ABSTRACT
The aim of the present study was to evaluate indigenous PGPR (Plant growth promoting rhizobacteria) previously isolated from Argentina's soybean fields for their in vitro antagonistic effects on the control of Fusarium tucumaniae and F. virguliforme, in two separated in vitro assays. In assay 1, the bacteria that showed the highest significant (P < 0.05) F. tucumaniae mycelial growth inhibition were strains Bacillus subtilis 54 (70%), B. cereus 13 (44%), B. cereus 7 (44%) and Chryseobacterium vietnamense 110 (42%). Despite their antagonistic activity, the strains identified as Stenotrophomonas malthophilia and B. cereus were not included in any further experiments, because of their potential hazard. In assay 2, strains 54, 110 and Pseudomonas fluorescens 9 and 115 were tested against F. tucumaniae and F. virguliforme. In this study, native bacterial strains isolated from Argentine Pampas were tested for the first time against these pathogens. All four bacterial strains significantly inhibited mycelial growth of F. virguliforme. Further studies on the effects of these strains on the growth of soybean plants and on the Sudden Death Syndrome (SDS) control will uncover the mechanisms and in vitro antagonism potential of these bacterial isolates.
Key words: Antagonism, plant growth promoting rhizobacteria (PGPR), Fusarium, Pseudomonas, Bacillus, Chryseobacterium vietnamense.
Soybean is the main crop in Argentina. In the last seasons, the area planted to soybean in Argentina was about 20 million hectares per year (Carmona et al., 2015). Sudden Death Syndrome (SDS) is a soybean disease caused by at least four Fusarium species, but in Argentina F. tucumaniae and F. virguliforme are the predominant. These are soil-borne pathogens commonly found in Argentina's pampas region and are important causes of crop losses (Scandiani et al., 2010). The fungus infects soybean roots and, under appropriate conditions, toxin-dependent symptoms develop in the aerial tissues after flowering and during pod fill, leading to rapid necrosis (Hartman et al., 2015). Under monoculture and no-till conditions in the argentine Pampas region the presence of SDS has intensified (Scandiani et al., 2010). Because of the difficulty in obtaining resistant soybeans varieties, the impossibility of fungicides to move towards the roots basipetally, wide host range of the pathogen and its ability to survive in the soil with resistance structures (chlamydospores), common management strategies such as genetic resistance, seed treatment with fungicides and crop rotation do not provide adequate control of SDS (Scandiani et al., 2010). In this context, biological control appears as an alternative and interesting tool.
Plant growth promoting rhizobacteria (PGPR) have been widely reported and recognized to have the potential for PGP and for their ability to antagonize the growth of fungal pathogens in crops such as maize, rice, potato, wheat and canola (Siddiqui et al., 2006). In soybean, PGPR were successfully tested against Macrophomina phaseolina (Simonetti et al., 2015) and Pythium ultimum (León et al., 2009).
Biocontrol PGPR are able to antagonize phyto-pathogenic fungi by different mechanisms (Siddiqui et al., 2006), including antibiosis, competition, mycoparasitism, degrading enzymes or induced resistance (Ahmad et al., 2008). PGPR could produce antibiotics or secrete lytic enzymes such as glucanases, proteases, cellulases and chitinases that degrade disease-causing fungi cells (Someya et al., 2007). Antibiotics produced by PGPR include volatile antibiotics (hydrogen cyanide, aldehydes, alcohols, ketones, and sulfides) and nonvolatile antibiotics such as polyketides (diacetyl phloroglucinol; 2,4 diacetylphloroglucinol and mupirocin), heterocyclic nitrogenous compounds (phenazine derivatives) and phenylpyrrole antibiotic (pyrrolnitrin) (Dilantha Fernando et al., 2005). PGPR could also antagonize by competition, for example by siderophore production. In addition, siderophores produced by PGPR could contribute to enhanced plant growth.
There are few reports on the antagonistic effect of bacterial isolates on soybean Fusarium species causing SDS (Xing and Westphal, 2007; Agaras et al., 2012). It is reported that the chance of finding bacterial strains effective for biocontrol increases if the isolates are obtained from pathogen suppressive soils and from the same environment in which they will be used (Cook and Baker, 1983). The aim of the present preliminary study was to evaluate indigenous PGPR previously isolated from soy fields in Argentina's pampas region for their in vitro antagonistic effects on the control of F. tucumaniae and F. virguliforme.
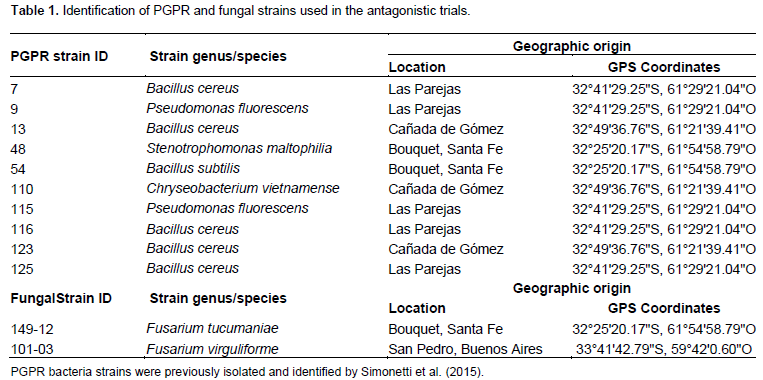
Ten PGPR strains that were previously isolated and identified by Simonetti et al. (2015) for their in vitro antagonistic capacity against M. phaseolina, were tested for their in vitro inhibitory capacity against the fungal pathogen F. tucumaniae (Table 1).
The fungal strains used in this study were originally isolated from infected soybean plants showing SDS root rot and provided by Centro de Referencia de Micología (CEREMIC), Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario (Table 1).
Assay 1
All bacterial strains were tested for their ability to inhibit the mycelial growth of F. tucumaniae149-12. Each bacterial isolate was streaked as a band on the edge of a PDA 90-mm diameter plate and incubated for 24 h at 28 ± 2°C. Then, a 6 mm diameter mycelial disc of F. tucumaniae 149-12 was taken from the margin of a growing colony and placed onto the centre of previously inoculated potato-dextrose-agar (PDA) plates. The Petri dishes were sealed by parafilm and incubated at room temperature in the dark. Plates containing only the fungal mycelial plug were maintained as control.
Assay 2
The bacterial strains that did not show genetic relationship with potentially hazardous bacteria in the assay 1 were tested for their ability to inhibit the growth of F. virguliforme 101-03 and F. tucumaniae 149-12 using in vitro dual-culture assay (Simonetti et al., 2012). Each bacterial isolate was prepared in nutrient broth (NB) and incubated for 48 h at 28 ± 2°C in order to use them in stationary phase. Fungi were maintained on PDA at 24 ± 2°C for one week. A 6 mm diameter mycelial plug was taken from the margin of a growing colony and placed centrally in a Petri dish containing PDA medium. Two drops (2 µL) of each bacterial culture previously prepared were placed in a straight line 3 cm away from the center of the plate and drops of sterile water served as control.
All these experiments were performed in triplicate. After incubation period of 11 days at 24 ± 2°C, mycelium growth inhibition was calculated as I=[(C-T)/C]×100, where C is the mycelium diameter in control, and T is the mycelium diameter in bacteria-inoculated plates.
Data were analyzed using analysis of variance and differences between means were tested using Tukey test with an overall risk level of 5%.
In assay 1, the bacteria that showed the highest significant (P < 0.05) F. tucumaniae 149-12 mycelial growth inhibition were strains 54 (70%), 7 (44%), 13 (44%), 110 (42%), 125 (34%), 123 (32%), 116 (31%) and 48 (30%) (Figure 1A and B). Despite their antagonistic activity, the strains identified as S. malthophilia (48) and B. cereus (7, 13, 116, 123 and 125) were not included in any further experiments because of their genetic relationship with potentially hazardous bacteria (Bottone, 2010; Brooke, 2012). For this reason only strains 9, 54, 110 and 115 were used in assay 2.
In assay 2 (Table 2), all four bacterial strains significantly inhibited mycelial growth of F. virguliforme 101-03. Strain 110 (C. vietnamense) exhibited the highest inhibition on the mycelial growth of F. virguliforme 101-03 (31.78%) (Figure 1D). On the other hand, the only strain that significantly inhibited mycelial growth of F. tucumaniae 149-12 was strain 54 (B. subtilis) (44%) (Figure 1C) and strains 9 and 115 (P. fluorescens) showed no significant effect, in accordance with assay 1. However, these strains (9 and 115) significantly inhibited mycelial growth of F. virguliforme 101-03 (22.48 and 19.4%, respectively).
Altogether, these results suggest that strain 54 (B. subtilis) displays antifungal features mainly towards F. tucumaniae 149-12, one of the causing agents of soybean SDS. The original contribution of this study is the isolation and testing of bacteria originating from the Pampas region.
These findings are in accordance with those of Xing and Westphal (2007), who found antagonism of B. subtilis against 12 isolates of F. virguliforme. On the other hand, our results differ with those found by Agaras et al. (2012) where Pseudomonas strain SMMP3 antagonized the growth of several pathogenic fungi, including the F. tucumaniae isolate CCC 132-11.
Because good results obtained in vitro cannot always be dependably reproduced under field conditions, these in vitro results should be confirmed by in planta experiments. In this way, Agaras et al. (2012) carried out both greenhouse and field trials using soybean seeds inoculated with Pseudomonas strain SMMP3. This inoculation treatment caused a reduction of SDS ratings (incidence, severity) and a decrease in the AUDPC (area under disease progress curves) values. Nevertheless, these effects were not statistically meaningful (P>0.05). This may be due to the many factors that affect the effectiveness of the bacteria in natural conditions (Badri et al., 2009). Isolated bacterial strains should be rhizospheric competent, able to survive and colonize in the rhizospheric soil (de Souza et al., 2015).
Further studies on the effects of this strain on the growth of soybean plants and on the SDS control will uncover the mechanisms and potential of this bacterial isolate. However, it has been previously described that most cases of naturally occurring biological control result from mixtures of antagonists, rather than from high populations of a single antagonist (Myresiotis et al., 2012). Moreover, root-infecting Fusarium species attack soybean seedlings in the first developmental stages, thus additional tests as for example seed treatment with multiple strain inoculation might be required to improve the degree of SDS control.
The authors have not declared any conflict of interests.
This work was funded by grant UBACyT 20020130100604BA Universidad de Buenos Aires, Argentina.
REFERENCES
|
Agaras B, Scandiani MM, Luque A, Carmona M, Wall L, Valverde C (2012). Biological control of the soybean sudden-death-syndrome fungus Fusarium tucumaniae by Pseudomonas sp. strain SMMP3. Taller "Interacciones planta-microorganismos", Sociedad Argentina de Microbiología General (SAMIGE), Mar del Plata, Argentina.
|
|
|
|
Ahmad F, Ahmad I, Khan MS (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163:173-181.
Crossref
|
|
|
|
|
Badri DV, Weir TL, van der Lelie D, Vivanco JM (2009). Rhizosphere chemical dialogues: plant-microbe interactions. Curr. Opin. Chem. Biol. 20(6):642-650.
Crossref
|
|
|
|
|
Bottone EJ (2010). Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23(2):382-398.
Crossref
|
|
|
|
|
Brooke JS (2012). Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25:2-41.
Crossref
|
|
|
|
|
Carmona M, Sautua F, Perelman S, Gally M, Reis E (2015). Development and validation of a fungicide scoring system for management of late season soybean diseases in Argentina. Crop Prot. 70:83-91.
Crossref
|
|
|
|
|
Cook RJ, Baker KF (1983). The Nature and Practice of Biological Control of Plant Pathogens. St Paul MN, Amer. Phytopathol. Soc., 539 p.
|
|
|
|
|
de Souza R, Ambrosini A, Passaglia LMP (2015). Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38(4):401-419.
Crossref
|
|
|
|
|
Dilantha Fernando WG, Nakkeeran S, Zhang Y (2005). Biosynthesis of antibiotics by PGPR and its relation in biocontrol of plant diseases. En: Siddiqui ZA, editor. PGPR: Biocontrol and Biofertilization. Dordrecht, The Netherland, Springer, pp. 67-109.
|
|
|
|
|
Hartman GL, Chang HX, Leandro LF (2015). Research advances and management of soybean sudden death syndrome. Crop Prot. 73:60-66.
Crossref
|
|
|
|
|
León M, Yaryura PM, Montecchia MS, Hernandez AI, Correa OS, Pucheu NL, Kerber NL, Garcıa AF (2009). Antifungal activity of selected indigenous Pseudomonas and Bacillus from the soybean rhizosphere. Int. J. Microbiol. Volume 2009, Article ID 572049, 9 pages. Myresiotis CK, Karaoglanidis GS, Vryzas Z, Papadopoulou-Mourkidou E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manage. Sci. 68:404-411.
|
|
|
|
|
Scandiani M, Aoki T, Luque AG, Carmona MA, O'Donnell K (2010). First Report of Sexual Reproduction by the Soybean Sudden Death Syndrome Pathogen Fusarium tucumaniae in Nature. Plant Dis. 94:1411-1416.
Crossref
|
|
|
|
|
Siddiqui ZA (2006). PGPR: Prospective Biocontrol Agents of Plant Pathogens. In. Siddiqui ZA, editor. PGPR: Biocontrol and Biofertilization. Dordrecht, The Netherland, Springer, pp. 111-142.
Crossref
|
|
|
|
|
Simonetti E, Hernández AI, Kerber NL, Pucheu NL, Carmona MA, García AF (2012). Protection of canola (Brassica napus) against fungal pathogens by strains of biocontrol rhizobacteria. Biocontrol Sci. Technol. 22:111-115.
Crossref
|
|
|
|
|
Simonetti E, Pin Viso N, Montecchia M, Zilli C, Balestrasse K, Carmona M (2015). Evaluation of native bacteria and manganese phosphite for alternative control of charcoal root. Microbiol. Res. 180:40-48.
Crossref
|
|
|
|
|
Someya N, Tsuchiya K, Yoshida T, Noguchi MT, Akutsu K, Sawada H (2007). Co-inoculation of an antibiotic-producing bacterium and a lytic enzyme-producing bacterium for the biocontrol of tomato wilt caused by Fusarium oxysporum f. sp. lycopersici. Biocontrol Sci. 12:1-6.
Crossref
|
|
|
|
|
Xing LJ, Westphal A (2007). Inhibition of Fusarium virguliforme by Prokaryotes in vitro. Subtrop. Plant Sci. 59:24-29.
|
|