ABSTRACT
To clearly delimit the members of the Bacillus subtilis group (BSG), is difficult using common phenotypic and genotypic methods. This study described the use of pheS and tuf gene as targets for interspecies discrimination within the BSG, and also to develop specific PCR and SNP primers for species and subspecies identification and differentiation. The average sequence similarity values of the pheS and tuf gene among type strains were 85.1 and 94.7%, respectively, and all members of the BSG could be clearly distinguished based on phylogenetic analyses of pheS gene sequence. In addition, the specific primers were designed according to pheS and tuf gene sequence. The primers were shown to specifically identify B. subtilis subsp. subtilis, B. amyloliquefaciens and Bacillus licheniformis, and clearly differentiate the subspecies of B. amyloliquefaciens using specific-PCR, combined with two-plex minisequencing method. In conclusion, we have successfully established a comparative sequence analysis and rapid molecular diagnosis techniques for determination of interspecies within the BSG.
Key words: Bacillus subtilis group (BSG), species and subspecies discrimination, comparative sequence analysis, specific Pcr, two-plex minisequencing.
The Bacillus subtilis group (BSG) contains more than 10 closely related taxa (Dunlap et al., 2015), and have some beneficial effects of BSG members as reported, like the production of enzymes, antibiotics, vitamins and fermented foods, which are commonly applied as animal feeds additives (Sorokulova et al., 2013; Kubo et al., 2011). Many studies have demonstrated that BSG strains have beneficial effects on production performance in domestic animals (Alexopoulos et al., 2004; Kritas et al., 2006; Knap et al., 2010; Ahmed et al., 2014). However, the species and subspecies determination of these phylogenetically related bacteria has long been problematic.
DNA-DNA hybridization (DDH) is the gold standard for bacterial species delineation (Stackebrandt et al., 2002), but this method is time-consuming, labor intensive, costly and difficult to use routinely in laboratories. To date, a comparative analysis on the 16S rDNA is a commonly used genotypic method for bacterial identification, and the strains that show at least 98.7% sequence similarity between the 16S rDNA are recognized to the same species (Stackebrandt and Ebers, 2006).
Unfortunately, poor discrimination has been observed in BSG, due to the high degree of similarity (reaching 99-100%) of the 16S rRNA gene sequences (Wang et al., 2007). In contrast, DNA sequences of housekeeping genes with a higher resolution seem to be more effective than the 16S rDNA and may act as an alternative to DDH, for species determination (Guo et al., 2012). The tuf gene encodes the elongation factor Tu associated with protein biosynthesis, which facilitates the aminoacyl-tRNA to the ribosomes during the translation process. Moreover, tuf is universally distributed, and the various copy numbers (one to three) per bacterial genome have been found (Ke et al., 2000). The tuf gene is ideally suited for inferring phylogenetic relationships between bacteria (Chavagnat et al., 2004; Picard et al., 2004). Phenylalanyl-tRNA synthase gene (pheS) has also been proposed as a useful molecular marker in the closely related species complex (Naser et al., 2005; Naser et al., 2007). In this study, we determined the utility of pheS and tuf genes sequences for species and subspecies discrimination in BSG, and as targets to develop specific primers for identification and differentiation.
Bacillus strains and culture conditions
All BSG type strains and isolates were obtained from Bioresource Collection and Research Center (BCRC) and are listed in Table 1. Bacillus strains were incubated aerobically on Nutrient agar (NA, Difco) for 24 h at 30°C.
Genomic DNA preparation and design of degenerate primers
The chromosomal DNA was extracted using the DNeasy Kit (Qiagen, Valencia, CA, USA), and the DNA concentration and purity were measured using an absorbance ratio of 260/280 nm and checked by agarose gel electrophoresis. By comparison with the pheS and tuf genes from the whole genome sequence in BSG species (Accession no: CP002905, AL009126, CP002207, FN597644, CP000560, CP000002), the degenerate primers, pheS-21F: 5’-CAYCCNGCHCGYGAYATGC-3’ and BasbpheS-416R: 5’-ARYACRTTCGGRTGAACCAT-3’, and Basbtuf-F1: 5’-CAAACTCGTGAGCACATYCT-3’ and Basbtuf-R1: 5’-CGTCAGTTGTACGGAARTAG-3’, were designed and targeted to the most conserved region of the gene.
Target gene amplification and DNA sequencing
The partial fragments of pheS and tuf genes of BSG related strains were amplified and sequenced using consensus degenerate primers. The thermal protocol was carried out under the following conditions: 5 min at 94°C; 30 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C; and 7 min at 72°C. The resulting amplicons were purified using the QIAquick PCR purification Kit (Qiagen Inc., Valencia, CA, USA) and sequenced with the BigDye Terminator v3.1 cycle-sequencing Kit on the 3730 DNA sequencer (Applied Biosystems and Hitachi, Foster City, CA, USA).
Intespecific bioinformatic analysis
The pheS and tuf gene sequences of all strains were aligned using the Clustal X program (version 1.8). The DNA sequence similarities were calculated using the MatGAT (version 2.02) (Campanella et al., 2003). Phylogenetic tree was performed with the PHYLIP (version 3.63) package, using the neighbour-joining method (Felsenstein, 2004; Kimura, 1980; Saitou and Nei, 1987).
Species and subspecies-specific primers design and PCR identification
The PCR oligonucleotide primers is specific for B. subtilis subsp. subtilis, B. amyloliquefaciens and Bacillus licheniformis were designed based on the pheS and tuf gene sequences, and all reference strains were used for specific PCR testing. The thermal protocol was carried out under the following conditions: 5 min at 94°C; 25 cycles of 1 min at 94°C, 1 min at 65°C, and 1 min at 72°C; and 7 min at 72°C.
Subpecies-specific SNaPshot mini-sequencing assay
The SNP primers specific for B. amyloliquefaciens subspecies were designed based on the pheS gene sequences. The mini-sequencing protocol and final data analysis were followed as previously described (Huang et al., 2014).
In this study, partial pheS and tuf gene fragments (approximately 410 bp and 750 bp) were successfully amplified from all BSG strains and were used for sequencing, by using degenerate primers. The average sequence similarity values for pheS and tuf genes among BSG type strains were mean 85.1 and 94.7%, respectively, which exhibit greater variation than 16S rDNA sequence (98.9%). The topology of the pheS tree showed all BSG members that could be discriminated (Figure 1). Nowadays, several molecular targets have been exploited to differentiate BSG members. The three targets (gyrA, gyrB and phoR) showed good resolution with a high discrimination power, and the average sequence similarity values of these targets were 83.7, 83.3 and 77.9%, respectively (Chun and Bae, 2000; Guo et al., 2012; Wang et al., 2007). Therefore, the pheS gene can be as an additional phylogenetic marker for differentiating among the BSG.
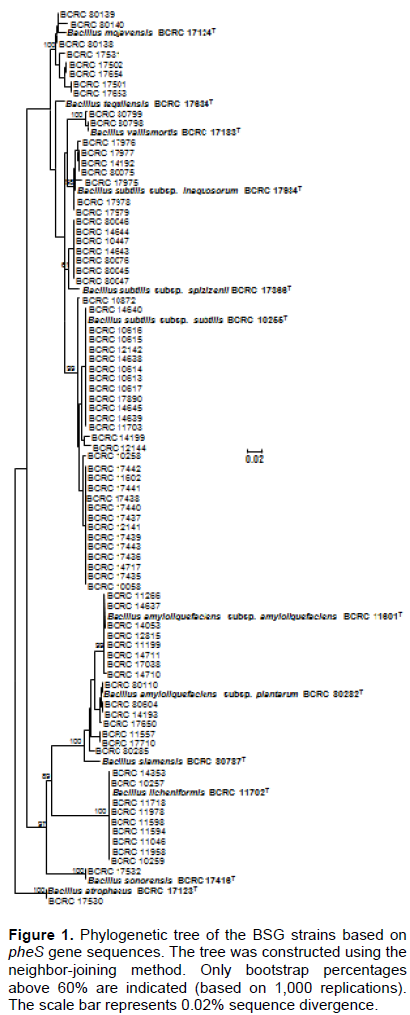
On the other hand, all DNA sequences were submitted to GenBank (accession number: KX987658-KX987837), and this accumulated sequence data could be applied to design specific primers for direct identification of particular microbials (Krawczyk et al., 2002). The species- specific primer has been established for B. subtilis based on Endo-beta 1,4-glucanase gene and ytcP (encoding a hypothetical protein similar to a ABC-type transporter) gene (Ashe et al., 2014; Kwon et al., 2009). To the best of our knowledge, there were no such studies, in the identification of B. subtilis at subspecies level. Four primer pairs were designed based on multiple alignments of the pheS and tuf sequences, and these primers successfully generated a single species and subspecies-specific band (202 bp, 145 bp, 112 bp and 194 bp) when used in PCR reactions with B. amyloliquefaciens, B. licheniformis and B. subtilis subsp. subtilis DNA (data not shown).
Moreover, the amplified fragments were sequenced, and the results demonstrated that the sequence agreed with what were expected. Annealing temperatures and additional PCR amplification cycles may influence PCR specificity (Krawczyk et al., 2002). In the present study, the most appropriate conditions for our primer pairs were an annealing temperature of 65°C and 25 cycles of PCR amplification. The specificity of these primer pairs were tested against the organisms indicated in Table 1.

Although B. amyloliquefaciens strains could be preliminary identified using species-specific PCR, but this method was not able to provide an accurate discrimination at the subspecies level. Afterward, a mini-sequencing assay was applied. The SNP specific primers spBamyphes-231f1 (5’-GCACGCTTGAATTGGTYGC-3’) and spBamyphes-306f1 (5’-GACTGACTGACTCRTTCACAGAGCCTTCTGTCGA-3’) were designed to anneal immediately, adjacent to the nucleotide at two subspecies-specific SNPs found at positions 231 and 306 in the alignment of all B. amyloliquefaciens pheS gene sequences (Table 2). Following the above, B. amyloliquefaciens species- specific amplicons containing two diagnosis sites were purified and subjected to a duplex mini-sequencing reaction. The results showed the presence of two peaks of the expected color and position in all samples (Figure 2; Table 1). Compared to other genotypic methods for strain differentiation of Bacillus spp. such as repetitive element palindromic PCR (rep-PCR), PCR restriction fragment length polymorphism (RFLP) and DNAsequencing (Banyko and Vyletelova, 2009; Freitas et al., 2008; Jeyaram et al., 2011; Shaver et al., 2002), the
mini-sequencing method is more direct and rapid due to its determined exact single nucleotide polymorphism at diagnosis sites.
All members of BSG can be clearly discriminated by housekeeping gene sequencing, and the developed specific primers can be successfully applied to quickly and accurately identify the B. subtilis subsp. subtilis, B. amyloliquefaciens subspecies and B. licheniformis using specific PCR combined with mini-sequencing assay.
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed ST, Islam M, Mun HS, Sim HJ, Kim YJ, Yang CJ (2014). Effect of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 93:1963-1971.
Crossref
|
|
|
|
Alexopoulos C, Georgoulakis IE, Tzivara A, Kyriakis CS, Govaris A, Kyriakis SC (2004). Field evaluation of the effect of a probiotic-containing Bacillus licheniformis and Bacillus subtilis spores on the health status, performance, and carcass quality of grower and finisher pigs. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 51:306-312.
Crossref
|
|
|
|
Ashe A, Maji UJ, Sen R, Mohanty S, Maiti NK (2014). Specific oligonucleotide primers for detection of endoglucanase positive Bacillus subtilis by PCR. 3 Biotech. 4:461-465.
|
|
|
|
Banyko J, Vyletelova M (2009). Determining the source of Bacillus cereus and Bacillus licheniformis isolated from raw milk, pasteurized milk and yoghurt. Lett. Appl. Microbiol. 48:318-323.
Crossref
|
|
|
|
Campanella JJ, Bitincka L, Smalley J (2003). MatGAT: an application that generate similarity/identity matrices using protein or DNA sequences. BMC Bioinform. 4:23.
Crossref
|
|
|
|
Chavagnat F, Haueter M, Jimeno J, Casey MG (2002). Comparison of partial tuf gene sequences for the identification of lactobacilli. FEMS Microbiol. Lett. 17:177-183.
Crossref
|
|
|
|
Chun J, Bae KS (2000). Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 78:123-127.
Crossref
|
|
|
|
Dunlap CA, Kim SJ, Kwon SW, Rooney AP (2015). Phylogenomic analysis shows that Bacillus amyloliquefaciens subsp. plantarum is a later heterotypic synonym of Bacillus methylotrophicus. Int. J. Syst. Evol. Microbiol. 65:2104-2109.
Crossref
|
|
|
|
Felsenstein J (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39:783-791.
Crossref
|
|
|
|
Felsenstein J (2004). PHYLIP (phylogenetic inference package), version 3.6. Distributed by the author. Department of Genome Sciences and Department of Biology, University of Washington, Seattle.
|
|
|
|
Freitas DB, Reis MP, Lima-Bittencourt CI, Costa PS, Assis PS, Chartone-Souza E, Nascimento AM (2008). Genotypic and phenotypic diversity of Bacillus spp. isolated from steel plant waste. BMC Res. Notes 1:92.
Crossref
|
|
|
|
Guo Q, Li S, Lu X, Li B, Stummer B, Dong W, Ma P (2012). phoR sequences as a phylogenetic marker to differentiate the species in the Bacillus subtilis group. Can. J. Microbiol. 58:1295-1305.
Crossref
|
|
|
|
Huang CH, Chang MT, Huang L, Chua WS (2014). Molecular discrimination and identification of Acetobacter genus based on the partial heat shock protein 60 gene (hsp60) sequences. J. Sci. Food Agric. 94:213-218.
Crossref
|
|
|
|
Jeyaram K, Romi W, Singh TA, Adewumi GA, Basanti K, Oguntoyinbo FA (2011). Distinct differentiation of closely related species of Bacillus subtilis group with industrial importance. J. Microbiol. Methods 87:161-164.
Crossref
|
|
|
|
Ke D, Boissinot M, Huletsky A, Picard FJ, Frenette J, Ouellette M, Roy PH, Bergeron MG (2000). Evidence for horizontal gene transfer in evolution of elongation factor Tu in enterococci. J. Bacteriol. 182:6913-6920.
Crossref
|
|
|
|
Kimura M (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120.
Crossref
|
|
|
|
Knap I, Lund B, Kehlet AB, Hofacre C, Mathis G (2010). Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 54:931-935.
Crossref
|
|
|
|
Kubo Y, Rooney AP, Tsukakoshi Y, Nakagawa R, Hasegawa H, Kimura K (2011). Phylogenetic analysis of Bacillus subtilis strains applicable to natto (fermented soybean) production. Appl. Environ. Microbiol. 77:6463-6469.
Crossref
|
|
|
|
Krawczyk B, Lewandowski K, Kur J (2002). Comparative studies of the Acinetobacter genus and the species identification method based on the recA sequence. Mol. Cell Probes 16:1-11.
Crossref
|
|
|
|
Kritas SK, Govaris A, Christodoulopoulos G, Burriel AR (2006). Effect of Bacillus licheniformis and Bacillus subtilis supplementation of ewe's feed on sheep milk production and young lamb mortality. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 53:170-173.
Crossref
|
|
|
|
Kwon GH, Lee HA, Park JY, Kim JS, Lim J, Park CS, Kwon DY, Kim YS, Kim JH (2009). Development of a RAPD-PCR method for identification of Bacillus species isolated from Cheonggukjang. Int. J. Food Microbiol. 129:282-287.
Crossref
|
|
|
|
Picard FJ, Ke D, Boudreau DK, Boissinot M, Huletsky A, Richard D, Ouellette M, Roy PH, Bergeron MG (2004). Use of tuf sequences for genus-Specific PCR detection and phylogenetic analysis of 28 Streptococcal species. J. Clin. Microbiol. 42:3686-3695.
Crossref
|
|
|
|
Naser SM, Thompson FL, Hoste B, Gevers D, Dawyndt P, Vancanneyt M, Swings J (2005). Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology, 51:2141-2150.
Crossref
|
|
|
|
Naser SM, Dawyndt P, Hoste B, Gevers D, Vandemeulebroecke K, Cleenwerck I, Vancanneyt M, Swings J (2007). Identification of lactobacilli by pheS and rpoA gene sequence analysis. Int. J. Syst. Evol. Microbiol. 57:2777-2789.
Crossref
|
|
|
|
Saitou N, Nei M (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425.
|
|
|
|
Shaver YJ, Nagpal ML, Rudner R, Nakamura LK, Fox KF, Fox A (2002.) Restriction fragment length polymorphism of rRNA operons for discrimination and intergenic spacer sequences for cataloging of Bacillus subtilis sub-groups. J. Microbiol. Methods 50:215-223.
Crossref
|
|
|
|
Sorokulova I (2013). Modern Status and Perspectives of Bacillus Bacteria as Probiotics. J. Prob. Health 1:e106.
Crossref
|
|
|
|
Stackebrandt E, Frederiksen W, Garrity GM, Grimont PA, Kampfer P, Maiden MC, Nesme X, Rossello-Mora R, Swings J, Truper HG, Vauterin L, Ward AC, Whitman WB (2002). Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047.
Crossref
|
|
|
|
Stackebrandt E, Ebers J (2006). Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 33:152-155.
|
|
|
|
Wang LT, Lee FL, Tai CJ, Kasai H (2007). Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA-DNA hybridization in the Bacillus subtilis group. Int. J. Syst. Microbiol. 57:1846-1850.
Crossref
|