Full Length Research Paper
ABSTRACT
Moringa stenopetala is a multipurpose tree with considerable economic and social potential as it has vital nutritional, industrial, and medicinal applications. The study was aimed to investigate the antimicrobial activities of M. stenopetala seed oil against pathogenic microorganisms. M. Stenopetala seeds were collected from three locations (Damba Gofa, Shelle, and Konso) and extracted using two different solvents (hexane and petroleum ether). Pathogenic microorganisms: bacteria (gram-positive, Staphylococcus aureus, and gram-negative Escherichia coli) and the fungal strains (Trichophyton mentagrophytes and Candida albicans) were used in this study. Standard procedures were followed to determine antimicrobial activities of M. stenopetala extract against pathogenic microorganisms. The result revealed that M. stenopetala seed extract has shown inhibitory activity against T. mentagrophytes fungi at the concentration ≥ 12.5% at all locations and both extraction solvents used. However, the extract did not show any inhibitory activity against tested bacteria and C. albicans fungi. The finding indicated that M. stenopetala seed could be used as an alternative to chemical fungicide to control T. mentagrophytes fungi. Further investigation is needed on the identification of compounds that inhibits the pathogenic microorganism.
Key words: Antimicrobial activity, bacteria and fungi, Moringa stenopetala seed, extract.
INTRODUCTION
World Health Organization (WHO) reported that 80% of the population in developing countries relies on medicinal plants to acquire primary health care needs (WHO, 2002). This is likely in Ethiopia where 80% of the human population and 90% of livestock depend on traditional medicines (Abebe, 2001). The majority of these come from plant sources, which are the main sources of antimicrobial molecules (Adnan et al., 2015). These include secondary metabolites synthesized by the plants, more likely phenolic compounds (Hu et al., 2021). In addition, they have an advantage over synthetic products due to fewer side effects (Adnan et al., 2015).
Furthermore, they are the source of new antimicrobial drugs due to the increment of microorganisms resistant to conventional antimicrobials (Silva and Fernandes Júnior, 2010).
Moringa stenopetala belongs to the Moringaceae family and it is one of the species of the thirteenth Moringa geniuses (NRC, 2001). It is an underutilized, fast-growing vegetable food crop indigenous to East African lowlands and southern Ethiopia (Abuye et al., 2003). In Ethiopia, M. Stenopetala is commonly known as Shiferaw (Amharic), Aleko, Aluko, Halako (Gamo Gofa), Kallanki (Benishangul), Telahu (Tsemay), Haleko, Shelchada (Konso) and Haleko (Burji) (UNIDO, 2015). In English, it is named as Africa Moringa tree, Ben oil tree, Cabbage tree, and Horse-radish tree (Demeulenaere, 2001). Various parts of Moringa are used for human food, fuelwood, livestock forage, medicine, dye, water purification, soil and water conservation, quality of cooking oil, green manure, and as a source of income for Moringa cultivators (Demeulenaere, 2001; Abay et al., 2015).
M. stenopetala is used traditionally as food and to treat malaria, hypertension, asthma, diabetes, common cold, wounds, retained placenta, and stomach problems (Mekonnen and Gessesse, 1998). The seeds show a flocculating property, important in purifying turbid water (Abuye et al., 2003; Prashith et al., 2016). It is a major source of oil which could be important for cooking, salad (Raghavendra et al., 2016), and for different industrial applications (Seifu, 2015).
Furthermore, the seed possesses coagulant activity is useful for clarifying water and possesses antimicrobial activity (Rani et al., 2018). M. stenopetala seed extraction using different extraction solvents like hexane and methanol exhibits inhibition against waterborne disease, caused by Salmonella typhii, Vibrio cholera, and Escherichia coli (Walter et al., 2011). This is mainly due to biologically active compounds of a plant relying on the type of solvent used in the extraction procedure (Seleshe and Kang, 2019).
Even though M. stenopetala has a remarkable role in the lives of a large population of Southern Ethiopian, there is a lack of research conducted on the antimicrobial activities of M. stenopetala seed extract in the study area. Furthermore, the growing pressure on food manufacturers to avoid the use of chemical preservatives needs to search for alternative preservatives. Therefore, the present study aimed to evaluate the antimicrobial activity of M. stenopetala seed solvent extract collected from different locations against four pathogenic micro-organisms, namely Staphylococcus aureus, Escherichia coli, Trichophyton mentagrophytes, and yeast Candida albicans.
MATERIALS AND METHODS
Samples collection
The identification of M. stenopetala used in this study was done with the help of a botanist from Arbaminch university and the dominance of M. stenopetala in the sites considered by the study (Abuye et al., 2003; Gebregiorgis et al., 2012; Seifu, 2015). Matured pods of M. stenopetala with similar color were collected from three locations in Southern Ethiopia; Gofa Zone (Demba Gofa district), Gamo Zone (Shelle district), and Segen Area Zone (Konso district) from January to February 2022. The locations were selected purposely based on the availability and abundance of M. stenopetala trees in the area.
Shelle district is located about 27 km from Arba Minch town and 532 km from Addis Ababa. Demba Gofa district is located 526 km from Addis Ababa. Konso district is located about 600 km southwest of Addis Ababa capital city of Ethiopia.
M. stenopetala seed powder preparation
The powder preparation was performed following the procedure indicated by Haile et al. (2019). Briefly, the matured seeds were separated from their pods and cleaned by removing the bark. The seeds with even appearance in size and shape were selected. The seeds were sun-dried to separate the husk from the seed kernel and the seed powder was prepared using a mechanical grinder. The powders obtained were sieved and then stored in polythene bags until extraction at Arba Minch University Chemistry laboratory.
Oil extracts preparation
The oil was extracted using a semi-continuous process; soxhlet procedure, through repeated washing (percolation) with n-Hexane and petroleum ether. Seed powders of 40 g were placed in a porous cellulose thimble. Then the timble was placed in an extraction chamber in between flask containing solvents of 150 ml and condenser. Heat was applied into the flask where the solvent evaporates into a condenser and converted to liquid that flows into the extraction chamber containing the sample. At the end of extraction, the remaining solvent in a flask is evaporated in an oven and the oil was collected (Adejumo et al., 2013).
Test organisms
The pathogenic microorganisms used in this study were gram-positive bacteria S. aureus and gram-negative bacteria E. coli; the fungal strains T. mentagrophytes and C. albicans (Yeast). The strains were clinical isolates obtained from Bacteriology and Mycotic disease reference laboratory of Ethiopian Public Health Institute, Addis Ababa, Ethiopia.
Inoculum preparation
The inoculum for bacteria was prepared from the stock cultures and sub cultured onto nutrient agar using a sterilized wire loop and incubated at 37oC for 24 h. Whereas the yeast and fungi were inoculated with Sabouraund Dextrose Agar (
Controls used in the study
Chloramphenicol for S. aureus and E. coli and Ketoconazole for T. mentagrophytes and C.albicans was used as a positive control but 5 % Tween 80 was utilized as a negative control.
Antimicrobial assay
Antibacterial activity of n-Hexane and petroleum ether extracts of M. stenopetala seed oil were evaluated by the modified agar well diffusion technique (Bauer et al.,1996). Standardized inoculum of bacterial and fungal culture suspension was uniformly swabbed on the Mueller Hinton Agar (MHA) (OXOID) and SDA (PARK) media respectively by using a sterile cotton swab. The inoculated plates were left at room temperature for 10 minutes to absorb any surface moisture before applying the extract. Thus, wells were aseptically punched on both MHA and SDA plates equidistant of 6 mm in diameter by using a sterile stainless still borer and labeled at the backside of the plates. Each well was filled with 100 μl of n-hexane and petroleum ether extracts at concentrations of 3.13, 6.25, 12.5, 25 and 50%. Accordingly, all plates were kept to settle down on a working bench for 1hr to allow proper diffusion of the extract into the media. The bacteria cultures were incubated at 37ºC for 24 h while the fungal culture was incubated at 25ºC for 72 h. The solvents that were used to reconstitute the extract were set up in parallel. Antimicrobial activity was determined by measuring the zone of inhibition around each well. For each extract duplicate trials were conducted against each organism.
Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration of the seed oil was determined against the test organisms by using the agar dilution technique (Griffin et al., 2000). This was conducted by mixing the sterile cooled at 45°C MHA and SDA media with different concentrations (4 and 2%) of n-Hexane and petroleum ether extract and poured into Petri dishes (90 mm) and left to solidify then the plates were left upside down at room temperature for 10 to 15 minutes to avoid moisture. In the same fashion controls without the extract were set up in parallel using 5% Tween 80 for negative control and Chloramphenicol and Ketoconazole for positive control. Mueller Hinton Agar and SDA were inoculated with the strains to confirm the viability of the culture. Followed by these 10 μl from each standardized bacterial and fungal suspension was taken and inoculated on the media that were incorporated with plant extracts. The plates were allowed to stand for 5 min and incubated at 37ºC for 24 h for bacteria and 25ºC for 72 h for fungi. The procedure was performed in duplicate at different concentrations of the extract.
Statistical analysis
The zone of inhibition around each disc was measured in mm and the results were presented as means ±SD using IBM SPSS Statistics software (version 25).
RESULTS
Antibacterial activity of M. stenopetala seed oil
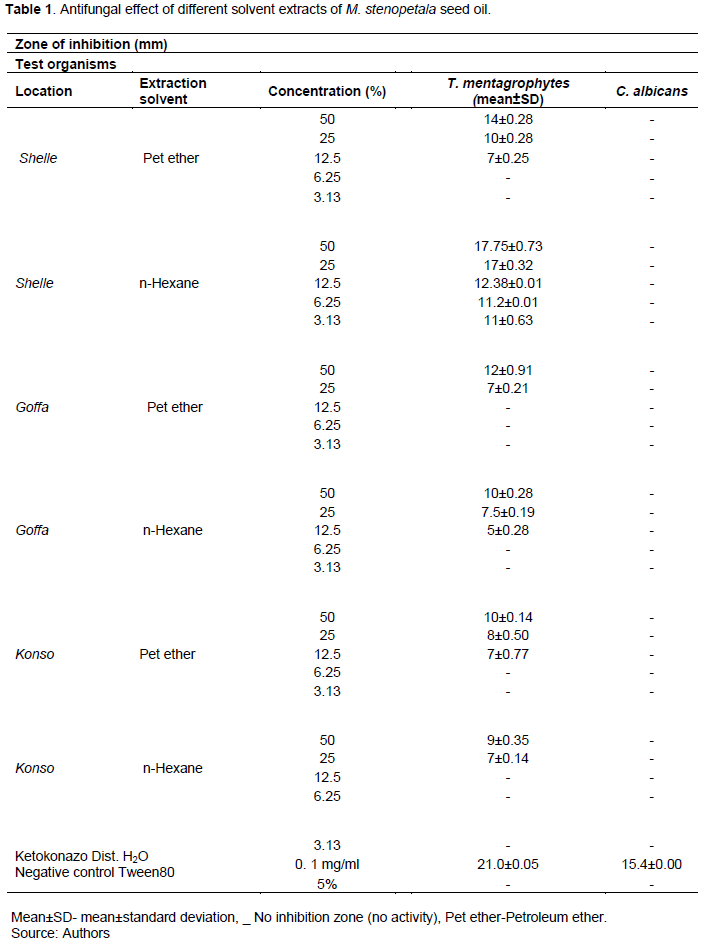
The results have shown that n-Hexane and petroleum ether extracts of M. stenopetala seed from the different locations used at different concentrations has shown no zones of inhibition of bacterial growth (Table 1). The inhibition zone for the standard drug chloramphenicol was 11.8 mm for E. coli and 14.0 mm for S.aureus.
Antifungal activity of M. stenopetala seed oil
Minimum inhibitory concentration of M. stenopetala seed oil
The finding of this research has indicated no inhibition for all tested microorganisms at the concentrations of 2 and 4%. Furthermore, no inhibitory effect was observed in the presence of 5% Tween 80 which was used as a negative control (Table 1).
DISCUSSION
The study did not show any inhibition activity of M. stenopetala extracts against bacteria. Previous study reported controversial results from the present study (Chekesa and Mekonnen, 2015); methanol crude extract and ethyl acetate extract of the M. stenopetala seeds showed the highest antibacterial activity, against S. aureus and E. coli but petroleum ether extract of the seeds only showed inhibition on S. aureus but not in E.coli. The resistance of E. coli to the extract matches findings from a study on the antibacterial activity of Moringa leaf extract to be ineffective against E.coli (Bhawasar et al., 1965; Peixoto et al., 2011). In line with the current study petroleum ether leaf extract of M.olifera didn’t show inhibition against S.aureus and E.coli isolated from urinary tract-infected patients (Abdalla et al., 2016).
The organisms that are included in this study are clinical isolates that are obtained from symptomatic patients. Hence, they may have a high chance of exposure to anti-bacterial agents that may bring change to the molecular and other factors. Therefore, the microorganisms are expected to be less sensitive compared to standard organisms with no chance of exposure to any antimicrobial agents. Moreover, a previous study (Rahman et al., 2008) reported that petroleum ether extract from the stem bark of M. oleifera did not show antibacterial activity in both E. coli and S. aureus. Furthermore, a study made by Shailemo et al. (2016) showed antimicrobial activity M. oleifera n-Hexane seeds and bark extracts against pathogens of water-borne diseases was lower than other solvents used for extraction. The inactivity of both extracts against bacteria might be because of the presence of polar compounds in the plant that can bind to the cytoplasmic membrane of the organism but since both the extracts are non-polar the activity of the compound becomes inactive against the tested organism (Boyd and Beveridge, 1981).
Both n-Hexane and petroleum ether extract of M. stenopetala seed showed antifungal activity against T. mentagrophytes at the concentration ≥ 12.5% except n-Hexane extract collected from Shelle which has shown antifungal activity at the concentration of ≥ 3.06. In line with the current finding, Dinesha et al. (2018) reported that Moringa seed kernel oil presented excellent antifungal activities. Furthermore, Anthonia (2012) reported that T. mentagrophyte growth was inhibited by inhibition zone of 22 mm using ethanolic extract M. oleifera leave. In other study, M. stenopetala methanolic leaf extract results in concentration dependent inhibition of mycelial growth of Aspergillus flavus (Kekuda et al., 2016).
The result has demonstrated an increase in the exteraction concentration resulted in gradual increases in the inhibition zone. Similar result has been reported by Prabakaran et al. (2018) for M. oleifera extract. Both n-Hexane and petroleum ether extract of M. stenopetalla seed has shown no antifungal activity against C. albicans (Table 1). This result was in line with a study conducted by Rahman et al. (2008) where petroleum ether extract from the stem bark of M. oleifera did not show antifungal activity against C. albicans. The inhibition zone for the standard drug Ketoconazole was 21.0mm for T. mentagraphyte and 15.4 mm for C. albican. In a study done by Lalas et al. (2012) Moringa peregrina seed oil extracted by n-Hexane a low activity to C. albicans was found compared to other microorganisms C. albicans was also found to be the most resistant compared to the tested organism for cold pressed and n-Hexane extracted Moringa peregrina seed oil (Osman et al., 2022). In our study both n-Hexane and petroleum ether extract did not show any activity against C. albicans this might be due to different species of Moringa.
Generally, the variations in the antimicrobial activities of different study reports could be due to differences in Moringa species, environment conditions, extraction methods, extraction solvent used, age and parts of Moringa used.
CONCLUSION
The results of the study revealed that M. stenopetala seed extract has shown the potential to inhibit the activities of T. mentagraphyte fungi even at a lower concentration. The result of the present study is promising as the M. stenopetala seed extract exhibited marked antifungal potential which could be used as an alternative to the fungicide chemical. Further studies need to be conducted with various pathogenic micro-organisms and extraction with more polar extraction solvents such as Carbon tetrachloride, chloroform, ethyl acetate, etc. Identification of compounds that are responsible to inhibit pathogenic microorganisms alsoneeds further investigation.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENT
This research was funded by Arba Minch University.
REFERENCES
|
Abay A, Birhane E, Taddesse T, Hadgu KM (2015). Moringa stenopetala Tree Species Improved Selected Soil Properties and Socio-economic Benefits in Tigray, Northern Ethiopia. Science, Technology and Arts Research Journal 4(2):68-78. |
|
|
Abebe D (2001). The role of medicinal plants in healthcare coverage of Ethiopia, the possible benefits of integration. Conservation and sustainable use of medicinal plants in Ethiopia, Medhin Z, Abebe D (eds.), Addis Abeba (Ethiopia) pp. 6-21. |
|
|
Abuye C, Urga K, Knapp H, Selmar D, Omwega AM, Imungi JK, Winterhalter P (2003). A compositional study of Moringa stenopetala leaves. East African Medical Journal 80(5):247-252. |
|
|
Adnan M, Tariq A, Akhtar B, Ullah R, AbdElsalam NM (2015). Antimicrobial activity of three medicinal plants (Artemisia indica, Medicago falcata and Tecoma stans). African Journal of Traditional, Complementary and Alternative Medicines 12(3):91-96. |
|
|
Anthonia OO (2012). Evaluation of Antimicrobial properties and nutritional potentials of Moringa oleifera Lam. leaf in South-Western Nigeria. Malaysian Journal of Microbiology 8(2):59-67. |
|
|
Bauer AW, Kirby WMM, Sherris JC, Turck M (1996). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology 45:493-496. |
|
|
Bhawasar GC, Guru LV, Chadda AK (1965). Antibacterial activity of some indigenous medicinal plants. Medicine and Surgery 5:11-14. |
|
|
Boyd L, Beveridge EG (1981). Antimicrobial activity of some alkyl esters of gallic acid (3, 4, 5,-trihydroxybenzoic acid) against Escherichia coli NCTC 5933 with particular reference to n-propyl gallate. Microbios 30(120):73-85. PMID: 6272069. |
|
|
Cheesbrough M (2002). Medical Laboratory Manual for Tropical countries ELBS edition. Tropical Health Technology Publications UK. pp. 2-392. |
|
|
Chekesa B, Mekonnen Y (2015). Antibacterial activity of Moringa stenopetala against some human pathogenic bacterial strains. Science, Technology and Arts Research Journal 4(2):190-198. |
|
|
Demeulenaere E (2001). Moringa stenopetala, a subsistence resource in the Konso district. Proceedings of the International Workshop Development Potential for Moringa Products, October 29-November 2, 2001, Dar- Es-Salaam, Tanzania, pp: 2-2. |
|
|
Dinesha BL, Nidoni U, Ramachandra CT, Naik N, Sankalpa KB (2018). Effect of extraction methods on physicochemical, nutritional, antinutritional, antioxidant and antimicrobial activity of Moringa (Moringa oleifera Lam.) seed kernel oil. Journal of Applied and Natural Science 10(1):287-295. |
|
|
Gebregiorgis F, Negesse T, Nurfeta A (2012). Feed intake and utilization in sheep fed graded levels of dried moringa (Moringa stenopetala) leaf as a supplement to Rhodes grass hay. Tropical Animal Health and Production 44(3):511-517. |
|
|
Griffin SG, Markham JL, Leach DN (2000). An agar dilution method for the determination of the minimum inhibitory concentration of essential oils. Journal of Essential Oil Research 12(2):249-255. |
|
|
Haile M, Duguma HT, Chameno G, Kuyu CG (2019). Effects of location and extraction solvent on physico chemical properties of Moringa stenopetala seed oil. Heliyon 5(11):e02781. |
|
|
Hu WZ, Guan YG, Ji YR, Yang XZ (2021). Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Bioscience 44:101435. |
|
|
Prashith K, Raghavendrab HL, Tifsehit S, Dereje D (2016). Antifungal and antiradical potential of Moringa stenopetala (Baker f.) Cufod (Moringaceae), Journal of Bioscience and Agriculture Research 11(1):923-929. |
|
|
Lalas S, Gortzi O, Athanasiadis V, Tsaknis J, Chinou I (2012). Determination of antimicrobial activity and resistance to oxidation of Moringa peregrina seed oil. Molecules: 17(3):2330-2334. |
|
|
Mekonnen Y, Gessesse A (1998). Documentation on the uses of Moringa stenopetala and its possible antileishmanial and antifertility effects. Ethiopian Journal of Science 21(2):287-295. |
|
|
NRC (2001). Moringa. Lost crops of Africa: Vegetables. II. Washington DC, USA: National Academies Press P 266. |
|
|
Peixoto JRO, Silva GC, Costa RA, Vieira GHF, Fonteles FAA, Dos Fernandes VRHS (2011). In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pacific Journal of Tropical Medicine 4(3):201-204. |
|
|
Prabakaran M, Kim SH, Sasireka A, Chandrasekaran M, Chung IM (2018). Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Bioscience 26:23-29. |
|
|
Raghavendra HL, Kekuda PT, Vijayananda BN, Duressa D, Solomon T (2016). Nutritive composition and antimicrobial activity of Moringa stenopetala (Baker f.) Cufod. Journal of Advances in Medical and Pharmaceutical Sciences 10(3):1-9. |
|
|
Rahman MS, Zerin L, Anwar MN (2008). Antibacterial and antifungal activities of Moringa Oleifera stem bark. Chittagong University Journal of Biological Sciences 3(1):109-117. |
|
|
Rani A, Zahirah N, Husain K, Kumolosasi E (2018). Moringa genus: A review of phytochemistry and pharmacology. Frontiers in pharmacology 9:108. |
|
|
Seifu E (2015). Actual and potential applications of Moringa stenopetala, underutilized indigenous vegetable of Southern Ethiopia: a review. International Journal of Agricultural and Food Research 3(4): 8-19. |
|
|
Seleshe S, Kang SN (2019). In Vitro Antimicrobial Activity of Different Solvent Extracts from Moringa stenopetala leaves. Preventive nutrition and food science 24(1):70-74. |
|
|
Shailemo DH, Kwaambwa HM, Kandawa-Schulz M, Msagati TA (2016). Antibacterial activity of Moringa ovalifolia and Moringa oleifera methanol, N-hexane and water seeds and bark extracts against pathogens that are implicated in water borne diseases. Green and Sustainable Chemistry 6(2):72-77. |
|
|
Silva NCC, Fernandes Júnior A (2010). Biological properties of medicinal plants: a review of their antimicrobial activity. Journal of venomous Animals and Toxins including tropical diseases 16(3):402-413. |
|
|
UNIDO (2015). Establishing Moringa based economic development program to improve the livelihood of rural women of Ethiopia. Accessed on September 1, 2022. |
|
|
Walter A, Samuel W, Peter A, Joseph O (2011). Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. African Journal of Microbiology Research 5(2):153-157. |
|
|
World Health Organization (WHO) (2002). Traditional Medicines Strategy 2002-2005. World Health Organization: Geneva. Accessed on September 5, 2022. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0