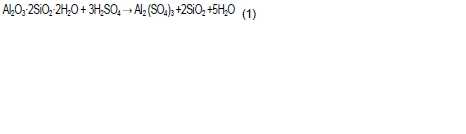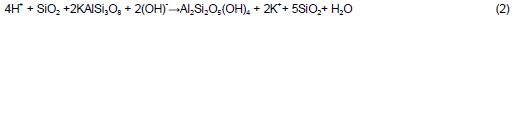ABSTRACT
This study aimed at finding out whether the property of plasticity of acid leached clays can be reversed by treatment with a suitable base. Studies were carried out on representative samples, which were taken from iron bearing clays from Mukurwe-ini, Nyeri County in Kenya (Latitude 00°34´00´´S, Longitude 37°03´00´´E). Characterization of the clay was done in its natural form, and after acid treatment, to determine its mineralogical and chemical composition. Natural clay was refluxed with sulphuric and hydrochloric acids of different concentrations at 100 and 200°C for 2 h followed by thorough washing with distilled water to remove the acid matrix. Atomic absorption spectroscopy, X-ray fluorescence spectroscopy, and X-ray diffraction, analysis techniques were used to determine the physico-chemical characteristics of natural and acid leached clays. The results indicate that SiO2, Al2O3, and Fe2O3 are the major components of Mukurwe-ini clay, MgO, CaO, K2O, TiO2, MnO, and Na2O are present in trace amounts. XRD characterization shows that Mukurwe-ini clays consist primarily of quartz, kaolinite, albite, and microcline minerals. Iron content was drastically reduced in the acid washed samples and X-ray diffraction (XRD) mineralogical analysis of base acid-activated clays showed enhanced levels of the mineral kaolinite in comparison to acid-activated clays (5.3 to 15.7%) a clear indication that the natural properties of the acid washed clay were restored by base treatment of acid washed samples. The Atterberg limits of the base treated samples closely compared with those of the natural clays.
Key words: Clay minerals, Atterberg limits, and plasticity.
Plasticity is the property that makes a body to show changes of its shape without rupture when an external force is exerted on it, so that when the deforming force is removed the acquired shape is maintained (Andrade et al., 2010; Keller, 1979). Plasticity is a very important property of clays in that distinguishes clay from other similar sized colloidal particles. An understanding of the factors, which determine it in soils, will aid in the interpretation of test data, and lead to a better understanding of the performance of clay soils in applications such as in ceramics and engineering. The factors that affect the plasticity of a soil are also likely to affect most of its other properties that are of interest to the engineers (Dumbleton and West, 1966). Plasticity in clays is attributable to a multiplicity of inter-related factors, which in most cases act together, that it is hard to pinpoint at a single factor as the cause of this important ceramic property. However, water of plasticity plays a key role in defining this property. Other factors that do affect clay plasticity include particle size, specific surface area, water characteristics, mineralogical composition, dispersion state of particles, and ceramic body temperature (Andrade et al., 2010). A keen study of structure of clays reveals that these minerals hold water in various forms. Water is confined in the pores in the clay particles by capillarity action. Adsorbed water is held in the interlamella layer of the sheet silicates. Either of these two forms of water is easily lost by drying or heating the clay between 100 to 200°C and it is easily regained under ordinary conditions. Water is also held in form of hydroxyls. Hydroxyl water is driven off from the clay minerals at elevated temperatures between 400 to 700°C, dehydroxylation results in oxidation of Fe2+ to Fe3+ hence the colorization of the clays at elevated temperatures (Stucki et al., 1984a). Chemical and physical properties of clay minerals are integrally linked to how the clay surface interacts with the different forms of water present in the minerals. Examples of such properties include; all of the adsorptive catalytic and cation exchange reactions, shrink-swell phenomena, plasticity, and catalysis (Schoonheydt and Johnson, 2006). Moisture content influences strongly the engineering properties of clays. When clays are subjected to high temperatures, they lose adsorbed and hydration water leading destruction of the clay structures (Gulgun, 2011). Cao et al. (2011) did a study on the effect of low concentration of NH4+ on adsorption of vermiculite from Hebei province, China and found that indeed the ammonium ion is adsorbed on the surface of the minerals. The plasticity index gives an indication of the degree of plasticity shown by clay body and is often be correlated with properties such as specific surface area, dry strength, and rheological behaviour. The plastic limit gives an estimate of the sorptive properties of clays and may be correlated with shrinkage on drying (Bain, 1971). Plasticity measurement is usually evaluated by means of the water of plasticity (Keller, 1979). The tests for Atterberg limits were developed as a means of distinguishing between clays and other soils. The "liquid limit" is the relatively high water content at which the soil changes from a liquid to a plastic state, and the "plastic limit" designates the relatively low water content at which soil changes from a plastic to a solid state (Dumbleton and West, 1966).
The procedures for determining the liquid and plastic limits are well established and are described in detail in publications of the American Society for Testing and Materials and of the British Standards Institutions (British Standard, 1377: Part 2 1990).The difference in water content between the liquid and plastic limits is defined as the "plasticity index" of the soil. High values of plasticity index mean that the clay is more plastic and compressible, hence the greater the shrinkage characteristics of the soil. The plasticity index has proven to be one of the most useful of all soil indices and is essential to the description of a cohesive soil (Andrade et al., 2010).Atterberg’s plasticity index (PI) provides a good aid in the examination of plasticity of ceramic raw materials consisting of clay mixtures and iron oxides.
Presence of inherent impurities has a big impact on the utility of clays. Iron is the fourth-most abundant element in Earth’s crust (6% of mass); next to oxygen, silicon and aluminium, its ubiquitous presence in clays should be no surprise (Stucki, 2006; Atkins et al., 2006). In order to make high quality ceramic products, clays with low shrinkage, good plastic properties and long vitrification range are used; however, large amounts of iron affect these vital properties (Grim, 1979). Structural and colloidal iron causes unwanted colour on clays when they are fired (Stucki et al., 1984b). Reduction of iron impurities is therefore of great importance for the usability of clay in many applications, particularly in ceramic, paper and catalysis industries where purity requirement are specifically high. When iron is confined in structural form, low concentrations are often tolerable. In order to make high quality ceramic products, clay with low iron content, preferably less than 1% is desirable (Karoki, 2009). Chemical-treatment remains one of the methods used to reduce the level of iron in clays. The method however leads to loss of clay’s natural structural properties such as plasticity that would render the treated materials unusable in many ceramic applications. The acid-treated and cation exchanged clays can be simply regarded as solid acids and act as heterogeneous catalysts. Their obvious benefits include low cost, ease of separation, reduced waste generations and environmental friendliness (Igbokwe et al., 2011). Acid-treated have large surface areas and swelling properties suitable for use in solid supports for inorganic reagents such as potassium permanganate, thallium (III) nitrate and both copper (II) and iron (III) nitrates (Yahiaoui et al, 2003). The process involves acid-activation of the clays with strong mineral acids like HCl, H2SO4 and HNO3.The activated material has improved specific surface area and acidity (Vaughan and Pattrick, 1995). The clays used should be free of iron and other metal ions, which may poison the catalysts. Acid–activated clays have been in use in petroleum industry in catalytic cracking of long chained fractions. They are extensively used in edible oil industry to remove offending smells and to decolorize the oils. White clay is used as filler and coating material in the paper industry, kaolinite minerals are used for this purpose (Grim, 1979). Common clays are used in manufacture of hydraulic cements where tetra calcium aluminiumferrite (C4AF) makes up 5-15% of normal Portland cement clinkers (Mohamed and Hesham, 2010). The level of iron in clays used in making Portland cement ranges between 0.5-6.0% of clay materials otherwise; the cement product will have undesired colour after calcination process (Neville, 2012).
In agriculture, activated clay catalysts are used as selective adsorbents of non-ionic organics such as aromatics and chlorinated hydrocarbons (Vaughan and Pattrick, 1995). Clays serve as inert carriers of most pesticide without affecting their potency or activity because of their inert nature. Clays for instance, bentonites make good backfill material for underground nuclear-waste repositories because of their inane ability to self-seal and retain contaminants when they are hydrated (Julia and Lawrence, 2011). Clays are extensively used in modification and improvement of road pavements to enhance viscosity and penetration of the soil surface (Grim, 1979).
Clay minerals hold significant amounts of aluminium in their structures. Clay deposits are now in use as alternative source of aluminium because of depletion of traditionally used alumina deposits (Karoki, 2009). The clay used for aluminium extraction should have high aluminium content; kaolinite clays are popular for this reason. It has already been mentioned elsewhere in this literature review that clays have adsorptive abilities and for this reason they find good use in beauty and pharmaceutical industries because they effectively adsorb toxins on the skin, for example talc is used in pastes, ointments and lotions for skin toning. Kaolinite clays are used to cure gastronomic disorders because they can absorb bacteria and other microorganisms in the stomach and intestinal lining of digestive systems.
The emphasis for future work is on advanced clay-based nanomaterials for use in new approaches to sustainable energy, green environment and human health. There is a shift from traditional use of clay-products in ceramics to nanocomposites with uses in the rapidly growing nanotechnology research on synthetic materials (Zhou and Keeling, 2013). For the current study; it was of interest to find out whether the property of plasticity can be reversed by suitable base treatment of the acid washed clays.
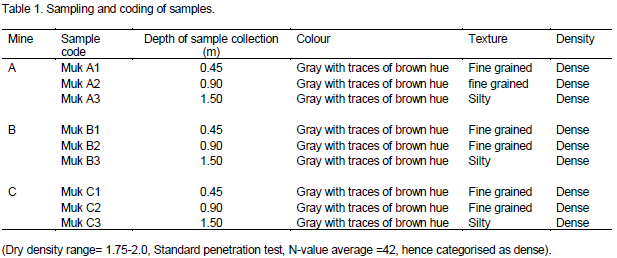
Clay samples used in this work came from a well-known deposit located to the Southeast of Mukurwe-ini sub-County, Nyeri County (Latitude 00°34´00´´S, Longitude 37°03´00´´E), and was collected on site. For each mine, three samples were collected at three depths namely 0.45, 0.9, and 1.5 m. The samples were packed in plastic containers and coded as indicated in the Table 1.
Instrumentation
Atomic absorption and X–ray spectroscopy techniques were used for elemental analysis of the raw and treated clays. Nature of minerals present in raw and treated clays was determined using X-ray diffraction. Calibration of Atomic Absorption Spectrophotometer (AAS) and X-ray fluorescence (XRF) instruments was done using standard rock samples SY-2, MRG-1, and MRG-2procured through Department of Mines and Geology, Ministry of Mining of Kenya.
Sample preparation
Raw clay samples were dried at 105°C in an oven for 6 h, and allowed to cool in desiccators. Samples of the clay so prepared were crushed in a TS 750 Siebtechnik laboratory disc mill to particle size less than 2.00 µm.
Chemical analysis of the clay
Preparation of samples for AAS and XRFS analysis was done using the methods described by Haruna et al. (2007) and Karoki (2009). The raw, acid and base treated samples were subjected to qualitative and quantitative phase analysis, for phase identification and quantification. This was done using a Bruker D2 Phaser. The results were given in form of spectra and the quantities of each mineral present in the sample.
Determination of plasticity index
The constancy limits of the raw and treated clay samples were done using British Standard, 1377: Part 2: 1990).
Treatment of clays with acids
Samples were air-dried and ground to a particle size of 2.00 µm. About 20 g of prepared sample were weighed into a 250 ml Pyrex conical flask and 100 ml of 12 M hydrochloric acid (HCl) added. The resulting slurry was heated at 100°C in a fume chamber, using an electrically heated hot plate for 2 h. Ice-cold distilled water was carefully added to the resulting slurry, which was then filtered using Whatman filter paper No 541 to separate acid matrix from the clay. This filtration was done under gravity. The clay was quenched thoroughly with distilled water. The treatment process was repeated with 10, 8, 6, 4 and 2 M HCl. Treatment was repeated at 200°C. The chemical and mineralogical composition was determined using AAS and XRFS (Onukwuli and Ajemba, 2012). These treatments were repeated with sulphuric (VI), H2SO4 acid.
Treatment of acids-treated clays with bases
Approximately 1000 g of dry clay sample was treated with 5000 ml of 12 M hydrochloric acid and heated to 100°C for two hours in a fume chamber. Ice-cold distilled water was added to the mixture, which was then filtered. The residual washed thoroughly with distilled water, dried in an oven at 105°C for 2 h to drive out any moisture present. The dry residue was then divided into 3 portions. A 300 g of the first portion was treated with 600 ml of 0.5 M ammoniumhydroxide (NH4OH) and allowed to stand for 24 h. The mixture was filtered and residual kept aside for plasticity test and X-ray diffraction analysis. A 300 g of the second portion was treated with same volume of 1.0 M NH4OH and handled as the first portion. The same amount of the third portion was mixed with 600 ml freshly prepared aluminium hydroxide solution (120.7 g of aluminium chloride hexahydrate (AlCl3.6H2O) was dissolved in 1000 ml of distilled water and equal volume of 1 M ammonium hydroxide solution added to precipitate the aluminiumhydroxide, Al(OH)3) and given the same treatment as the other portions. Plasticity of the three residues was then determined using the cone penetrometer method. Composition of the base treated clay samples was done using X-ray diffraction analysis (XRD).
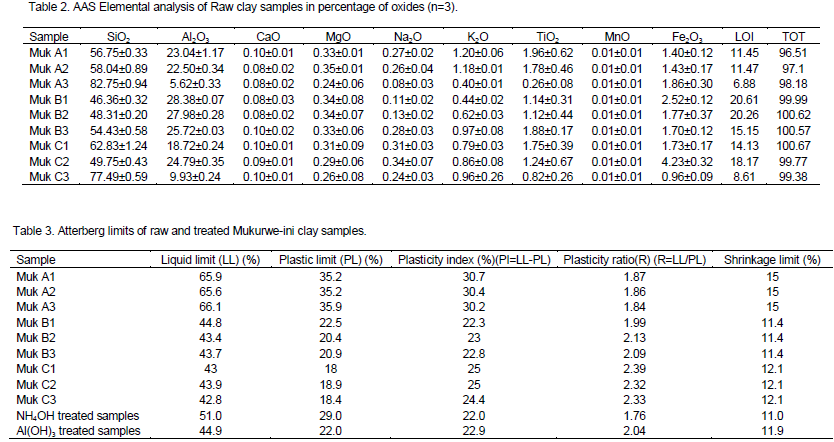
Result of elemental analysis of raw clay samples
The results show that Mukurwe-ini clays contain silica and alumina as major quantities; however, MgO, CaO, Na2O, K2O, and TiO2 are present in trace amounts. Iron is also present in the range 1.4 to 4.2% (Table 2). This clay therefore, cannot be used in manufacture of high-grade ceramic products such as white porcelain, glossy paper, and others where clays with less than 1% iron content is required (Karoki, 2009). Furthermore, the SiO2/ Al2O3 ratio greater than one is suggestive of a clay suitable not for bleaching but for zeolite development (Usman et. al., 2012).
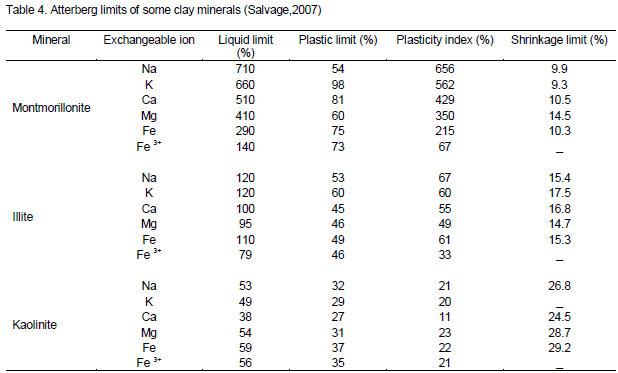
Analysis using Atterberg limits
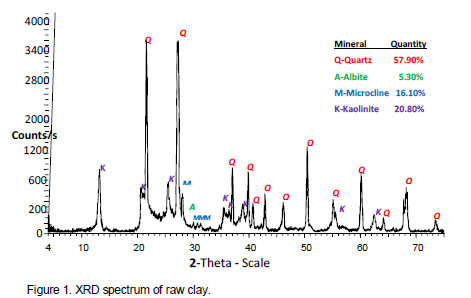
The results of determination of Atterberg limits of raw and base-treated samples shows the clays have medium plasticity (Table 3). Comparison of these results with similar work available in literature (Table 4) places Mukurwe-ini clay among kaolinite group but with isomorphic substitution of Al with Na, Mg, K, Ca, and Fe in the clay structure. Using the results of this study, it was interesting to note that the Atterberg limits of the treated clays were highly comparable to those of natural clays(Table 3). This result confirms that the treatment given to the acid washed clays restored the property of plasticity of the treated materials.
XRD analysis of raw Mukurwe-ini clay
Bulk XRD spectrum analysis shows that the raw clay consists of the minerals quartz, albite, microcline, and kaolinite. Phase quantification of the minerals by XRD gave quartz (57.90%), albite (5.30%), microcline (16.10%), and kaolinite (20.80%). The raw clay exhibited well-defined peaks at 2θ values at 12°, 25°, and 38°, which are typical reflections of the clay mineral kaolinite (Panda et al., 2010) and which correspond to d values of 7.17Å and 3.58Å characteristic of the mineral kaolinite (Harris and White, 2007). The spectrum is illustrated in Figure 1.
Analysis of acid washed clays
AAS Analysis of acid washed samples
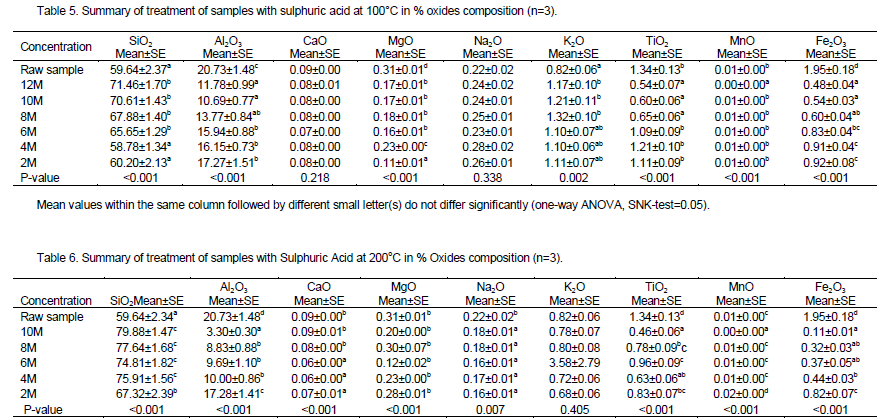
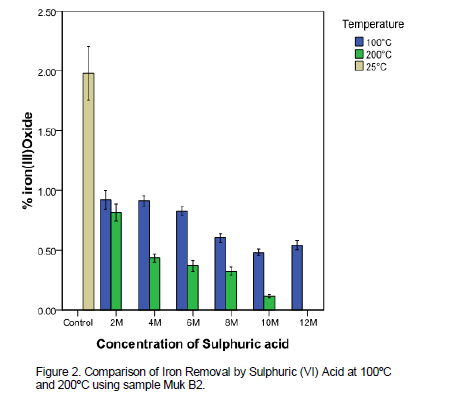
The results of AAS analysis of the acid washed samples are shown in Tables 5 and 6. It was noted that the chemical composition of the clays changed substantially after acid treatment. Figure 2 provides a summary of changes of iron levels after acid treatment. As the concentration of the acid increased, there was marked increase in dissolution of iron as shown by the its reduced levels compared to the raw (control) sample(Figure 2).
After acid treatment, there was a reduction of Al2O3, MgO, CaO, Na2O, Fe2O3, and TiO2, however K2O is retained the acid matrix. Acid attack caused fast exchange of hydrated exchangeable cations with H+, which then attack the structural OH- groups (Martin et al., 2012). Potassium cations may have reacted with amorphous silica in the matrix to produce potassium feldspar, which is resistant to acid attack (Motlagh et al., 2011). Silica content in the acid washed clays increased. This was caused by leaching of Al3+from octahedral layer due acid attack; at higher acid concentrations (above 8 M), there was significant reduction of alumina due severe leaching of the clay structure leading to de-alumination of the clay (Panda et al., 2010). According to Panda et al. (2010), the reaction between kaolinite and sulphuric acid is described by Equation (1).
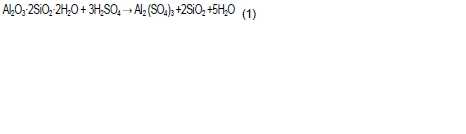
Formation of amorphous silica and dissolution of Al3+as in Equation (1) accounts for the increase of silica content and attendant reduction of alumina in the acid treated samples. Loss of ignition of the clays increased after acid treatment. This is due to an increase of amorphous silica that made water adsorption higher (Panda et al., 2010). Clays subjected to acid dissolution at a higher temperature (Table 6) showed high Si/Al ratios, a sign of extensive destruction of the mineral structures caused by discharge of Al3+ from octahedral layers (Bowanko and Jozefaciuk, 2002).
XRD analysis of acid washed samples
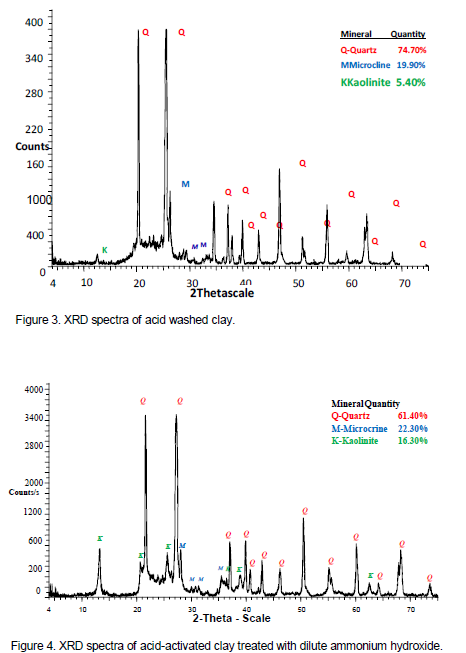
Analysis of the XRD diffractogram indicates dissolution of the minerals kaolinite and albite in the acid, however quartz and microcline are largely unaffected by the acid treatment. Phase quantification after acid treatment was quartz (61.40%), microcline (22.30%), and kaolinite (5.40). Dissolution of the minerals in the acid converted the clay into amorphous silica and as a consequence loss of its plasticity. The retention of the reduced peak means the structure of the clay was only partially affected. The retained peak opened an opportunity of restoring back the lost properties of the acid washed clays (Figure 3). The preserved peak opened an opportunity of restoring back the lost properties of the acid washed clays. The spectra is shown in Figure 3.
XRD results of base treated acid-activated clays
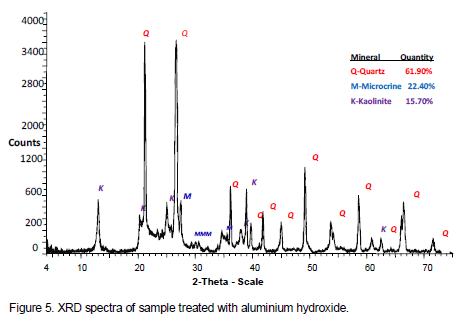
The result of analysis of base treated acid-activated showed restoration of kaolinite reflections at its typical 2? values, though with a slightly reduced intensity. The acid-activated clay treated with ammonium hydroxide had the following phase quantities; quartz (61.70%), microcline (22.30%), and kaolinite (16.30%). The protonated amorphous silica phase reacted with hydroxyl ions of the base to form the mineral kaolinite, hence increase of kaolinite from 5.40% in the acid washed clay to 16.30% in the base acid-activated material (this can be explained by Equation (2)). The diffractograms for acid washed material subjected to ammonium hydroxide and aluminium hydroxide treatment are shown in Figures 4 and 5 respectively.
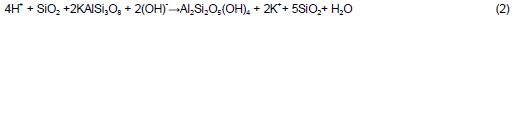
The XRD spectra of raw and phase quantification of raw and base acid-activated materials are comparable in many ways, an indication of reversal of physico-chemical properties of the acid-washed samples by exposure to the chosen bases. This fact was further reinforced by comparison of the Atterberg limits of the raw and treated materials, which bear very strong similarities (Table 3).
This study has established that the major minerals in Mukurwe-ini clay include quartz (57.69%), kaolinite (20.80%), albite (5.30%), and microcline (16.10%), evidently kaolinite is the only clay mineral present in this clay. From the XRD analysis of the clay, sodium and potassium are retained in the clay as the minerals albite (NaAlSi3O8) and microcline (KAlSi3O8) respectively, commonly called the feldspars. Presence of Kaolinite mineral in the clay makes it suitable for use in ceramics. This study has also shown that the quality of the clay is noticeably improved by acid treatment by reducing its iron content to less than 1%, which is ideal for making high-grade ceramics or in other specialized applications such as in paper industry, catalysis, filler material, white cement and many others where clay purity is required. As the only clay mineral present, kaolinite imparted plasticity on the clay, plasticity index was found to range between 22.3 to 30.7% for the raw samples. The base treated acid-activated material had a PI value of 22.0, which is comparable to those of raw samples a clear reversal of plasticity lost during acid thermal treatment. High levels of silica (57.90%) and feldspars (21.40%) means the geological processes of clay minerals formation is still ongoing in the study area, furthermore this is a sign of geologically young residual clays.
The authors have not declared any conflict of interest.
REFERENCES
Andrade A, Dachamir H, Hazim A (2010). Measuring and modeling plasticity of clay. Mater. Res. 13(3):395-399.
Crossref |
|
|
|
Atkins P, Overton T, Rourke J, Weller M, Armstrong F (2006). Shriver & Atkins Inorganic Chemistry (Fourth Edition). New York: Oxford University Press pp. 168, 338, 628. |
|
|
|
Bain A (1971). A plasticity Chart as an aid to the identification and assessment of industrial Clays. Clay and Clay minerals. 9(1):1-17. |
|
|
|
Bowanko G, Jozefaciuk G (2012). Effect of acid and alkali treatments on surface areas and adsorption energies f selected minerals. clay and clay minerals. 50(6):771-783. |
|
|
|
British Standard 1377: Part 2 1990. "Soil testing Mechanics," Laboratory Testing Manual 2000, Central Materials Laboratory, Ministry of Works, The United Republic of Tanzania. |
|
|
|
Cao W, Liao L, Zhohui S, Wang X, Zhiguo X (2011). Adsorption of low concentration Ammonium onto Vermiculite from Hebei Province, China. Clay and Clay Minerals 59(4):446-458. |
|
|
|
Dumbleton MJ, West G (1966). Some factors affecting the relation between the clay minerals in soils and their plasticity. Clay and clay Minerals 6:179-193. |
|
|
|
Gulgun Y (2011). The effect of temperature on characteristics of Kaolin and Bentonite. Sci. Res. Essays 6(9):1928-1939. |
|
|
|
Harris W, White N (2007). Methods of soil analysis. Part 5. Mineralogical methods. SSSA Book Series No.5, Soil Society of America, Madison, USA. 4:36. |
|
|
|
Haruna K, Onoja P, Chiroma M (2007). Characterization of Mayo-Belwa clays. Leonardo Electronic J. Practices Technol. 6(11):123-130. |
|
|
|
Igbokwe K, Olebunne L, Nwakandu S (2011). Effect of Activation Parameters on conversion In Clay-Catalyzed Esterification of Acetic acid. Int. J. Basic appl. Sci. (IJBAS-IJENS) 11(5):1-8. |
|
|
|
Julia NR, Lawrence NW (2011). Hydration behaviour of MX80.bentonite in a confined volume system: Implications for backfill design. Clay and Clay Minerals 59(4):640-653. |
|
|
|
Karoki K (2009). Analysis and treatment of clays from Mwea to assess their value as a source of Aluminium and Ceramic products. (Master of Science Un-Published), Kenyatta, Nairobi, Kenya. pp. 3-31. |
|
|
|
Keller D (1979). Clays. In Kirk, E. and Othmer, F. (Ed), Encyclopedia of Chemical Technology (Third Edition). New York. John Wiley and Sons 6:190-206. |
|
|
|
Mohamed AA, Hesham GI (2010). Variation of Feed Chemical Composition and Its Effect on Clinker Formation–Simulation Process. Proceedings of the World Congress on Engineering and Computer Science 2010 Vol II WCECS 2010, October 20-22, 2010, San Francisco, USA. |
|
|
|
Motlagh KM, Youzbashi AA, Rigi AZ (2011). Effect of acid activation on structural and bleaching properties of a Bentonite. Iranian J. Mat. Sci. Eng. 9(4):50–56. |
|
|
|
Neville AM (2012). Properties of Concrete (Fifth Edition), London, Prentice Hall Chapter 1 Safaribooksonline.com Web ISBN-10:0-273-78633-78634. |
|
|
|
Onukwuli D, Ajemba R (2012). Evaluation of the Effects of acid activation on adsorptive properties of Clay from Ukpor in bleaching Palm oil. Int. J. Multidisciplinary Sci. 3(5):46-52. |
|
|
Panda AK, Mishra BG, Mishra DK, Singh RK (2010). Effect of sulphuric acid treatment on the physic-chemical characteristics of kaolin clay. Colloid and surfaces A: Physiochemical and Engineering Aspects 363(2010):98-104.
Crossref |
|
|
|
Salvage PF (2007). Evaluation of possible swelling potential of soils. Proceedings of the 26th Southern African Transport Conference (SATC, 2007). Pretoria, South Africa pages 277 – 282 ISBN 1-920-01702-x. 9-12 July 2007 online at repository.up.ac.za/bitstream/handle/2263/5947/040.pdf. Accessed 20/6/2013. |
|
|
Schoonheydt R, Johnson T (2006). Surface and Interface Chemistry of Clay Minerals. In Lagaly, G., Theng, K., Bergaya, F., (Ed.) Handbook of Clay Science. Development in Clay Science (Vol.1). Amsterdam: Elsevier Ltd. Chapter 3.
Crossref |
|
|
|
Stucki W, Golden C, Charles R (1984a). Effects of reduction and re-oxidation of structural iron on the surface charge dissolution of Smectites. Clay and Clay minerals. 32(5):350-356. |
|
|
Stucki W, Golden C, Charles R, Philip L (1984b). Effects of oxidation state of octahedral iron on Clay swelling. Clay and Clay Minerals 32(5):357-362.
Crossref |
|
|
Usman MA, Ekwueme KI, Alaje TO, Mohammed AO (2012). Research article, Characterization, acid activation, and bleaching performance of Ibeshe clay, Lagos, Nigeria. International Scholarly Research Network volume 2012, Article ID 658508,5 pages. Doi:10.5402/2012/658508. Accepted 11 January, 2012.
Crossref |
|
|
|
Vaughan J, Patrick D (1995). The Mineralogy Society Series. Mineral Surfaces. London: Chapman Hall. pp. 303-323. |
|
|
Yahiaoui A, Mohammed B, Hachemaoui A (2003). An Acid Exchanged Montmorillonite Clay-Catalyzed Synthesis of Polyepichlorhydrin. Int. J. Mol. Sci. 4:548-561.
Crossref |
|
|
Zhou C, Keeling J (2013). Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Appl. clay sci. 74:3-9.
Crossref |