber officinale) belongs to the family Zingiberaceae. Ginger is the common name for this plant. Ginger has analgesic, sedative, antipyretic and antibacterial properties (O’Hara et al., 1998).
Although the antimicrobial activity of the plant species investigated in this study have been well documented, there is still dearth of information as regarding the antimicrobial activity of these plants against E. coli O157:H7. The development of drug resistance in human pathogens against commonly used antibiotics has necessitated a search for new antimicrobial substances from other sources including plants that can aid in treating infection associated with E. coli O157:H7.
Source of plant materials and antibiotic drugs
All plants were obtained from Jos North Local Government Area of Plateau State, Nigeria and authenticated in the Department of Plant Science and Technology, Faculty of Natural Sciences, University of Jos, Nigeria. The antibiotic drugs were purchased from a pharmaceutical shop.
Preparation of plant extracts
Plant extracts were prepared by cold percolation method described by Akinpelu and Onakoya (2006). The various test plant species were well dried under the shade and then ground into fine powder using an electrical blender. A portion of 250 g each of the plants powder was separately soaked in 300 ml of 95% ethanol and another portion in 300 ml of sterile distilled water in glass containers and then covered with their lids. The plants soaked in ethanol were kept at room temperature those soaked in water were kept at refrigeration temperature (to prevent spoilage) for the period of 7 days to permit full extraction of the active ingredients or the chemical components. The fluids were then filtered using whatman No 1 filter paper into beakers. The extracts were obtained by oven drying the filtrate at 50°C and then kept in refrigerator pending analysis.
Phytochemical screening of the medicinal plant extracts
The phytochemical screening of the ethanolic extracts of the plant parts mentioned above was carried out in order to elucidate the chemical constituents such as alkaloids, balsam, cardiac glycosides, flavonoids, phenols, saponins, tannins, terpenes and steroids, responsible for their antimicrobial and therapeutic activities. The plant extracts were screened for the presence of these agents using standard qualitative procedures described by Trease and Evans (1989) and Sofowora (1993).
Source of microorganism
The culture of E. coli O157:H7 was obtained from the Microbiology Unit of the Department of Plant Science, University of Jos, Nigeria. The organism was isolated from human stool sample. The bacterial culture was maintained on nutrient agar slant and kept in refrigerator prior to use.
Sensitivity test
Before carrying out the antimicrobial tests, five grams (5 g) of each of the extracts was weighed separately and dissolved in 10 ml of sterile distilled water to produce a solution of 500 mg/ml. A serial doubling dilution was then carried out for each of the solutions to obtain concentrations of 250, 125, 62.5 and 31.25 mg/ml (Taura and Oyeyi, 2009). The same concentration levels were prepared using antibiotic drugs.
The antimicrobial activity of each of the plant extracts was determined using agar well diffusion method (Irobi et al., 1996; Akande and Hayashi, 1998). The bacterial isolates were subcultured three times in fresh Tryptone Soya Broth (TSB) in order to obtain a more vigorous population. The stocks were incubated at 37°C for 24 h. A 0.5 ml of the standardized portion of the new culture was aseptically transferred into Petri dishes containing nutrient agar and left for about 20 min to allow the microorganisms fix on the medium. Wells where extracts were to be introduced into the plates were carefully marked using sterile cork borer (6 mm diameter) and small drops of extract of various concentrations (500, 250, 125, 62.5 and 31.25 mg/ml) were added into the wells. A well was also made at the central portion of the agar medium and drops of sterile distilled water and or 95% ethanol were placed therein to serve as controls. The plates were incubated at 37°C and the zones of inhibition were measured after 24 h. The growth inhibitory effects of the plant extracts were compared with that of 3 standard antibiotics, namely; ampicillin, ciprofloxacin, and streptomycin at the same concentrations as the plant species. The presence of zones of inhibition was regarded as the evidence of antimicrobial action. The zones of inhibition were measured with a ruler at right angles across the zones to find the average diameter in millimeters.
Determination of minimum inhibitory concentration (MIC)
The MIC was determined for the plant species that showed inhibitory effect against the test organism. The macro broth dilution method (utilizing nutrient broth) was used for the determination of MIC. Serial doubling dilutions of the plant extracts and the standard antibiotics were prepared according to the method described by Taura and Oyeyi (2009) in test tubes containing 2 ml of broth to arrive at concentrations of 125 mg/ml, 62.25 mg/ml, 31.25 mg/ml, etc. A drop of the standardized inoculum corresponding to a 1:100 dilution of the overnight broth culture of the organism was then introduced into each of the tubes. The tubes were then incubated at 37°C overnight after which they were examined for signs of turbidity, which would indicate the growth of the test organism. Control tubes were also included alongside with the incubated tubes (for easy reading) as demonstrated for antibiotics by Norrel and Kessley (1997). The controls were prepared as follows:
A = Positive (growth) controls - seeded tube but no extract
B = Negative (sterility) - Unseeded tube with extract
C = Negative (sterility) - Unseeded tube without extract
Statistical analysis
Each test was replicated twice for each plant’s extract and antibiotic at various concentrations. The data obtained from the study were subjected to statistical analysis using analysis of variance (ANOVA) and the least significant difference (LSD) was used to test whether there was a significant between the means or not. Statistical package employed was IBM SPSS software, version 22.
Table 1 shows the phytochemical profile of the ethanolic extracts of the twenty plant species. The results showed that alkaloids, balsam, flavonoids, saponins, cardiac glycosides, phenols, tannins, terpenes and steroids were present in the crude extracts of the plant species. However, two of the plants (A. vera and E. hirta) did not possess all the chemical compounds, while the rest of the plants contained all. It was observed also that some of the plant species contained these compounds in appreciable amounts and others had them in trace amounts.
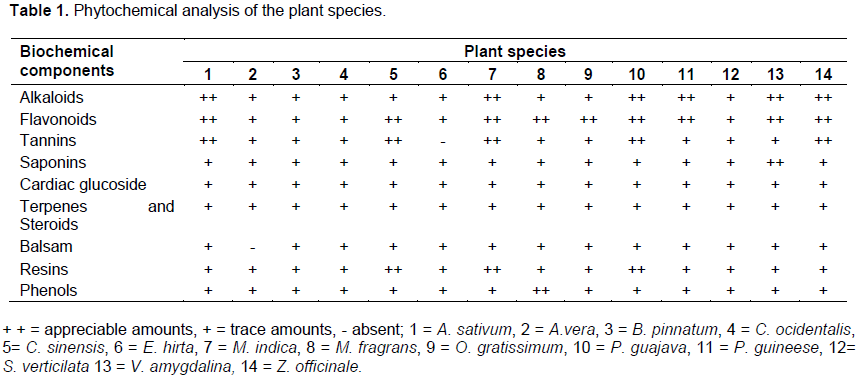
The antimicrobial activity of ethanolic and water extracts of 14 plant species and of 3 commonly used antibiotic drugs on human isolate of E. coli O157:H7 are presented in Tables 2 and 3 respectively. All the 14 plants showed inhibitory effect against the growth of the bacterial isolate at various degrees (Table 2). The results also reveal that among these 14 plants species, the ethanolic and water extracts of P. guajava exhibited the highest inhibitory effect against bacterial isolate with growth inhibition mean zone diameters of 29.9 and 26.0 mm respectively at a high concentration of 500mg/ml. These were closely followed by the ethanolic extracts of A. sativum, Z. officinale, V. amygdalina and M. indica with mean zones of inhibition of 21.2, 20.8, 20.3 and 19.9 mm respectively at 500 mg/ml concentration. The results also show that the ethanolic and water extracts of A. sativum, M. indica, P. guajava, V. amygdalina and Z. officinale inhibited the growth of the test organism at all the concentrations employed in this study. It was observed that the ethanolic extracts of P. guineese, S. verticillata C. sinensis, and water extract of M. fragrans also showed promising activity against the test bacterium by producing moderate mean zones of inhibition of 16.9, 15.3 15.0 and 15.8 mm, respectively at 500 mglml. On the other hand, the crude extracts of 4 plant species namely; A. vera, B. pinnatum, C. occidentalis and O. gratissimum exhibited relatively minor effect on the growth of E. coli O157:H7 isolate with zone diameters ranging between 5.7 and 8.7 mm for their ethanolic extracts and 3.6 and 6.2 mm for their water extracts at 500 mg/m.
The results also show that both ethanolic and water extracts of A. vera exhibited analogous activity on the test organism with mean zones of inhibition of 6.0 and 5.6 mm respectively at 500 mg/ml. The ethanolic extract of E. hirta showed only slight activity on the organism with mean zones of inhibition of 2.3 and 4.5 mm at 250 and 500 mg/ml, respectively, while the water extract of the same plant showed no activity at all the concentrations (Table 2).
When the results of the activity of water (aqueous) and ethanolic extracts of the plant species against the test organism were compared statistically, it was observed that there was a significant difference (P<0.05) between the aqueous and ethanolic extracts with respect to the degree of their inhibitory effect, with the latter having higher inhibition than the former. The results in Table 2 also indicate that the plant extracts that inhibited the growth of the test organism decreased in effectiveness as the extract concentration decreases. Thus at a lowest concentration of 31.25 mg/ml most of the plant extracts showed little or no activity against the test organism. At higher concentrations of 250 and 500 mg/ml most of the plants that exhibited activity had better zones of inhibition.
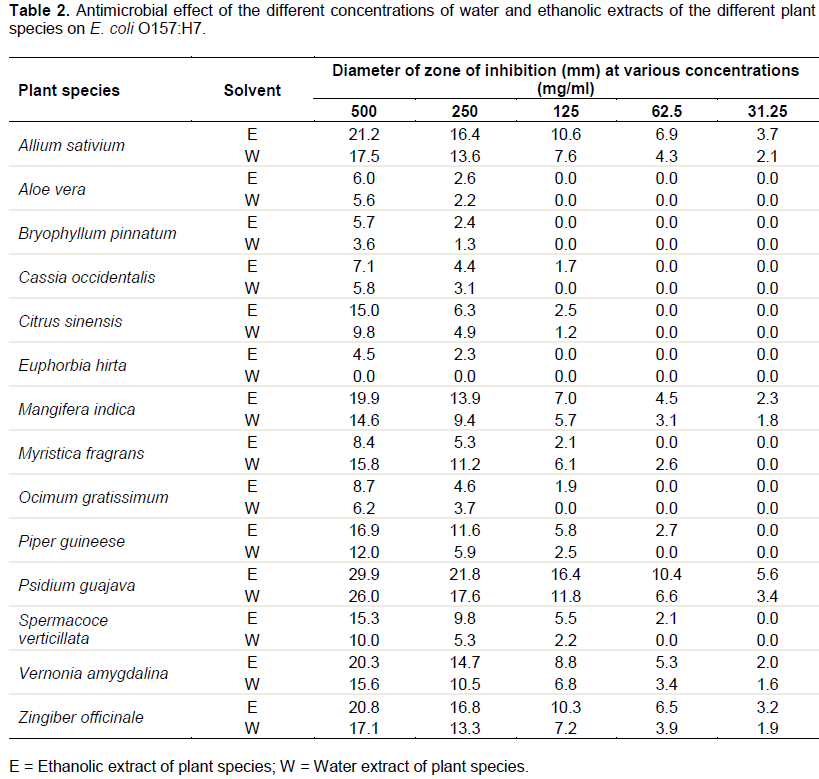
Table 3 shows the sensitivity of E. coli O157:H7 to 3 different standard antibiotic drugs. The results reveal that 3 of the antibiotics, namely, ciprofloxacin, streptomycin and ampicillin showed inhibitory effects against the test organism with zones of inhibition of 38.6, 30.2 and 22.3 mm respectively at 500 mg/ml. Statistical analysis of the results in Table 3 indicated that there was significant difference between the zones of inhibition exhibited by some of the antibiotics against the test organism with regard to various concentrations.
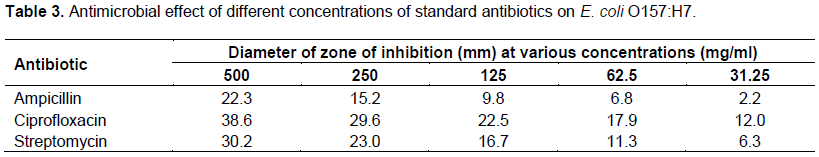
On comparing the results of the antimicrobial tests of the plant drugs and that of the standard antibiotic drugs against the test organism (Tables 2 and 3), it was observed that only one of the standard antibiotic drug, ciprofloxacin that inhibited the growth of the test organism more than all the plant drugs at all the concentrations employed in this study. Comparison of the results in Tables 2 and 3 also reveal that extracts of P. guajava showed antimicrobial activity against the test organism that is comparable to that of streptomycin. In the same manner, extracts of A. sativum, M. inidca, V. amygdalina, and Z. officinale exhibited activity against the test organism almost similar to ampicillin.
The minimum inhibitory concentration (MIC) of the plant extracts and that of the antibiotic drugs that showed activity against the test organism was determined and the results are presented in Tables 4 and 5 respectively. The MIC results in Table 4 reveal that some of the plant species exhibit equal or similar MIC on the test organism, while the MIC results of the rest of the plants varied from one another. Out of the 14 plant species that their MICs were determined in this study, the ethanolic and water extracts of P. guajava had the lowest MIC values of 0.25 and 0.5 mg/ml respectively. These were followed by the MIC results of A. sativum, M. inidca, V. amygdalina and Z. officinale, which manifested equal but also low MICs of 1 and 2 mg/ml for their ethanolic and water extracts respectively. The results in Table 4 also reveal that the ethanolic and water extract of C. sinensis, M. fragrams, P. guineese and S. verticillata presented mordrate MIC values ranging between 4 and 8 mg/ml. On the other hand, the extracts of A. vera, B. pinnatum, E. hirta, C. occidentalis and O. gratissimum presented high MIC values that varied between 16 and 62.5 mg/ml. The MIC results of the plant extracts show that ethanolic extracts of most of the plant species exhibited a lower MIC values than their water extracts (Table 4).The results in Table 5 show that ciprofloxacin had the lowest MIC value (0.125 mg/ml). This was followed by streptomycin (0.25mg/ml), while ampicillin had the highest MIC value of 1 mg/ml.
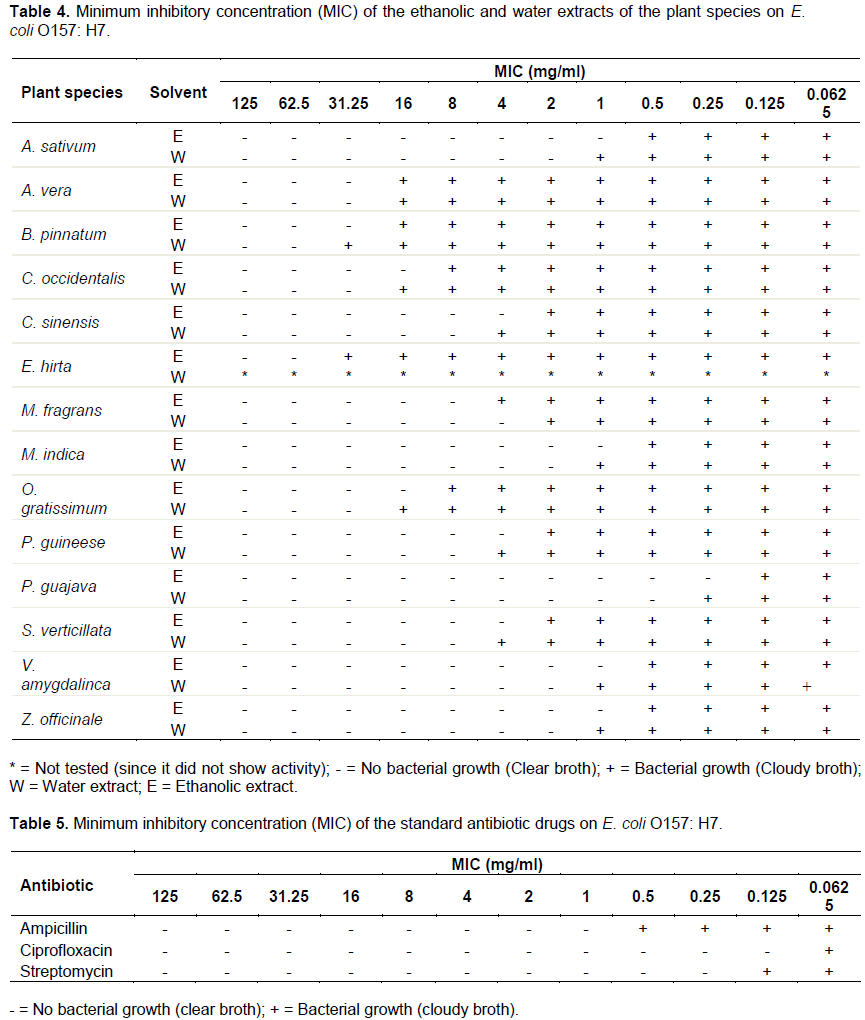
A comparison between the MIC results of the plant extracts with those of the standard antibiotics reveal that the standard antibiotic (ciprofloxacin) had a lower MIC value than the MIC values of the plant species (Table 4 and 5). The results show that the ethanolic extracts of P. guajava presented the same MIC value with that of streptomycin (0.25 mg/ml) against E. coli O157:H7. However, the ethanolic and water extracts of P. guajava manifested better MICs against the test organism than ampicillin (1 mg/ml). The results also show that that ethanolic extracts of A. sativum, Z. officinale, V. amygdalina and M. indica manifested the same MIC (1 mg/ml) with standard antibiotics ampicillin against the test organism.
The results of the phytochemical screening obtained in this study may have been affected by the type of solvent used for extraction. Only ethanolic extracts of the plant species were used to determine the type of chemical compounds present in them. Certain plant–based drugs have presented high yield of extractable chemical substances in their water extract (Rojas et al., 2006). This shows that water, and not always alcohol, can serve as the best solvent for extracting certain chemical compounds from some plants. Another factor that could have affected the type of chemical compounds present in the plant species is the source of the plants. It is known that environmental stress and mineral deficiency (e.g. boron shortage) affect phenolic anabolism and tannins present in the plants (Harbone, 1993). The presence of the phytochemical compounds in appreciable quantities in most of the plant species suggests that such could be useful in curing infections associated with E. coli O157:H7 most especially those that have antibacterial medicinal potentials. For example, Jin-Hyung et al. (2011) reported that flavonoid (phloretin) inhibited E. coli O157:H7 biofilm formation and ameliorates colon inflammation in rats.
Recent research has focused on natural plant products as alternatives for disease control in both developing and developing countries (Tona et al., 1998; Keita et al., 2000; Todar, 2005). The significance of the inhibitory effects of crude extracts of various plant species against E. coli O157:H7 have been demonstrated by the results from this study. Among the plant extracts tested in this work, those of the leaves of P. guajava (guava) showed the most remarkable inhibitory effect against the test organism (E. coli O157:H7). The largest zones of inhibition caused by the extracts of leaves of P. guajava against the test organism further supported the effective use of guava to control diarrhoea, dysentery and gastro-enteritis (Arima, 2003). In addition guava is known to be rich in phytochemical compounds such as flavonoids, phenols, terpenes, and essential oils. Much of guava’s therapeutic activity is attributed to these compounds (Holetz, 2002). Following P. guajava in order of inhibitory effects against E. coli O157:H7 are A. sativum, Z. officinale, V. amygdalina and M. indica. Phytochemical constituents such as alkaloids, flavonoids, saponins, terpenes and steroids are some of the major compounds present in these plants and they are known to exhibit antimicrobial properties. Thus, the plants also showed high antimicrobial properties against the bacterial isolate. The present finding confirms the scientific studies carried out on some of these plant species as having effectiveness in inhibiting the growth of certain bacterial isolates in vitro (Ali, 1994; Tona, 1999; Nwokedi et al., 2003; Akinpelu and Onakoya, 2006; Okorondu et al., 2006). The present study also agrees with work of Eman and Hoda (2008) that reported that garlic powder was able to induce strong inhibitory effect on the growth of E. coli O157:H7.
It is interesting to note that P. guajava, A. sativum, Z. officinale, V. amygdalina and M. indica were the only plant species that exerted inhibitory effect on the test bacterial isolate at all the concentrations considered in this research. This suggests that the compounds responsible for the antibacterial activity were present in appreciable quantity at all the concentrations as to have been able to inhibit the growth of the test organism. The extracts of C. senensis, P. guineese, S. verticillata and M. fragrans also showed promising activities against the test microorganism by producing moderate zones of inhibition on the culture plates. The results obtained from this study thus compliment earlier reports by Soforowa (1993), Burkill (2000) and Shafran et al. (1996) that justify that some of these plants showed antimicrobial activity against some pathogenic microorganisms associated with diarrhoea.
The results of this investigation also reveal that some of the plant species, namely, A. vera, B. pinnatum, C. occidentalis, E. hirta and O. gratissimum exerted very minimal antimicrobial effect on the test organism. The relatively low inhibitory activity exhibited by these plants extracts against the test organism is surprising as this contradicts previous reports (Tella, 1986; Erique, 1988; Iwu, 1993; George and Roger, 1998). These reports showed that these plants have strong antimicrobial activities against both gram-positive and gram-negative bacteria. The reduced effectiveness of these plant extracts observed in this study may be due to low concentration of phytochemical constituents or that the method of extraction did not yield high concentrations of the chemicals that could have had reasonable effects on the test organism.
The findings of the present study showed that the ethanolic extracts of all the plant species exhibited a higher degree of antimicrobial activity when compared to the water extracts with the exception of A. vera and M. fragrans. The higher susceptibility of the test bacterial isolate to ethanolic extracts of most of the plant species is not surprising as previous studies have reported ethanol to be a better solvent than water for extracting secondary metabolites (which are inhibitory to microorganisms) from most plants (Olukoya et al., 1993; Okorondu et al., 2006). Caceres et al. (1993) tested guava leaf extract obtained with three solvents of different polarities (n-hexane, acetone and ethanol). They discovered that ethanolic extract of the plant was most efficient against the pathogenic enterobacteria tested. The parity in the activity and spectrum of extracts as a result of the nature of solvents lend more weight to the findings of Obi and Onuoha (2000). These workers reported high recovery of alkaloids and essential oils with ethanol than with water. Thus, it could be that some of the active principles responsible for medicinal property of the plants may not be extractable using only water as solvent. The higher inhibitory activity exhibited by ethanolic extracts than the water extracts by most of the plant species also correlated with the preparations of medicinal plants by traditional medical practitioners (TMP) who use rum and liquor to extract the active plant compounds. Furthermore, the reason why the ethanolic extracts of most of the plant drugs exerted more antibacterial effect against E. coli O157:H7 isolate than their aqueous (water) extracts could be due to the fact that the antibacterial activity of the plants seemed to depend on their polar constituents with the ethanolic extracts being more polar than the aqueous extracts (Sofowora, 1993).
Both water and ethanol were found to be suitable solvents for the extraction of bioactive agents from A. vera. Their ethanolic and water appeared seems to exert similar antimicrobial effect on the test organism. This suggests that the polar constituents of the ethanolic and water extracts of this plant are similar in activity (Sofowora, 1993). It is interesting to note that the use of water extract of nutmeg (M. fragrans) for anti-diarrhoeal activity, normally practiced in India folk medicine (Shidore et al., 1985) is confirmed in this study, as the water extract of the plant seems to show more activity against the test organism than the ethanolic extract.
The results of this study show that only the ethanolic extract of the leaf of E. hirta was slightly effective against the E. coli O157:H7 isolate. The water extract of the same plant showed no activity at all. This may be attributed to the fact that ethanolic extract contained a small quantity of the bioactive agents which had little effect on the organism. Another reason could be that the polar constituents that could have exerted effect on the organism were not present in the water extract (Sofowora, 1993) that was why no inhibition was encountered.
It was observed that the test plants that showed activity against the E. coli O157:H7 isolate became more effective in inhibiting the organism as the concentration of the plant extracts increased. This suggests that the antibacterial activity of the crude plant extracts appeared to be dosage dependent. Hence, the concentrated decoction may be an effective therapy against diarrhoegenic agents. This finding may be useful in dosage administration. However, it reflects the problem of drug administration by traditional healers in which the dosage of the unrefined herbal preparations is often very small to make any meaningful impact or too large, which may be harmful to the body system (Nwokedi et al., 2003). Meanwhile, it may be pertinent to continue cooperation with traditional healers to regulate and standardize the dosage of herbal medicines they administer to the patients that patronize them.
The sensitivity test of E. coli O157:H7 to different standard antibiotics that served as positive control showed that all the three (ciprofloxacin, streptomycin and ampicillin) were active against the organism. The findings of this study are in agreement with former studies by Akinpelu and Onakoya (2006) and Mawak and Ashamu (2006) in which streptomycin and ampicillin acted against certain bacterial isolates including E. coli O157:H7. In addition, Antai and Anozie (1987) observed that ciprofloxacin inhibited the growth of many serotypes of pathogenic E. coli.
On comparing the results of the antimicrobial activity of the extract of the plant species with those of the standard antibiotics, it was observed that none of the plant extracts was more active against E. coli O157:H7 than ciprofloxaxin. However, the extracts of some of the plant species (A. sativum, M. indica P. guajava, V. amygdalina and Z. officinale) presented antimicrobial activity comparable to that of other standard antibiotics (streptomycin and ampicillin). This finding agrees with the work of Gnan and Demello (1999) who compared the effects of the extracts made of guava leaves and fruits at a concentration of 6.5 mg/ml upon test organism to those of conventional antibiotics (chloramphenicol, cefoxitin and metaxotin) and found that the results were comparable. Akinpelu and Onakoya (2006) revealed also that extracts of P. guajava compared favourably with a standard antibiotic (streptomycin) when tested against certain gastrointestinal organisms. In another study Rojas et al. (2006) reported that the water extract of Jacaranda mimosifolia and Piper pulchrum showed a higher activity than standard antibiotic drug (gentamycin sulphate) against Bacillus cereus. In addition, Irobi et al. (1996) found that leaf extracts of P. guajava were more efficient than oxytetracycline for treating acute diarrhoea in humans. The present findings and the reports of previous workers have confirmed the fact that some of the herbal preparations used by the traditional healers actually possess medicinal potency similar to standard antibiotics. Many of the herbal remedies of old have since been adopted and adapted by conventional Western allopathic medicine, simply due to the fact that they are effective. Thus the inestimable value of medicinal plants to health care systems in the world has increasingly become appreciated (Egunyomi, 2015). In some remote communities in Colombia, traditional healers claim that their medicine is cheaper and more effective than modern medicine (Rojas et al., 2006). Rojas et al. (2006) also reported that patients of rural communities who rely mostly on traditional medicine claimed to have a reduced risk to get infectious diseases from resistant pathogen than people in urban areas treated with synthetic antibiotics. However, if they are treated in a hospital the chance of contracting a nosocomial infection may increase. Thus, one way to prevent antibiotic resistance of pathogenic species according to Akinpelu and Onakoya (2006) is by using new compounds that are not based on existing synthetic antimicrobial agents. Drug resistance has been a source of grave concern in clinical practice (Antai and Anozie, 1987). It is regrettable to note in a typical Nigerian setting, the escalation of antibiotic resistance of pathogenic organisms due to the uncontrolled use of antibiotics and the common practice of self-medication. This suggests that the Nigerian Society for Microbiologists and the Nigerian Medical Association should embark on a nationwide programme of public enlightenment through the mass media, on the dangers of antibiotic abuse.
A close examination of Tables 4 and 5 reveals that, apart from the standard antibiotic drug (ciproflaxacin) which had a lower MIC value than all the extracts of the plant species, the other two standard antibiotics (streptomycin and ampicillin) had equal MIC values with the extracts from some of the plant species. The low MIC value exhibited by ciproflaxacin could be attributed to the fact that it is among the latest antibiotic drugs not yet exposed to bacterial resistance. Thus, E. coli O157:H7 was found to be highly sensitive to the drug. Among the plant species, P. guajava, A. sativum, Z. officinale, V. amygdalina and M. indica could be regarded as the first choice plants whole extracts for inhibiting the growth of E. coli O157:H7, since they compared favourably with standard antibiotics. It was also observed that the extracts of four plants, namely C. senensis, M. fragrans, P. guineese and S. verticillata showed relatively low MIC values against the test organism. This suggests that the four plants could also serve as alternative sources of herbal medicines for treating infection caused by the pathogen. Again, the extracts of plants such as A. vera, B. pinnatum, C. occidentalis, O. gratissimum and E. hirta may not be effective for the treatment of the infection associated with the test organism, as they exhibited high MIC values against the test organism. Generally, the ethanolic extracts of most of the plant species had lower MIC values than the water extracts of the same plants. This shows that the chance to find antimicrobial agents was more apparent in ethanol than in water extracts of the same plants.
The results of the antimicrobial activity and that of the MIC of the various the crude extracts of the plant species revealed that out of the 14 plants species examined, only 9 inhibited the growth of E. coli O157:H7 substantially, with P. guajava showing the best antimicrobial activity. The inhibition of E. coli O157:H7 in vitro by the crude extracts of these plants species, points to the fact that they were actually fortified with bioactive principles. The crude extracts of some of the plant species also compared favourably with antibiotic drugs by exhibiting similar antimicrobial activity. Furthermore, as the extracts of the plant species contain phytochemical constituents, it therefore suggests that they could be important source of antimicrobial drugs which could be beneficial in curbing the spread of infectious disease associated with E. coli O157:H7.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdelrahim SI (2002). Antimicrobial activity of Psidium guajava L. Fitoterapia. 73(7- 8):713-715.
|
|
|
|
Akande JA, Hayashi Y (1998). Potency of extract contents from selected tropical chewing sticks against Staphylococcus aureus and Staphylococcus auricularis. World J. Microbiol. Biotechnol. 14:235-238.
Crossref
|
|
|
|
Akinpelu DA, Onakoya TM (2006). Antimicrobial activities of medicinal plants used in folklore remedies in South-Western States of Nigeria. Afr. J. Biotechnol. 5(11):1078- 1081.
|
|
|
|
Akobundu IO (1998). A handbook of West Africa Weeds (2nded.). Agriculture Printer at INTEL. Ibadan, pp. 564.
|
|
|
|
Ali M (1994). Volatile oils. In: Textbook of Pharmacognosy.CBS publisher and distributors. India, pp. 143-184.
|
|
|
|
Antai SP, Anozie SO (1987). Incidence of infantile diarrhoea due enteropathogenic Escherichia coli in Port Harcourt metropolis. J. Appl. Bacteriol. 62:227-229.
Crossref
|
|
|
|
Arima H (2003). Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biotechnol. Biochem. 66(8):1727-1730.
Crossref
|
|
|
|
Bangbose SOA (1980). Research into African Medicinal Plants. J. Phytochem. 27:2-5.
|
|
|
|
Belongia EA, Osterholm MT, Soler JT, Ammend DA, Braun JE, MacDonald KL (1993). Transmission of Escherichia coli O157:H7 infection in Minnesota child day–care facilities. J. Am. Med. Assoc. 269:883-888.
Crossref
|
|
|
|
Burkill HM (2000). The Useful Plants of West Tropical Africa. Royal Botanic Garden. Kew UK, P 686.
|
|
|
|
Caceres A, Fletes L, Aguilar L (1993). Plants used in Guatemala for treatment of gastrointestinal disorders. J. Ethnopharm. 38:31-38.
Crossref
|
|
|
|
Centres for Disease Control and Prevention (2003). Escherichia coli O157:H7 general information, frequently asked questions:What is Escherichia coli O157:H7? Cragg GM, Newman DJ (2013). Natural products: a continuing source of novel drug leads. Biochim. Biophs. Acta. 1830:3670-3695.
|
|
|
|
David B, Wolffender JL, Dias DA (2015). The pharmaceutical industry and natural products, historical status and new trends. Phytochem. Rev. 14(2):299-315.
Crossref
|
|
|
|
David M (1997). Anti-microbial activity of garlic. Anti-microbial agents and chemotherapy. J. Ethnopharm. 74:113-123.
|
|
|
|
Doyle MP, Padhye VV (1989). Escherichia coli O157:H7. In M.P. Doyle (ed.). Food borne bacterial pathogens. Marcel Dekker New York, pp. 235-281.
|
|
|
|
Duke AJ (1985). Handbook of Medicinal Herbs. CRC Press Inc. Florida. P 355.
|
|
|
|
Dupont H, Timsit JF, Souweine B, Gachot B, Wolff M, Regnier B (1996). Antimicrobial susceptibility of Escherichia coli O157:H7 and other enterohaemorrhagic Escherichia coli isolated in Italy. Lancet. 15:351-353.
|
|
|
|
Edeoga HO, Okwu DE, Mbaebie BO (2005). Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 4:685-688.
Crossref
|
|
|
|
Egunyomi A (2015). Value of medicinal plants. A scale dependent on time and race. Nigeria J. Bot. 28(1):1-14.
|
|
|
|
Ekuadzi E, Dickson R, Fleischer T, Pistorius L, Gibbons S. (2014). Flavonoid glycosides from the stem bark of Margaritaria discoidea demonstrate antibacterial and free radical scavenging activity. Phytother. Res. 28(5):784-787.
Crossref
|
|
|
|
Eman MA, Hoda MZ (2008). Studies on the effect of garlic preparation on Escherichia coli O157:H7 causing enteritis in lambs. Egyptian J. Clin. Pathol. 21(4):102-129.
|
|
|
|
Erique G (1988). Natural Remedies for Health and Well Being (6th ed). Ovit publishing company. Mexico, P 359.
|
|
|
|
Erodgrul OT (2002). Antibacterial activities of some plant extracts used in folk medicine. Pharm. Biol. 40:269-273.
Crossref
|
|
|
|
Gairola S, Sharma J, Bedi YS (2014). A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medical plant use. J. Ethnopharmacol. 155(2):925-986.
Crossref
|
|
|
|
Galvez J, Zarzuelo A, Crespo ME, Lorente MD, Ocete MA, Jimenez J (1993). Antidiarrhoeic activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med. 5:333-336.
Crossref
|
|
|
|
Garland S (1984). The Herb and Spice Book. Frances Lincoln Publishers Ltd. London, pp. 20-27.
|
|
|
|
George DP, Roger MD (1998). Encyclopedia of Medicinal Plants. Macmillian press. London. P 630.
|
|
|
|
Gill LS (1988). Ethnomedical Uses of Plants in Nigeria. Uniben Press, Benin city. pp. 276.
|
|
|
|
Gnan SO, Demello MT (1995). Inhibition of Staphylococcus aureus by Goiaba extracts. J. Ethnopharm. 68:103-108.
Crossref
|
|
|
|
Gordon MC, David JN (2013). Natural Products: A continuing source of novel drug leads. Biochim. Biophs. Acta. 1830(6):3670-3695.
Crossref
|
|
|
|
Gricilda SF, Molly T (2001). Study of anti-diarrhoeal activity of four medicinal plants in castor oil induced diarrhoea. J. Ethnopharm. 76:73-76.
Crossref
|
|
|
|
Griffin PM, Tauxe RV (1991). The epidemiology of infections caused by Escherichia coli O157:H7, other enterohaemorrhagic Escherichia coli, and the associated haemolytic uraemic syndrome. Epidemiol. Rev. 13:60-98.
Crossref
|
|
|
|
Harbone JB (1993). Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. Chapman and Hall, London. P 279.
|
|
|
|
Harborne JB, Marbry TT, Marbry H (1975). The Flavonoids. Champan and Hall., London. P 86.
Crossref
|
|
|
|
Hartog MGL, Feskens EJM, Hollman PCH, Katau MB (1993). Dietary antioxidant flavonoids and risk of coronary heart disease in the Zutphen elderly study. Lancet 342:1007-1011.
Crossref
|
|
|
|
Holetz FB (2002). Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Oswaldo Cruz. 7:1027-1031.
Crossref
|
|
|
|
Hugo JA, Russel AV (1997). Plasmodium Resistance to Azadirachta indica extract. J. Biochem. 3(2):244-250.
|
|
|
|
Hutchison ML, Walters LD, Moore A, Crookes KM, Avery SM (2004). Effect of length of time before incorporation on survival of pathogenic bacteria present in livestock wastes applied to agricultural soil. Appl. Environ. Microbiol. 70: 5111-5118.
Crossref
|
|
|
|
Irobi ON, Mo–Young M, Anderson WA, Daramola SO (1996). Antimicrobral activity of bark extracts of Bridelia ferruginea. Int. J. Pharm. 34(2):87-89.
Crossref
|
|
|
|
Iwu MM (1993). Handbook of African Medical Plants. CRC Press Inc. Boca Raton. pp. 223-224.
|
|
|
|
Jin-Hyung L, Sushil CR, Jung-Ae K, Moo HC, Hyungdon Y, Chang-Soo L, Jintae L (2011). Apple flavonoid phloretin inhibits Escherichia coli O157:H7. Biofilm and ameliorates colon inflammation in rats. Infect. Immun. 79(12):4819-4827.
Crossref
|
|
|
|
Karch H, Strocbine NA, O'Brien AD (1986). Growth of Escherichia coli in the presence of trimethoprim–sulfamethoxazole facilitates detection of shiga–like toxin producing strains by colony blot assay. Microbiol. Lett. 27:347-349.
|
|
|
|
Keita MS, Vincent C, Jean-Pierre S, Arnasou IT, Belanger A (2000). Efficacy of essential oil of Ocimum gratissimum (African basil) applied as an insecticidal fumigant and powder to control Callosobrochus maculatus (Fab) (Coleoptera: Buchidae). J. Stored Prod. Res. pp. 339:349.
|
|
|
|
Kim HH, Samadpour M, Grimm L, Clausen CR, Besser TE, Baylor M, Kobayashi JM, Neil MA, Schoenknecht FD, Tarr PI (1994). Characteristics of antibiotic – resistant Escherichia coli O157:H7 in Washington State. J. Infect. Dis. 170:1606-1609.
Crossref
|
|
|
|
Kloucek P, Polesny Z, Svobodova B, Vlkova E, Kokoska L (2005). Antibacterial screening of some Peruvian medicinal plants used in Calleria District. J. Ethnopharm. 99:309-312.
Crossref
|
|
|
|
Kokwaro JO (2000). Medicinal Plants of East Africa. East Africa Literature Bureau. Nairobi, P 246.
|
|
|
|
Lawson LD (1998). Garlic: A Review of its Medicinal Effects and Indicated Active Compounds. In: Phytomedicines of Europe the Chemistry and Biology Activity L. D. Lawson and R. Bauer (ed). ACS symposium series American Chemical Society. Washington DC. pp. 691.
Crossref
|
|
|
|
Lin J (2002). Anti-diarrhoeal evaluation of some medicinal plants used by Zulu traditional healers. J. Ethnopharm. 79(1):53-56.
Crossref
|
|
|
|
Lothar MD (2000). Escherichia coli, Antibiotics, and the hemolytic–uremic syndrome. The New Eng. J. Med. 342:1990-1991.
Crossref
|
|
|
|
Lozoya X (2002). Intestinal anti-spasmodic effect of a phytodrug of Psidium guajava foliage in the treatment of acute diarroheic disease. J. Ethonopharm. 83(1-2):9-24.
Crossref
|
|
|
|
Mawak JD, Ashamu TO (2006). Occurrence of entropathogenes in traditional weaning cereal pastes in Jos, Nigeria. Niger. J. Microbiol. 20(3):1288-1295.
|
|
|
|
Mukherjee PK, Saha K, Murugesan T, Mandal SC, Pal M, Saha BP(1998). Screening of anti-diarrheaol profle of some plant extracts of a specific region of Wet Bengal. Indian J. Ethnopharm. 60:85-89.
Crossref
|
|
|
|
Murray M (1995).The Healing Power Herbs. (2nded.). A Prima Publishing Company New York, P 400.
|
|
|
|
Norrel SA, Keesley KE (1997). Microbiology Laboratory Manual–Principles and Applications. Prentice Hall, New Jersey, pp. 123-126.
|
|
|
|
Nwokedi VC, Itelima JU, Ogaraku AO (2003).The antimicrobial activities of extracts of Veronia amygdalina and Telfairia occidentalis on some bacteria. W. Afr. J. Biol. Sci. 14:43-47.
|
|
|
|
O'Hara MD, Mary MS, David K, Kim F, Kathi K (1998). A review of 12 commonly used medicinal herbs. Arch. Family Med. 7:523-536.
Crossref
|
|
|
|
Obi VI, Onuoha C (2000). Extraction and Characterization Methods of Plants and Plant Products. In Biological and Agricultural Techniques. JN Ogbulie and OJ Ojiako.Websmedia Publications. Owerri. pp. 271-286.
|
|
|
|
Ojo OA, Ajiboye BO, Oyinloye BE, Ojo AB (2014). Prophylactic effect of ethanolic extract of Irvingia gabonesis stem bark against cadmium-induced toxicity in rats. Advan. Pharmaceut. 8 p.
|
|
|
|
Okorondu SI, Braide W, Ogbulie TE, Akujobi CO (2006). Antimicrobial and phytochemical properties of some traditional species. Niger. J. Microbiol. 20(3):1301 – 1308.
|
|
|
|
Olajide OA, Awe SO, Makinde JM (1999). Pharmacological Studies on the Leaf of Psidium guajava. Fitoterapia, 70:25-31.
Crossref
|
|
|
|
Olukoya DK, Idika N, Odugbemi TO (1993). Antibaterial activitiy of some medicinal plants. Nigerian J. Res. Ethopharm. 39:69-72.
|
|
|
|
Omonkhua AA, Onoagbe, IO, Akinlosotu, OB, Ajayi TS, ADU K (2015). Hypoglycaemic and hypolipid effects of total saponins fraction extracted from Irvingia gabonesis (Aubry Lecomte Ex O'roprke) Baill. Stem Bark in rats. Niger. J. Bot. 28(1):109-118.
|
|
|
|
Otshudi AL, Foriers A, Vercruysse A, Van Zeebroeck A, Lauwers S (2000). In vitro antimicrobial activity of six medicinal plants traditionally used for treatment of dysentery and diarrhea in Democratic Republic of Congo. Phytomed. 7:167-177.
Crossref
|
|
|
|
Pavia AT, Nicholas CR, Green DP, Tauxe RV, Mottice S, Greene KD, Wells JG, Siegler RL, Brewer ED, Hannon D (1990). Haemolytic-uraemic syndrome during an outbreak of Escherichia coli O157:H7 infection in institutions for mentally retarded persons: clinical and epidemiological observations. J. Pediatr. 116:544-551.
Crossref
|
|
|
|
Proulx F, Turgeon JP, Delage G, Lafleur L, Chicoine L (1992). Randomized, control of trial of antibiotic therapy for Escherichia coli O157:H7 enteritis. J. Pediatrics. 121:299 -303.
Crossref
|
|
|
|
Rees LP, Minney SF, Plummer NT, Slater JH, Skyrme DA (1993). A quantitative assessment of the antimicrobial activity of garlic (Allium satium). World J. Microbiol. Biotechnol. 9:303-307.
Crossref
|
|
|
|
Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Herbert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML (1983). Haemorrhagic colitis associated with a rare Escherichia coli serotype. New Eng. J. Med. 308:681- 685.
Crossref
|
|
|
|
Rojas JJ, Veronica J, Ochoa Ocampo SA, Munoz JF (2006). Screening for antimicrobial activity of ten medicinal plants used in colombian folkloric medicine. A possible alternative in the treatment of non-nosocomial infections. Complem. Alter. Med. 6:1- 8.
Crossref
|
|
|
|
Ross ZM, O'Gara E. A, Hill DJ, Sleightholme HV, Maslin DJ (2001). Antimicrobial properties of garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with garlic oil sulphides and garlic powder. Appl. Environ. Microbiol. 67(1):475-480.
Crossref
|
|
|
|
Shafran I, Meurer N, Thomas FD (1996).The use of nutmeg in the treatment of refractory diarrhoea in patients with Crohn's diseases. New Eng. J. Med. 296:694.
|
|
|
|
Shidore PP, Mujumdar SM, Shrotri DS, Mujumdar AM (1985). Anti-diarrhoeal and anti-inflammatory activity of nutmeg extracts. Indian J. Pharm. Sci. 10:188-190.
|
|
|
|
Sofowora A (1993). Medicinal Plants and Traditional Medicine in Africa. John Wiley Inc. Manchester. pp. 256.
|
|
|
|
Swerdlow DL, Woodruff BA, Brady RC, Griffin PM, Tripen S, Donell HD, Geldreich D, Payne BJ, Meyer A, Wells JG, Greene KD, Bright M, Bean NH, Blake PA (1992). Waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhoea and death. Ann. Inter. Med. 117:812- 819.
Crossref
|
|
|
|
Tanaka T, Ishida N, Ishimatsu M, Nonaka G (1992). Tannins and related compounds. CXVI. Six new complex tannins, guajavins, psidinins and psiguavin from the bark of Psidium guajava L. Chem. Pharmacol. Bull. 40(8):2092- 2098.
Crossref
|
|
|
|
Taura DW, Oyeyi TI (2009). In vitro antibacterial activity of the combinations of Ethanolic extracts of Annova comosus, Allium sativum and Aloe barbadensis in comparison with ciprofloxacin. Biol. Environ. ScI. J. Trop. 6(1):146-151.
|
|
|
|
Tella A (1986). The effects of Azadirachta indica in acute Plasmodium berghei malaria. Nigeria Med. J. 7:258-263.
|
|
|
|
Thomas GB, David MD, Swerdlow MD, Patricia L, Griffin MD. (1995). Escherichia coli O157:H7 and haemolytic uraemic syndrome. New Eng. J. Med. 333(6):364-368.
Crossref
|
|
|
|
Todar K (2005). Antimicrobial Agents Used in Treating Infectious Diseases. University of Wisconsin Press. Madison. pp. 1- 8.
|
|
|
|
Tona L (1999). Biological screening of traditional preparation from some medicinal plants used as antidiarrhoeal in Kinshasa, Congo. Phytomedicine, 55(6):34-40.
Crossref
|
|
|
|
Tona L, Kambu K, Ngimbi N, Cimanga K, Vlietinck AJ (1998). Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharm. 61(1):57- 65.
Crossref
|
|
|
|
Trease GE, Evans WC (1989). Text book of Phamacognosy (15thed.). W. B. Samber publishing company Toronto, pp. 125.
|
|
|
|
Veeramuthu D, Muniappan A, Savarimuthu I (2006). Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. Complem. Alter. Med. 6(35):1- 7.
|
|
|
|
Wachsmuth IK, Evins GM, Fields PI (1997). The molecular epidemiology of cholera in Latin America. J. Infect. Dis. 167:621-626.
Crossref
|
|
|
|
Wong CS, Jelacic S, Rebecca L, Sanda L, Watkins P, Tarr I (2000). The risk of the haemolytic-uraemic syndrome after antibiotic treatment of Escherichia coli O157:H7 Infections. New Eng. J. Med. 342:1930-1936.
Crossref
|



