ABSTRACT
Phenotypic and molecular characterization of multiple antibiotic resistant Gram-negative bacteria in urine samples of pregnant women in Mother and Child Hospital, Nigeria was reported. In the study, 407 apparently healthy pregnant women were recruited. Structured questionnaire was administered to the patients to obtain their socio-demographic information and the medical history. Urine samples were collected, processed and analysed using standard microbiological procedures. Detailed identification of the bacteria isolates was done using biochemical characterization using Bergey’s Manual of Determinative Bacteriology and Analytical Profile Index (API) Kit. The antimicrobial susceptibility testing of the bacteria isolates was carried out using the Kirby-Bauer’s disk diffusion technique. Detection of the beta lactamase resistance genes (bla CTX-M and Tet A) was done by polymerase chain reactions (PCR) with appropriate primers. The following Gram-negative bacteria were recovered comprising Pseudomonas aeruginosa 48 (34.0%), Escherichia coli 30 (21.3%), Klebsiella sp. 27 (19.1%), Proteus sp. 15 (10.6%), Salmonella sp. 8 (5.7%), Providencia rettgeri, 4 (2.8%) and Enterobacter cloacae 4(2.8%) and other enterobacteriaceae 5 (3.5%). Resistance of the isolates to antibiotics used varied greatly among the isolates. Resistance to antibiotics was highest with P. aeruginosa having 100% to augumentin, tetracylines, amoxicillin, nitrofurantoin, cotrimoxazole, ceftriazone, cefixime (97.9%) and cefuroxime (95.8%). There was diversity in the multiple antibiotic resistance (MAR) patterns among the isolates with 12 different MAR patterns observed. The selected P. aeruginosa profiled for resistance genes harboured bla-CTX-M (585bp) and Tet A (954bp) genes. The multiple antibiotic resistant bacteria recovered could pose great health challenge to the pregnant women and the unborn foetus.
Key words: Gram negative bacteria, antibiotics, enterobacteriaceae, resistance genes.
Bacteriuria can be defined as the appearance or presence of bacteria causing diseases in the urethra, bladder, and pelvis of the kidney. Bacteria such as Escherichia coli, Enterococcus faecalis Klebsiella species, Proteus mirabilis, Pseudomonas aeruginosa and Streptococcus agalactiae are some of the causative agent of urinary tract infections (UTI). Blockage of the urinary tract, catheter usage, deficiency in oestrogen, genetic predisposition and sexual intercourse are predominant risk factors for urinary tract infection (Tigabu et al., 2020). Asymptomatic bacteriuria (ASB) is the detection of 105CFU/ml of one or more 46 bacterial species, irrespective of pyuria, in a urine specimen from a pregnant women patient without any symptoms of a urinary tract infection (UTI) (Willey et al., 2020). The relatively upsurge of ASB in pregnancy, the consequences encountered by the women and their pregnancies, avoidance of treatment with undesired outcomes, screening and treatment of ASB in pregnancy with justification. There are variations in the frequency of pathogens isolated and antimicrobial resistance patterns based on different geographical regions. Asymptomatic bacteriuria occurs in 2 to 15% of pregnant women. Therefore if the ASB remains untreated, over 30% of mothers will develop acute pyelonephritis, and this has been associated with low birth weight and premature birth (Sujatha and Nawani, 2014; Smaill and Vazquez, 2019).
It is recommended that screening for bacteriuria should routinely be undertaken in the first trimester of pregnancy to identify women who are at risk in order to prevent undesirable end result as bacteriuria which is one of the risk factor in pregnant women. The importance of the microbiological analysis of urine samples collected from patients that appears healthy cannot be over emphasized.
Multiple antibiotic resistant Gram-negative bacteria are bacteria which have developed resistant to many common commercial used antibiotics. Their habitat is in the bowel and therefore causes no harm or any problems but can cause infections in the urine, skin wounds and blood when left untreated. These multidrug resistant Gram-negative bacteria infections pose a serious threat in the clinical settings with limits to the choice of antibiotics in the treatment and management of infections in patients that are hospitalized, and more especially patients in intensive care unit. Overuse and misuse of antibiotics and problems and wrong infection control practices have led to the development of multiple resistant Gram-negative bacteria infections. This study investigates into the phenotypic and molecular characterization of multiple resistant Gram-negative bacteria among pregnant women attending antenatal of Mother and Child Hospital in Ondo, Nigeria.
Isolation of bacteria
Urine samples of four hundred and seven apparently healthy pregnant women attending antenatal clinic at Mother and Child hospital in Ondo Town were collected between July 2015 and January 2016. Samples were cultured on Centrimide and MacConkey agar (Lab M Ltd, UK) by streaked plate method, incubated at 37°Cfor 24 h for isolation of P. aeruginosa and other Gram-negative bacteria. Preliminary identification of isolates was by morphological, cultural characteristics and biochemical tests. Bacteria isolates were further re-confirmed using analytical profile index (API) 20E test kit (bioMérieux, Inc., France).
Antibiotic susceptibility testing
Susceptibility test of the isolates to antibiotics was carried out by the Kirby-Bauer’s disc diffusion method (Bauer et al., 1966). Antibiotic discs (Abtek Biological limited, UK) which include; gentamycin (10 µg), augmentin (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), cefuroxime (30 µg), nitrofurantoin (200 µg), cotrimoxazole (25 µg), ofloxacin (5 µg), amoxicilin (25 µg), tetracycline (30 gµ), ciprofloxacin (5 µg), ofloxacin (5 µg), ceftriazone (30 µg) and cefixime (5 µg) were carefully placed on Mueller-Hinton agar plates previously seeded with 24 h old culture (0.5 Mcfarland’s standard- 107cfu/ml). The plates were incubated at 37°C for 24 h and diameter of zone of inhibition was measured by a transparent calibrated ruler to the nearest millimetre and the results interpreted according to the guidelines of Clinical Laboratory Standard (CLSI, 2013). Multiple antibiotic resistance was defined as resistance to more than two class of antibiotics.
Molecular characterization of multiple antibiotic resistant isolates
Twelve representative multiple antibiotic resistant bacterial isolates selected on the basis of their antibiotype were profiled for detection of resistance bla CTX (585bp) and Tet A (954bp) genes by polymerase chain reaction (PCR) using appropriate primers as depicted in Table 1. The DNA of the bacteria isolates was extracted using boiling method at 100°C for 7 min in water bath, cold shocked in ice for 2 min. The PCR thermocycling conditions include initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s for bla CTX (585 bp) gene and 43°C for 30 s for Tet A (954 bp) gene, extension at 72°C for 1 min and final extension at same temperature for 5 min. The bands were then visualized with a short wave ultraviolet trans illuminator and photographed gel bioimaging system.
Table 2 shows the baseline characteristics of the subjects. The women recruited were between the age brackets of 15 and 49 years. Among the 407 pregnant women, 36(8.8%) was in their first trimester, 171(42.0%) in second trimester and 200(49.1%) in third trimester. Six (1.5%) of the subjects was single, while 401(98.5%) was married. Also, 37(9.1%) was students, 75(18.4%) was public or civil servants, 6(1.5%) was professionals, 253(54.4%) was artisans or traders and 6(3.9%) was dependent or unemployed.
Five pregnant women within the age bracket 21-32 years suffering from urinary tract infection served as control and were all in their second and third trimesters. Table 3 depicted the percentage distribution of the bacteria isolates recovered viz; P. aeruginosa (34.0%), E. coli (21.3%), Proteus sp (10.6%), Salmonella sp (5.7%), Klebsiella sp (19.1%), P. rettgeri (2.8%), E. cloacae (2.8%) and other enterobacteriaceae 5 (3.5%). Table 4 shows the percentage occurrence of bacteria isolated from pregnant women in relation to their occupation. The percentage distribution of the bacterial isolates varies with occupation. There was no bacterial isolates recovered from pregnant women who are professionals/ managers.
Table 5 shows the antibiotic resistance profiles of the bacterial isolates. Resistance to antibiotics varies greatly among the organisms. Resistance to beta-lactam class of antibiotics was generally high among the organisms. P. aeruginosa showed highest resistance most antibiotics tested namely augmentin (100%), tetracyclines (100%), amoxicillin (100%), ceftriaxone (100%), cotrimoxazole (100%), nitrofurantoin (95.8%), cefuroxime (95.8%) and cefixime (97.9%). E.coli were only resistant to augmentin (93.3%).
Table 6 showed the multiple antibiotic resistance pattern of P. aeruginosa and Klebsiella ornithinolytica isolates. All P. aeruginosa and K. ornithinolytica isolates recovered from this study were multidrug resistance. The isolated bacteria were resistant to multiple antibiotics which ranges from four to seven classes. K. ornithinolytica (9.5%) were resistant to four class of antibiotics. P. aeruginosa (97.9%) were multi-resistant to six different class, 2.1 to 7% and 4.2 to 5% different class of antibiotics. Meanwhile, both K. ornithinolytica and P. aeruginosa exhibited 2 different multiple antibiotic resistance patterns each (Table 6).
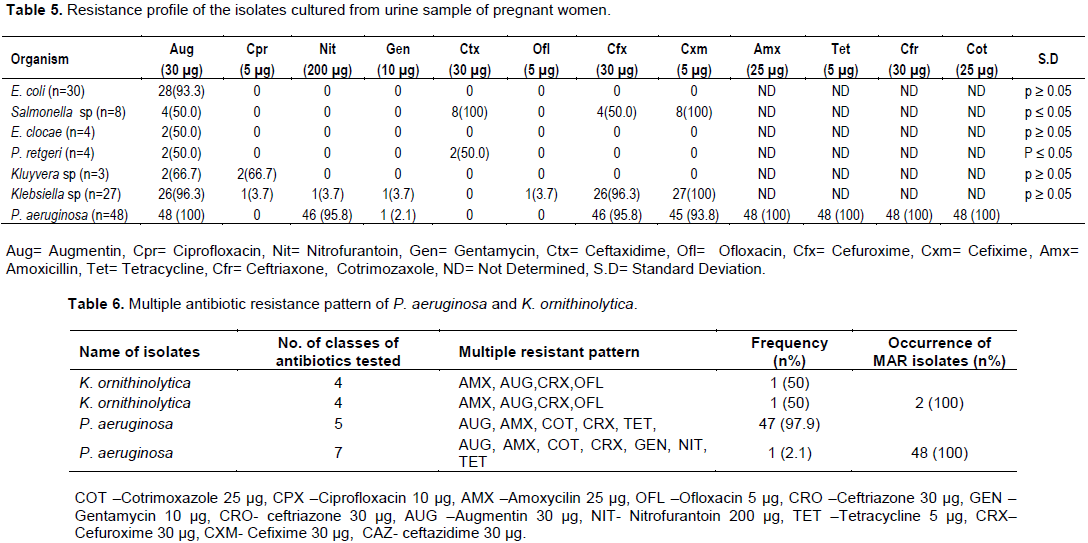
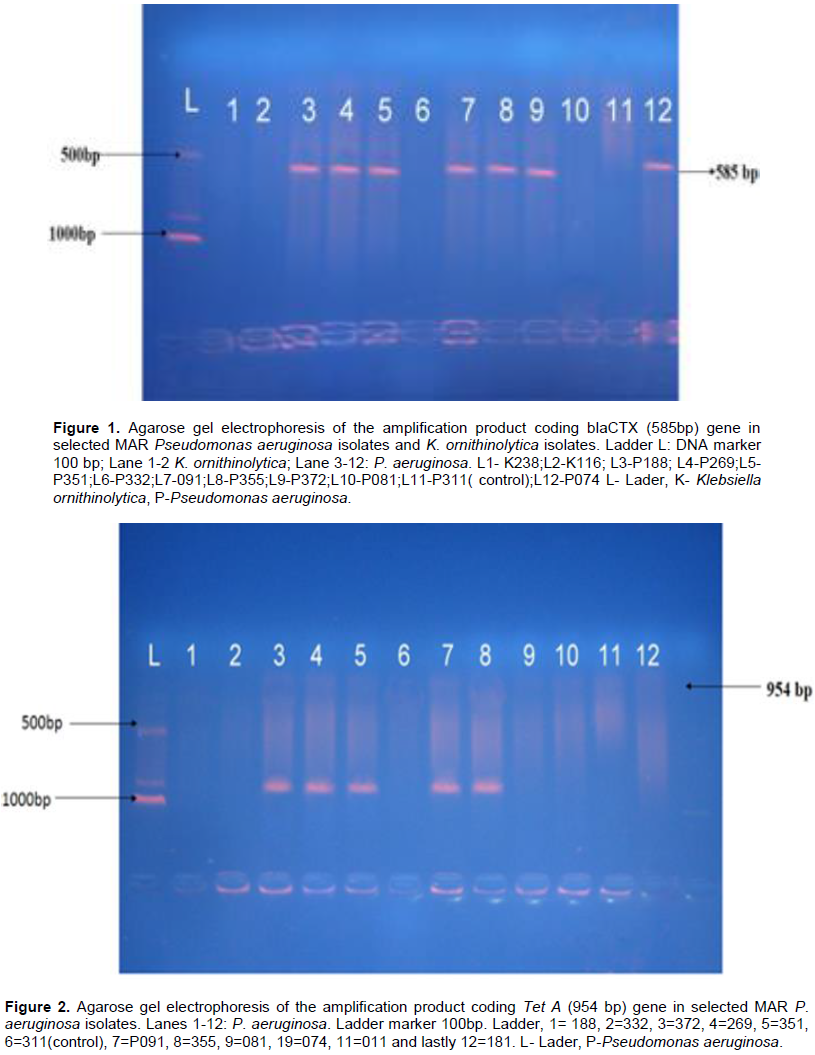
Figures 1 and 2 showed agarose gel electrophoresis of the amplification product coding bla CTX (585 bp) and Tet A (954) genes in selected MAR P. aeruginosa and K. ornithinolytica isolates. In Figure 1, seven of the 12 representative isolates that were resistance to beta lactam antibiotics as depicted by Lanes 3, 4, 5, 7, 8, 9, and 12 harboured blaCTX resistance gene of molecular weight of 585 bp. Lanes 1-2 represent K. ornithinolytica which harboured no blaCTX resistance gene. Lane 11 represents the control (patient suffering from urinary tract infection) which also harboured no blaCTX resistance gene. Figure 2 shows the agarose gel electrophoresis of TetA genes in selected 12 representatives multiple antibiotic resistant P. aeruginosa. Five of the 12 representative isolates that were resistant to tetracycline antibiotics as depicted by Lanes 3, 4, 5, 7 and 8, harboured Tet A resistance gene of molecular weight of 945 bp. Lane 11 represents control (patient suffering from urinary tract infection).
This study showed the prevalence of 36.4% bacteria amongst the sampled population of 407 pregnant women attending ante-natal clinic at Mother and Child Hospital Ondo town Nigeria. This level of prevalence is at variance to other researchers’ reports. Olamijulo et al. (2016) reported a prevalence of 14.6% in a study carried out in 556 pregnant women in Lagos University Teaching Hospital, Nigeria. It is also at variance with Nguefack et al. (2019) who also reported a prevalence of 9.9% in a three hospitals in the developing country.
The prevalence of bacteria in increasing order in this study, P. aeruginosa (34.0), E. coli (21.3), Klebsiella sp (19.1), Proteus sp (10.6), Salmonella sp (5.7), other enterobacter (3.5), Providencia rettgeri (2.8) and Enterobacter clocae (2.8) is in contrast to Olamijulo et al. (2016) who reported that Klebsiella sp is the most common pathogen isolated in urine of pregnant women. Gram-negative bacteria are responsible for more than 85% cases of UTI and are the dominant causative agents, also they are the normal flora of the intestinal tract especially the rectum which is very close to the urethral orifice (Anyamene et al., 2002; Obiogbolu, 2004).
Bacteria were predominantly recovered among women of ages (19-32) in their second and third trimesters with none in the first trimester. This finding is quite similar to Durowaiye et al. (2011) who reported the Women in the third trimester were observed to have the highest prevalence 18.2% than those in their second and first trimester. This could be because of changes that occur in the anatomic site and physiology that is been experienced by pregnant women during the stages of pregnancy. Because of the uterus expansion there is increase in the hormonal effects which can together lead to invasion of microorganisms. These hormonal changes reduce the muscular tone of the uterus and induce the mechanical pressure from the gravid uterus and this may leads to urinary stasis which encourages the multiplication of bacteria in urine since urine is an excellent culture media for bacteria growth (Obiogbolu, 2004). Pregnant women within the age of 20-39 years old age group had the highest prevalence of bacteria. This shows that these women are at the risk of developing urinary tract infection in future if not or properly treated. The bacteria are responsible for asymptomatic bacterial infection are of faecal origin and they colonize the periurethral region.
The percentage of Proteus sp, P. retgeri, E. coli, Klebsiella sp, P. aeruginosa, Salmonella sp and E. clocae was predominantly high among pregnant women who were farmers/ artisans/traders. This high prevalence among these categories of women could be as a result of consumption of contaminated food, socio-economic status, attitude to personal hygiene and educational exposure.
All the bacterial isolates in this study namely; K. ornithinolytica, E. coli, E. clocae, Salmonella sp, and P. rettgeri were highly sensitive to nitrofurantoin, gentamycin, and ofloxacin except P. aeruginosa. Similarly, E. coli were relatively sensitive to ceftaxidime, cefixime, ciprofloxacin, gentamycin, nitrofuraintoin and cefuroxime. This finding is comparable to Olamijulo et al. (2016) who reported in their study that Gram-negative bacteria showed high sensitivity rate to gentamycin and ofloxacin. This could be because gentamycin belongs to the class aminoglycoside where antibody binds to the subunit of the bacteria ribosome, interruption of protein synthesis thereby preventing bacteria from performing vital roles needed for survival. Therefore, gentamycin can be used in the treatment of these Gram-negative bacteria in
urinary tract infection.
Antimicrobial susceptibility testing in this study revealed the high resistance of P. aeruginosa to amoxicillin which belongs to penicillin class (beta lactam group) by the isolates recovered. Similar results were reported in ast studies by Sabharwal (2012). This could be a result of misuse and excessive usage of antibiotic and increase in the spread of beta lactamase producing isolates. Sensitivity of P. aeruginosa to flouroquinolones (ciprofloxacin (100%) and ofloxacin) were significant in this study thereby revealing the potency of the antibiotics against the urinary tract pathogens. However, widespread usage may lead to resistance against fluoroquinolones (Gupta et al., 2005).
P. aeruginosa in this study showed high resistance to nitrofurantoin (95.8%) which is in contrast to a report that nitrofurantoin, is a urinary antiseptic, and found to have a better susceptibility and considered safe by Peterson and Andriole (1997) and Christensen (2000).
The result from the antimicrobial susceptibility profile in this study is closely related to that obtained by Akingbade et al. (2012), who reported high resistance rate of P. aeruginosa to the following antibiotics: amoxicillin (92.7%), oxacillin (88.2%), cotrimoxazole (77.3%), erythromycin (72.7%), and tetracyclines (70.9%), while they also reported a relatively low resistance to gentamicin as recorded in the present study. Similarly, they reported a low resistance to ceftazidime (20%), gentamicin (26.4%) by P. aeruginosa, while a sharp contrast pattern was observed in the present study with high sensitivity to ceftazidime (97.9%), gentamycin (97.9%) and ciprofloxacin (100%), but high resistance to tetracycline (100%), amoxicillin (100%), cotrimoxazole (100%) and ceftriaxone (100%). Also, Akingbade et al. (2012) recorded a high resistance to ofloxacin (60.0%) which is in contrast to the present study in which a relatively high sensitivity to ofloxacin (100%) was observed.
P. aeruginosa is known to be an opportunistic pathogen which is a leading cause of morbidity and mortality rate in immunocompromised individuals including pregnant women. P. aeruginosa has become extremely difficult to eradication because of its remarkable capacity to resist different antibiotics. Strains of P. aeruginosa have been known to utilize high levels of intrinsic and acquired resistance mechanisms to attack most antibiotics. Biofilm-mediated resistance and formation of multidrug-tolerant persister cells have been a recent characterized mechanism for adaptive antibiotic resistance which is responsible for recalcitrance and relapse of infections (Pang et al., 2019). The discovery and development of alternative therapeutic strategies that present novel avenues to fight P. aeruginosa infections are increasingly demanded and gaining more attention. According to findings from this study, all P. aeruginosa recovered from the patients were multiple antibiotic resistant. This high prevalence of multiple antibiotic resistance (MAR) is more alarming and its consistent with previous researchers’ findings (Pharmd et al., 2018; Shah et al., 2015). Resistivity of the isolates to various antibiotics could be because of overuse of antibiotics and self-medication resulting in drug resistance especially by P. aeruginosa.
The detection of beta-lactamase resistance genes in P. aeruginosa in the study is undoubtedly partly responsible for the high resistance rate phenotypically observed particularly against most of the beta-lactams antibiotics used. These genes code for acquired extended spectrum beta-lactamases which are involved in the resistance against beta-lactams and are located in transferable genetic elements such as plasmids or transposons of the organisms (Giedraitien? et al., 2011) and often on integrons (Kotsakis et al., 2010; Zhao et al., 2009; Nordmann et al., 2012). Ogbolu et al. (2013) reported a prevalence of 30.8, 15.4, and 23.1% for blaTEM, blaSHV and blaCTX-M genes, respectively from P. aeruginosa isolates recovered in their studies. In this study, blaCTX-M was also detected in the organism. This study detected the presence of TetA resistance gene among P. aeruginosa.
In conclusion, the varieties of bacteria isolated in this study have great implication on the health status of pregnant women and their unborn fetuses. Antibiotics such as gentamycin, ciprofloxacin, ofloxacin and ceftazidime play a great role in the treatment of bacteria in pregnant women. Health education on personal hygiene should be emphasized by the physicians of antenatal care to all pregnant women, especially those of low socio-economic level.
The authors have not declared any conflict of interests.
REFERENCES
|
Adesiyan IM, Bisi-Johnson MA, Ogunfowokan AO, Okoh AI (2019). Incidence and antimicrobial susceptibility fingerprints of Plesiomonas shigelliodes isolates in water samples collected from some freshwater resources in Southwest Nigeria. Science of the Total Environment 665:632-640.
Crossref
|
|
|
|
Akingbade OA, Balogun SA, Ojo DA, Afolabi RO, Motayo BO, Okerentugba PO, Okonko IO (2012). Plasmid Profile Analysis of Multidrug Resistant Pseudomonas aeruginosa isolated from Wound Infections in South West, Nigeria. World Applied Sciences Journal 20(6):766-775.
|
|
|
|
|
Anyamene CO, Stellamaris N, Muoneke Umerie GNC (2002). Bacterial isolates associated with urinary tract infections in Akwa and environs. Journal of Applied Science 5(4):3092-3098.
|
|
|
|
|
Christensen B (2000). Which Antibiotics are Appropriate for Treating Bacteriuria in Pregnancy? Journal of Antimicrobial Chemotherapy 46:29-34.
Crossref
|
|
|
|
|
Clinical and Laboratory Standards Institute (CLSI) (2013). Performance standards for antimicrobial susceptibility testing. Eighteen Informational Supplements 23:62-64.
|
|
|
|
|
Colom K, Perez J, Alonso R, Fernandez-Aranguiz A, Larino E, Cisterna R (2003). Simple and reliable multiplex PCR assay for detection of blaTEM, bla (SHV) and blaOXA-1 genes in Enterobacteriaceae. Federation of European Microbiology Society Microbiology Letter 223(2):147-51.
Crossref
|
|
|
|
|
Durowaiye MT, Onaolapo JA, Oyi AR (2011). Preliminary Study on Asymptomatic Bacteriuria in Pregnant women Attending Antenatal Clinics in Three Hospitals in Kano, A North-West City in Nigeria. Nigerian Journal of Pharmaceutical Sciences 10(2):15-21
|
|
|
|
|
Giedraitien? A, Vitkauskien? A, Naginiene R, Pavilonis A (2011). Antibiotic Resistance Mechanisms of Clinically Important Bacteria. Medicina 47(3):137-146.
Crossref
|
|
|
|
|
Gupta K, Hooton T, Mand Stamm WE (2005). Isolation of Fluoroquinolone Resistant Rectal Escherichia coli After Treatment of Acute Uncomplicated Cystitis. Journal of Antimicrobial Chemotherapy 56(1):243-246.
Crossref
|
|
|
|
|
Kotsakis SD, Papagiannitsis CC, Tzelepi E, Legakis NJ, Miriagou V, Tzouvelekis LS (2010). GES-13, a β-lactamase variant possessing Lys-104 and Asn-170 in Pseudomonas aeruginosa. Antimicrobial Agents Chemotherapy 54:1331-1333.
Crossref
|
|
|
|
|
Nguefack CT, Ebongue CO, Chokotheu CN, Ewougo CE, Njamen TN, Mboudou E (2019). Clinical presentation, risk factors and pathogens involved in bacteriuria of pregnant women attending antenatal clinic of 3 hospitals in a developing country: a cross sectional analytic study. BMC Pregnancy and Childbirth 19:143.
Crossref
|
|
|
|
|
Nordmann P, Boulanger AE, Poirel L (2012). NDM-4 Metallo-B-Lactamase with Increased Carbapenemase Activity from Escherichia coli. Antimicrobial Agents of Chemotherapy 56(4):2184-2186.
Crossref
|
|
|
|
|
Obiogbolu CH (2004). Incidence of Urinary Tract Infection amongst Pregnant women within Akwa Metropolis. A B.Sc. Project in the Department of Applied Microbiology and Brewing, Nnamdi Azikwe University, Awka, Anambra State, Nigeria. P 55.
|
|
|
|
|
Ogbolu DO, Daini OA, Ogunledun A, Alli OAT, Webber MA (2013). Dissemination of IncF plasmids carrying beta-lactamase genes in Gram-negative bacteria from Nigerian Hospitals. Journal of Infection in Developing Countries 7(5): 382-390.
Crossref
|
|
|
|
|
Olamijulo JA, Adewale CO, Olaleye O (2016). Asymptomatic bacteriuria among antenatal women in Lagos, Journal of Obstetrics and Gynaecology 36(6):722-725.
Crossref
|
|
|
|
|
Pang Z, Raudonis R, Glick BR, Lin T, Cheng Z (2019). Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnology Advances 37(1):177-192.
Crossref
|
|
|
|
|
Peterson TF, Andriole VT (1997) Detection, significance and therapy of bacteuria in pregnancy.Update in the managed health care era. Infectious Diseases Clinics of North America 11:593-608.
Crossref
|
|
|
|
|
Pharmd JG, Pharmd KG, Pharmd LN, Pharmd CM (2018). Multidrug-Resistant Pseudomonas aeruginosa Infections: Hard to Treat, But Hope on the Horizon? Contagion 3:1.
|
|
|
|
|
Sabharwal ER (2012). Antibiotic Susceptibility Patterns of Uropathogens in Obstetric Patients. American Journal of Medical Science 4:316-319.
Crossref
|
|
|
|
|
Shah DA, Wasim S, Abdullah FE (2015). Antibiotic resistance pattern of Pseudomonas aeruginosa isolated from urine samples of Urinary Tract Infections patients in Karachi, Pakistan. Pakistan Journal of Medical Science 31(2):341-345.
|
|
|
|
|
Smaill FM, Vazquez JC (2019). Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Systematic Review P 11.
Crossref
|
|
|
|
|
Sujatha R, Nawani M (2014). Prevalence of Asymptomatic Bacteriuria and its Antibacterial Susceptibility Pattern Among Pregnant Women Attending the Antenatal Clinic at Kanpur, India. Journal of Clinical and Diagnostic Research 8(4):1-3.
Crossref
|
|
|
|
|
Tigabu A, Ferede W, Belay G, Gelaw B (2020). "Prevalence of Asymptomatic Bacteriuria and Antibiotic Susceptibility Patterns of Bacterial Isolates among Cancer Patients and Healthy Blood Donors at the University of Gondar Specialized Hospital", International Journal of Microbiology.
Crossref
|
|
|
|
|
Willey Z, Jacob JT, Burd EM (2020). Targeting Asymptomatic Bacteriuria in Antimicrobial Stewardship- The Role of the Microbiology Lab. Journal of Clinical Microbiology 58(5):18-18.
Crossref
|
|
|
|
|
Zhao WH, Chen G, Ito R, Hu ZQ (2009). Relevance of resistance levels to carbapenems and integron-borne blaIMP-1, blaIMP-7, blaIMP-10 and blaVIM2 in clinical isolates of Pseudomonas aeruginosa. Journal of Medical Microbiology 58:1080-1085.
Crossref
|
|