ABSTRACT
The effects of long-term fertilizer management on soil enzyme activities and soil microbe population under double-cropping paddy fields in southern China was studied. The purpose of this study was to explore the changes of soil enzyme activities and soil microbe population as related to mineral fertilizer and manure and rice residue based on a long-term field experiment. The experiment was initiated in 1986 and consisted of five treatments: without fertilizer (CK), mineral fertilizer (MF), rice residue plus mineral fertilizer (RF), low manure rate plus mineral fertilizer (LOM), and high manure rate plus mineral fertilizer (HOM). The cropping system consisted of barley (Hordeum vulgare L.), early rice (Oryza sativa L.) and late rice. In 2013-2014, soil samples were collected from the 0-20 cm layers to determine soil enzyme activities and soil microbe abundance during barley growth phases. The results indicated that during the barley growing season, the enzyme activities were higher in the HOM, LOM and RF than in the CK. The treatments of HOM, LOM and RF also improved the numbers of aerobic bacteria, actinomycetes and fungi. During barley growth phases, combined application of manure, crop residue and chemical fertilizer improved soil enzyme activities and soil microbe population.
Key words: Alkaline phosphatase, arylamidase, β-glucosidase, Hordeum vulgare L., manure, microbial abundance, mineral fertilizer, rice residue.
Soil microbes play an important role in the ecosystem functioning and are important in maintaining the soil fertility, nutrient cycling and organic matter decomposition (Pastor et al., 1984), which are dependent on the composition of soil microbial communities (Robertson et al., 2000; Singh et al., 2010), and relative to microbial diversity, community structure.Soil enzymes play important role in energy transfer and they are very important for soil quality and crop growth, which are used as indices for soil microbial activity and fertility (Benitez et al., 2000; Dick, 1994; Tabatabai, 1994). Soil enzymes respond to soil practice changes more quickly than other soil factors, therefore it was used as early indicators of biological changes.The soil enzyme reflects soil functional diversity, which was affected by environmental conditions and ecological factors, such as soil microorganisms, plants and animals (Nannipieri et al., 2004). Soil enzymes include arylamidase, alkaline phosphatase, β-glucosidase and arylsulfatase, which were important for transformation of C, N, P and S (Tabatabai, 1994).
Recently, some studies have shown that the soil enzymes and microbial activities were affected by field management, such as soil tillage and crop residue management (Ekenler and Tabatabai, 2003; Wu et al., 2004), application of fertilizer and organic matter (Carmine et al., 2004; Tejada et al., 2006; García-Gil et al., 2000), crop rotations (Hamido and Kpomblekou, 2009; Bandick and Dick, 1999), pH, ionic strength and natural organic matter (Kyriakopoulos et al., 2006). However, the effects of long-term fertilizer management on soil enzyme activities and soil microbial abundance under double-cropping paddy fields in southern China should be studied.
In recently years, the traditional fertilization practices have been changing in China’s major rice production regions. However, the studies on the effects of long-term fertilizer management on soil enzyme activities and soil microbe population under double-cropping paddy fields in southern China were less. Therefore, the purpose of this study was to explore the changes of soil enzyme activities and soil microbe abundance in a double-cropping rice system as related to the application of manure, crop residue plus mineral fertilizer, and mineral fertilizer based on a long-term field experiment.
Sites and cropping system
The experiment was started in October 20, 1986, at Ning Xiang County (28°07' N, 112°18' E, and altitude 36 m) of Hunan Province,
China. Under a continent monsoon climate, the annual mean precipitation is 1553 mm and potential evapotranspiration is 1354 mm. The monthly mean temperature is 17.2°C. Soil texture of the plough layer (0–20 cm) is silt clay loam with 13.71 sand and 57.73% silt. At the beginning of the study, the characteristics of the surface soil (0–20 cm) are as follows: soil organic carbon (SOC) 29.4 g kg-1, total nitrogen 2.0 g N kg-1, available N 144.1 mg kg-1, total phosphorous 0.59 g P kg-1, available P 12.87 mg kg-1, total potassium 20.6 g K kg-1, and available potassium 33.0 mg kg-1. There are three crops in a year, barley (Hordeum vulgare L.), early rice (Oryza sativa L.), and late rice. Barley is sown in the middle of November and is harvested in early May of the following year. Early rice is then transplanted, and harvested in the middle of July. The growing season of late rice lasts from late July to the end of October.
Experimental design
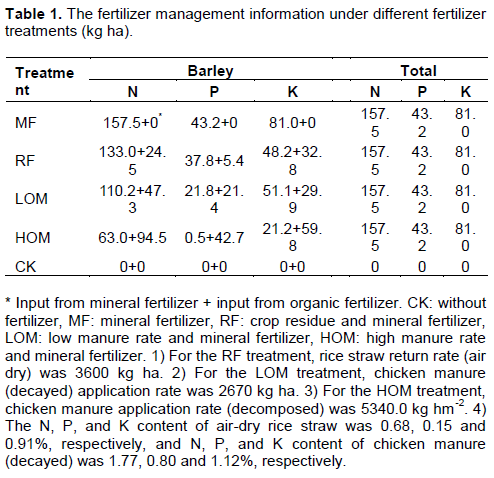
There were five treatments: control (without fertilizer, CK), mineral fertilizer (MF), rice residue and mineral fertilizer (RF), low manure rate and mineral fertilizer (LOM), and high manure rate and mineral fertilizer (HOM). The experiment ensured all fertilized treatments received equal N rate (the amount of N in mineral fertilizer plus that from rice residue or manure). The fertilizer management information are listed in Table 1. Barley is sown in November 15, 2013, and is harvested in May 7, 2014. Early rice is transplanted in May 9, and harvested in July 24. Late rice was transplanted in July 26, and harvested in October 17. Before barley sowing, manure and air–dried rice residue were incorporated into soil surface. Before barley sowing and rice transplanting, the soil were taken tillage, and the cultivation depth was about 20 cm. For barley, 70% of mineral N fertilizer was applied at seeding, and the 30% of N fertilizer was applied at top dressing. The phosphorus and K fertilizers were applied at seeding. The seeding rate application was 250.0 kg ha with each treatment. There were three replications and each plot size was 66.7 m2 (6.67 × 10 m). We referred to the data for the individual cropping periods as 2013–2014, after 27 consecutive years of position experiment.
Soil sampling and measurements
Data were collected from October 2013 to May 2014. In each plot, soil samples in the ploughed layer (0–20 cm) were collected from the centre of four hills of barley plants by using a drill at different barley growth stages, such as the seedling stage, tillering stage, jointing stage, heading stage and maturity stage. Three subsamples were collected from each plot.
The soil samples were passed through a 2-mm sieve and kept moist in a refrigerator at 4°C until analysis. Arylamidase (EC 3.4.11.2) activity was assayed by incubating 1.0 g moist soil with 3.0 mL of 0.1 M THAM buffer (pH 8.0) and 1.0 mL of an 8.0 mM solution of l–leucine β–naphthylamide hydrochloride (Acosta–Martínez and Tabatabai, 2000). Alkaline phosphatase (EC 3.1.3.1), β–glucosidase (EC 3.2.1.21) and arylsulfatase (EC 3.1.6.1) activities were determined as described by Tabatabai (1994), and the activity was reported as μg p–nitrophenol g-1 h-1. All measurements in the laboratory were repeated for three times. Meanwhile, colony forming units (CFUs) of soil aerobic bacteria, actinomycetes and fungi were enumerated by Wu et al. (2004). Colony forming units (CFUs) of soil aerobic bacteria were enumerated by a 10-fold dilution plate technique. And the number of aerobic bacteria was identified by spreading 100 μl of diluted sample on LB agar medium. Three replicates of the inoculated agar plates were incubated at 28°C for 3 days for bacteria, after which colonies were counted.
Statistical analysis
All data were expressed as mean ± standard error. The data of different treatments at the same growth stage were analyzed as a randomized complete block, using the ANOVA procedure of SAS (SAS Institute, 2003). Mean values were compared using the least significant difference (LSD) test, and a probability value of 0.05 was considered to indicate statistical significance.
Dynamics of alkaline phosphatase activity during barley growth stages
Under different fertilization treatments during the barley growth, alkaline phosphatase activity in the MF, RF, LOM and HOM soils was higher than that in the control plot (Figure 1). In the barley growing season, the order of alkaline phosphatase activity was: HOM>LOM>RF> MF>CK, and there were significant differences (P <0.05) between HOM, LOM, RF, MF and CK. In the barley growing season, the alkaline phosphatase activity under different N treatments was in the range of 93.07–221.79, 110.41–240.13, 115.60–246.85, 121.53–256.77 and 76.92–203.64 μg p-nitrophenol g-1 soil h-1, respectively. The highest activity was detected at the tillering stage (TS) (Figure 1).
Dynamics of β-glucosidase activity during barley growth stages
During barley growing stages, the activity of soil β-glucosidase was significantly affected by manure, rice residue and mineral fertilizer additions. The highest β-glucosidase activity was observed with HOM and the lowest activity with CK (Figure 2), and the order of β-glucosidase activities was as follows: HOM>LOM> RF>MF>CK. In the barley growing season, there was a significant difference (P < 0.05) in β-glucosidase activity under the HOM and MF treatment at the seedling stage (SS), TS, jointing stage (JS), heading stage (HS) and maturity stage (MS).
Dynamics of arylsulfatase activity during barley growth stages
During barley main growing stages, soil arylsulfatase activities with different treatments was in the range of 26.45–32.56, 29.72–34.30, 30.05–35.68, 31.52–36.97 and 24.10–31.45 μg p–nitrophenol g-1 soil h-1, respectively (Figure 3). There was no significant difference (P > 0.05) in arylsulfatase activities between the MF and CK, but the arylsulfatase activities in HOM and LOM was significantly (P < 0.05) higher than that in MF, CK at the main growth stages of barley.
Dynamics of arylamidase activity during barley growth stages
The application of the manure, rice straw and mineral fertilizers significantly affected soil arylamidase activity (Figure 4). At 0–20 cm soil depth, there was no significant difference (P > 0.05) in soil arylamidase activity under the HOM, LOM and RF, but these activities were significantly (P < 0.05) higher than that in CK in all growth stages of barley. In the barley growing season, arylamidase activity changed in the range of 22.75–35.54, 24.97–37.67, 25.62–38.95, 26.47–39.88 and 20.42–32.01 μg p-nitrophenol g-1 soil h-1, respectively.
Enumeration of aerobic bacteria, fungi and actinomycetes in soil
During barley growth stage, there were significant differences (P < 0.05) in the numbers of aerobic bacteria, fungi and actinomycetes between HOM, LOM, RF, MF and CK. The order of number of aerobic bacteria was RF>MF>HOM=LOM>CK at the barley main growing stages (Table 2). Also, the numbers of soil fungi and actinomycetes with manure, rice straw and mineral fertilizer were significantly higher (P < 0.05) than the control at different barley stages. And the number of soil actinomycetes and fungi decreased in the order HOM>LOM>RF>MF>CK in the barley growing season.
Soil enzyme activities under different fertilizer N management during barley growth
Soil enzymes responded to management practices more quickly than other soil factors, therefore, it was used as early indicator of biological changes. Wu et al. (2014) showed that the activity of soil enzymes was positively correlated with concentrations of soil available phosphorus and available nitrogen in continuous cropping soil. At the barley maingrowthstages, there were significant differences (P > 0.05) in alkaline phosphatase activity between HOM, LOM, RF, MF and CK treatments. The results showed that alkaline phosphatase activity were affected by application of manure and residue management practices. Temporal variations in activity, induced by manure and crop residue decomposition, may promote the enzymes by the microbial biomass. Increase of alkaline phosphatase activity in HOM, LOM, RF and MF treatments suggested that there was a stimulus of P-supplying mechanisms to the microbial community with fertilizer or manure, crop-derived inputs (Ritz et al., 1997). And there were significant differences (P > 0.05) in alkaline phosphatase activity under the HOM and MF treatments at the main growth stages of barley. And the difference in the enzyme activity was in response to mineral fertilizer, manure, and straw incorporation or crop rotation practices used in this study.
β-Glucosidase is the enzyme for degradation of organic compounds in soil which plays a crucial role in the C cycle in soils. β-Glucosidase releases important energy sources for microorganisms. Bandick and Dick (1999) and Ekenler and Tabatabai (2003) showed that the activity of β-glucosidase was affected by different residue management practices. These studies showed that there are significant difference in β-glucosidase activity among the mineral fertilizer, rice residue and mineral fertilizer, manure and mineral fertilizer and without fertilizer treatments at the barley main growth stages. There are close relationship between glucosidases and β-glucosidase activity, which glucosidases hydrolyzed to glucose. The increase of β-glucosidase activity and glucosidases with HOM, LOM and RF treatments was possibly related to the increased mineralization of organic matter added with the manure and rice residue, which may provide the higher C substrates for β-glucosidase and glucosidases activity.
Arylsulfatase is an extracellular enzyme which plays an important role in sulfur (S) recycle for soil and plant (Tejada et al., 2006). This study showed that the soil activity of arylsulfatase with HOM, LOM, RF treatments were higher than that of the MF, CK treatments at the barley main growth stages. These observations indicated that manure, rice residue and mineral fertilizer made higher contribution to the SOM than without fertilizer, which may explain the higher immobilization in rhizosphere soil containing manure, rice residue and mineral fertilizer and fertilizer than in rhizosphere soil containing without fertilizer. In the present study, differences in soil arylsulfatase activity were related to the quantity of substrate contained in the organic and rice straw incorporation.
Soil arylamidase plays an important role in N mineralization in soils (Acosta-Martínez and Tabatabai, 2000; Castellano and Dick, 1988). Previous studies demonstrated that the arylamidase activity was affected by residue management (Deng and Tabatabai, 1997). In this study, statistical analyses suggested that arylamidase activity was affected by different fertilizer management (Figure 4). Dick et al. (1988) reported that the arylamidase activity decreased with long-term addition of inorganic N, whereas the activity increased with crop residues additions in wheat-fallow system. The low activity of arylamidase at the main growth stages of barley might be related to the fertilizer management. The reason maybe the difference in decomposable organic material in the manure, crops straw-returned soil which favored soil enzyme activity (Luo et al., 2011; Sharma and Arora, 2011).
Soil microbial abundance in response to fertilizer N management
Enami et al. (2001) suggested that soil microbial community structure was affected by rice straw. Kyriakopoulos et al. (2006) also showed that soil microbial community structure was affected by pH, ionic strength and natural organic matter. It was discovered that the numbers of soil aerobic bacteria, actinomycetes and fungi increased by application of manure and rice residue. In the present study, as compared to the without manure soil, the numbers of soil aerobic anaerobic microorganisms were increased with manure and rice straws treatments (Table 2). This might be due the difference of decay rates in the manure and crops straws-returned soil which favored aerobic bacteria. As a result, the numbers of aerobic bacteria with HOM, LOM, RF treatments were increased at barley main growth stages. Some studies indicated that soil fungi are important for the formation and stabilization of soil aggregates; however, soil fungi are also sensitive to environmental change (David et al., 2012; Li et al., 2012). This research indicated that the number of soil fungi decreased following HOM>LOM>RF>MF>CK at barley main growth stages. The reason may be that the changes of soil environmental on fungal diversity could influence ecosystem function via decomposition of manure and crops straws, so the soil fungi populations were increased by application with manure and rice straws at barley main growth stages. Present results also indicate an increase of actinomycetes number with manure and rice residue application at barley main growth stages. And the differences in the different treatments may have resulted in the differences in the numbers of aerobic bacteria, actinomycetes and fungi between the applied with manure, rice straws and without organic input treatments. And the further studies could be helpful to better understand these changes in the number of soil microbial impact on the soil microbial functions and nutrient availability.
Soil microbial abundance and enzyme activities are the main driving factors for organic matter decomposition and play an important role in soil nutrient transformation. This study indicated that soil enzyme activities and soil microbial abundance were affected by the application of manure, crop residue and mineral fertilizer practices during barley growth. Combined application of manure, crop residue and mineral fertilizer stimulated soil β-glucosidase, alkaline phosphatase, arylsulfatase and arylamidase activities. The numbers of soil microbes also increased when mineral and organic fertilizers were added. The application of mineral fertilizer was beneficial for microbial abundance, but the enhancement was lower than that of HOM, LOM and RF treatments. Therefore, these findings indicated that soil enzyme activity and soil microbe population were improved by using HOM, LOM and RF treatments. As a result, it is the best way to maintain soil fertility by using HOM, LOM and RF fertilization models.
There is no conflict of interest.
REFERENCES
|
Acosta–Martínez V, Tabatabai MA (2000). Arylamidase activity of soils. Soil Sci. Soc. Am. J. 64:215-221.
Crossref
|
|
|
|
Bandick AK, Dick RP (1999). Field management effects on soil enzyme activities. Soil Biol. Biochem. 31:1471-1479.
Crossref
|
|
|
|
|
Benitez ER, Melgar H, Sainz M, Nogales R (2000). Enzyme activities in the rhizosphere of pepper (Capsicum annuum L.) grown with olive cake mulches. Soil Biol. Biochem. 32:1829-1835.
Crossref
|
|
|
|
|
Carmine CC, Magda DRP, Maria R, Pacifico R (2004). Effects of municipal solid waste compost amendments on soil enzyme activities and bacterial genetic diversity. Soil Biol. Biochem. 36:1595-1605.
Crossref
|
|
|
|
|
Castellano SD, Dick RP (1988). Distribution of sulfur fractions in soil as influenced by management of organic residues. Soil Sci. Soc. Am. J. 52:1403-1407.
Crossref
|
|
|
|
|
David JB, Kurt AS, Juan CL, Jared LD (2012). Soil fungi influence the distribution of microbial functional groups that mediate forest greenhouse gas emissions. Soil Biol. Biochem.53: 112–119.
Crossref
|
|
|
|
|
Deng SP, Tabatabai MA (1997). Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biol. Fert. Soil 24: 141–146.
Crossref
|
|
|
|
|
Dick RP (1994). Soil Enzyme Activities as Indicators of Soil Quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (Eds.). Defining soil quality for a sustainable environment. American Society of Agronomy, Madison, WI, Pp.107-124.
|
|
|
|
|
Dick RP, Rasmussen PE, Kerle EA (1988). Influence of long-term residue management on soil enzyme activity in relation to soil chemical properties of a wheat-fallow system. Biol. Fert. Soil 6:159-164.
Crossref
|
|
|
|
|
Ekenler M, Tabatabai MA (2003). Tillage and residue management effects on β–glucosamidase activity in soils. Soil Biol. Biochem.35:871-874.
Crossref
|
|
|
|
|
Enami Y, Okano S, Yada H, Nakamura Y (2001). Influence of earthworm activity and rice straw application on the soil microbial community structure analyzed by PLFA pattern. Eur. J. Soil Biol. 37:269-272.
Crossref
|
|
|
|
|
García-Gil JC, Plaza C, Soler-Rovira P, Polo A (2000). Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 32:1907-1913.
Crossref
|
|
|
|
|
Hamido SA, Kpomblekou AK (2009). Cover crop and tillage effects on soil enzyme activities following tomato. Soil Till. Res. 105:269-274.
Crossref
|
|
|
|
|
Kyriakopoulos G, Doulia D, Hourdakis A (2006). Effect of ionic strength and pH on the adsorption of selected herbicides on amberlite. Int. J. Environ. Anal. Chem. 86(3&4):207-214.
Crossref
|
|
|
|
|
Li YJ, Chen X, Shamsi IH, Fang P, Lin XY (2012). Effects of irrigation patterns and nitrogen fertilization on rice yield and microbial community structure in paddy soil. Pedosphere 22(5):661-672.
Crossref
|
|
|
|
|
Luo YJ, Wang ZF, Gao M, Wei CF (2011). Effects of conservation tillage on organic carbon, nitrogen and enzyme activities in a hydragric anthrosol of Chongqing, China. Energy Procedia 5:30-36.
Crossref
|
|
|
|
|
Nannipieri P, Kandeler E, Ruggiero P (2004). Enzyme Activities and Microbiological and Biochemical Processes in Soil. In: Burns RG, Dick RP (Eds.). Enzymes in the environment. Activity, Ecology and Applications. Dekker, New York, pp.1-33.
|
|
|
|
|
Pastor J, Aber JD, McClaugherty CA, Melillo JM (1984). Above–ground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecol. 65:256-268.
Crossref
|
|
|
|
|
Ritz K, Wheatley RE, Griffiths BS (1997). Effects of animal manure application and crop plants upon size and activity of soil microbial biomass under organically grown spring barley. Biol. Fert. Soil 24: 372-377.
Crossref
|
|
|
|
|
Robertson GP, Paul EA, Harwood RR (2000). Greenhouse gases in intensive agriculture: contributions of individual gases to the radiative forcing of the atmosphere. Science 289:1922-1925.
Crossref
|
|
|
|
|
SAS Institute (2003). SAS Version 9.1.2 2002–2003. SAS Institute Inc., Cary, NC.
|
|
|
|
|
Sharma RK, Arora DS (2011). Solid state degradation of paddy straw by Phlebia floridensis in the presence of different supplements for improving its nutritive status. Int. Biodeter. Biodegr. 65:990-996.
Crossref
|
|
|
|
|
Singh BK, Bardgett RD, Smith P, Reay DS (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 8:779-790.
Crossref
|
|
|
|
|
Tabatabai MA (1994). Soil Enzymes. In: Page AL, Miller RH, Keeney DR (Eds.), Methods of soil analysis. American Society of Agronomy, Madison, pp. 775-833.
|
|
|
|
|
Tejada M, Garcia C, Gonzalez JL, Hernandez MT (2006). Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 38:1413-1421.
Crossref
|
|
|
|
|
Wu JX, Dong YH, Li JG, Jiang JF (2014). Influences of different fertilizing strategies of continuous cropping on lettuce yield, soil nutrients and enzymatic activities in greenhouse cultivation. J. Food Agric. Environ. 12(2):661-665.
|
|
|
|
|
Wu WX, Ye QF, Min H, Duan XJ, Jin WM (2004). Bt–transgenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol. Biochem. 36:289-295.
Crossref
|
|