ABSTRACT
Acha (Digitaria exilis) starch was chemically modified using sodium hypochlorite (3.5% active chlorine) as oxidative agent. The physicochemical properties of the native acha starch and oxidized derivative were investigated. Oxidation improved the water and oil absorption capacity of the native acha starch and also the emulsion capacity was significantly improved. The bulk density, foam capacity and solubility reduced upon oxidation. The granule morphologies investigated using scanning electron microscopy showed significant difference in the ratio of crystalline and amorphous regions. However, oxidation of native acha starch did not affect the shape, appearance and structural arrangement of the starch granules. The granules were polygonal in shape with size range of 6 to 8.57 µm. The infra spectra showed additional band at 3600 cm-1 for the oxidized derivative indicating hydroxyl group stretching vibration of carboxylic acid. This indicates that the oxidation of native acha starch was successfully carried out. Improved physicochemical properties impacted upon oxidative modification is quite desirable in impacting greater stability and less retrogradation and seneresis of the native acha starch. Thus, oxidized acha starch will find suitable applications in food, pharmaceutical, paper and textile industries as good dispersants, emulsifying agent, surface sizing, adhensive, disintegrants, excipients and preparation of biopolymer based flocculants.
Key words: Modified starch, starch oxidation, physicochemical properties, scanning electron microscopy, starch granules.
The genus Digitaria referred to as fonio, family Poaceae, is one of the smallest cereal grains indigenous to most West African countries. It is one of the primary cereals of southern Sudan and Ethiopia. It is classified as millet but unlike other millets, it is low in protein (Jideani and Akingbala, 1993). They are consumed whole or milled into flour and can be processed into a variety of preparations such as gruels, porridges, beverages etc. (Coda et al., 2010).
To obtain oxidized starch, the most common approach is treating the native starch with a variety of oxidizing agents such as alkali metal hypochlorite. Oxidation improves whiteness and reduces microbiological content. In addition, the hydrogen bonding reduces the tendency to retro-gradation producing soft- bodied gels of high clarity.
The oxidation reaction are controlled by many factors such as the amount of alkali metal hypochlorite used, the
pH, the temperature, and the use of metal and/or bromide ions as catalyst. The oxidative reactions lead to the introduction of carboxyl and carbonyl groups, and to the degradation of the starch molecule.
The degradation of the starch molecule during oxidation leads to a lower viscosity of a solution (or dispersion) of the oxidized starch, which is usually desired of an oxidized starch. It has been found that the degradation occurs to a farther extent at neutral pH of about 7 to 7.5 than at alkaline pH such as pH of 9 or higher. In other words, in order to obtain an optimal yield of oxidized starch providing a dispersion of low viscosity, the oxidation reaction should preferably be carried out at neutral pH.
In practice, the pH during oxidation of starch using an alkali metal hypochlorite is chosen at 8.5 or higher, dependent mostly on the desired viscosity of the oxidized starch. Alkali metal hypochlorite often used as oxidizing agent is relatively cheap and large oxidizing power. The reaction temperature at which the starch is treated with an oxidizing agent is preferably chosen between 20 and 50°C.
The duration of alkaline treatment lasts at least 30, more preferably at least 60 min. Oxidized starch is used in the paper industry as coating binders, surface sizing and adhesives. It is also as emulsifying agent, protective colloid for providing desired stability and also to improve weaving operation in the textile industry by improving abrasion resistance of the warp yarn sizing. Arabic gum may be substituted in confectioneries by an oxidized starch to provide excellent stability of the food product, leading to a more clear food product.
Oxidation of acha starch
25 g of starch was dissolved into 100 ml of distilled water and the pH of starch solution adjusted to 10 to 11 with sodium hydroxide solution. The starch slurry was heated to a temperature of 30°C and 20 ml of sodium hypochlorite solution (3.5% active chlorine) added to the starch solution dropwise over a period of 20 min with stirring. During the addition of the reagent and the course of reaction the pH of the slurry was maintained at the desired value with NaOH or HCl solution. The mixture was stirred under the defined conditions above (temperature of 30°C) and then terminated after 2 h by addition of sodium metabisulphite. Filtered and suspended in distilled water and the pH adjusted to 6.5 to 7.0 and oven dried at 50°C.
Determination of physicochemical properties
Solubility
The native starch and modified starch samples (2 g each) were suspended in 20 ml of distilled water. Then heated to 70°C for 30 min with continuous shaking. The mixture was then centrifuged at 4000 rpm for 15 min. An aliquot of supernatant (5 ml) was evaporated at 105°C and weighed. The solubility of starch is the ratio in mass (g) of the dried supernatant to the initial mass (g) of dried starch.
Water and Oil absorption capacity
1 g of native and modified starch was weighed into test tubes. 10 ml of distilled water (and 10 ml of groundnut oil in the second test tube) were added, and then heated in a water bath at 60°C for 30 min. The starch slurry was centrifuged at 1000 rpm for 15 min and the supernatant carefully decanted and the weight of the starch paste taken.
WAC/OAC= weight of starch paste/weight of dry starch sample.
Bulk densities of native and modified starch
2 g each of native Acha starch and the modified starch were placed in a 10 ml measuring cylinder and the volume occupied by the sample without tapping recorded. The bulk density is the ratio of the weight to volume occupied.
The pH of starch
The pH of 1% w/v slurry of both the native starch and modified starch were determined using a pH meter.
Least gelation concentration of starch
8 samples each for native and modified starches (1 to 16% w/v) were prepared in test tubes with 5 ml of distilled water. The starch solutions were mixed using magnetic stirrer for 5 min and heated for 30 min at 80°C in a water bath followed by rapid cooling under running cold water. Further cool at 4°C for 2 h. Least gelation concentration was determined as that conc. when the samples from the inverted test tube did not fall down or slip.
Pasting properties of the starch
The pasting property of native starch and modified sample was carried out using Brookfield viscometer.
Foam capacity of starch
2 g of native Acha starch and each of modified starch were homogenized in 100 ml of distilled water using a magnetic stirrer for 5 min. The homogenate was poured into a 250 ml measuring cylinder and the volume occupied was recorded after 30 s. The foam capacity is expressed as the percent increase in volume.
Emulsion capacity of starch
2 g of native and modified acha starch were dispersed in 25 ml of distilled water using a magnetic stirrer for 30 s. After complete dispersion, 25 ml of vegetable oil (groundnut oil) was added gradually and the mixing continued for another 30 s. Then centrifuged at 1600 rpm for 5 min. The volume of oil separated from the sample was read directly from the tube. Emulsion capacity is the amount of oil emulsified and held per gram of sample.
Starch granules morphology
The starch granule morphology of both the native starch and modified starch were obtained using scanning electron microscopy (sem).
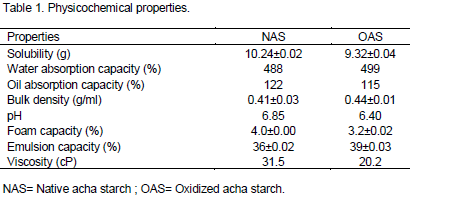
Solubility
The results of solubility of native acha starch (NAS) and oxidized starch (OAS) are shown on Table 1. The solubility expressed as gram per 100 g of starch (g/100 g) reduced from 10.24 value observed with the native acha starch to 9.32 for oxidized starch. The decrease in solubility of oxidized samples is probably due to introductions of bulky functional groups reducing the mobility of starch molecules. This pattern agreed with the results of Olu-owolabi et al. (2014). Thus, modifications altered this physical property (that is, solubility) of native acha starch.
Water absorption capacity
The water absorption capacity of native acha starch and chemically modified sample is presented in Table 1. Oxidation improved water absorption capacity of native acha starch. This may be attributed to the incorporation of carboxyl functional groups on the starch molecules which enhanced binding capacity more than the native starch. The increase in water absorption capacity following oxidation is very important especially in the application of this starch either as a drug carrier or disintegrant in tablets and capsule formulation (Emeje et al., 2012).
Oil absorption capacity
The result of oil absorption capacity of native acha starch and modified sample is presented in Table 1. The values expressed as percentage varied from 122 observed with native acha starch to 115 observed for oxidized sample. This may be attributed to the functional groups incorporated onto the starch molecule following chemical modification. This agrees with the report of Sathe and Salunkhe (1981) that acetylation and oxidation do not improve oil absorption capacity of great northern bean. This increase in starch crystallinity restricted access of oil into the granule of the starch. This is because chemical modification is thought to occur in the amorphous region of the starch molecules leading to increase in starch crystallinity.
pH of starch slurry
The result of pH of starch slurries of native and modified acha starch are presented in Table 1. The pH values reduced from 6.85 to 6.40. A reduction on pH value was observed following modification by oxidation. Reduction in pH values of oxidized samples may be attributed to the incorporation of acetyl functional group to the starch molecule thereby increasing the acidity of starch molecules.
Bulk density
The bulk density of native acha starch and modified sample are presented on Table 1. The bulk density values were in the range of 0.39 to 0.50. A reduction of bulk density was observed with all chemical modifications of the native acha starch. The least bulk density was observed with the acid thinned sample (0.39). The reduction in bulk density might be attributed to increased crystallinity following chemical modification. Increase crystallinity is characteristic of more ordered state and this might impact greater stability on the modified samples. Thus retrogradation of native acha starch as well as seneresis may be improved upon modification. This reduction in bulk density is in agreement with Emeji et al. (2012). This improved physical property following chemical modification of native acha starch is desirable in food and pharmaceutical applications as good dispersant and preparation of biopolymer based flocculants.
Foam capacity
The result of foam capacity of native acha starch and modified samples are presented in Table 1. The foam capacity of oxidized sample was reduced to 3.2% from 4% observed with native acha starch. Reduction in foam capacity following oxidation could find application as an emulsifier in the food industries (Ihegwuagu et al., 2009).
Emulsion capacity
The emulsion capacity of native acha starch and oxidized samples is presented on Table 1. The emulsion capacity increased in the modified samples from 36 to 39%. This suggests that chemically modified acha starch are better emulsifying agent due to the introduction of functional groups in the starch molecules increasing the binding force of the starch granules.
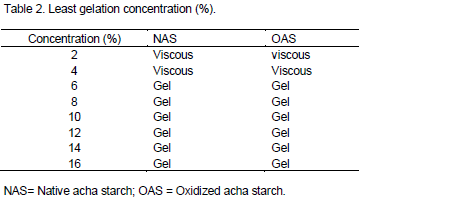
Least gelation concentration
The gelation properties of native acha starch (NAS) and oxidized samples are presented in Table 2. The least gelation concentration remained at 6%.
Starch pasting properties
The pasting properties of NAS and modified samples are presented in Table 1. These are shown as viscosities (Cp) at room temperature using Brookfield Viscometer. The pasting viscosities of modified samples were reduced from 31.5 centipoise for native acha starch to 20.2 centipoise for oxidized sample. The pasting viscosity reduced by about 30% on modification. In such instances where high content of starch is desirable in order for the starch to be able to form a gel as in wine gums and liquorice the pasting property of native starch would be too viscous during heating. In such applications, we can take advantage of oxidized starch. Also in instant soups compositions, thin boiling starches are often used as filler.
Starch granule morphology
Starch is stored in most green plants as minute granules in the leaves, stems, roots, fruits and seeds. Such starch is stored traditionally for the future use of the plant. The granule morphology of NAS and modified samples are shown in Figure 1. These morphologies were investigated using scanning electron microscopy at 15kv accelerating voltage and 2500 magnification each. Though increased crystalline region is observed on modifications, it is obvious that modifications may not have destroyed the shape, appearance and structural arrangements of the starch. The starch granules retain its polygonal shape with sizes ranging from 6 to 8.57 µm.
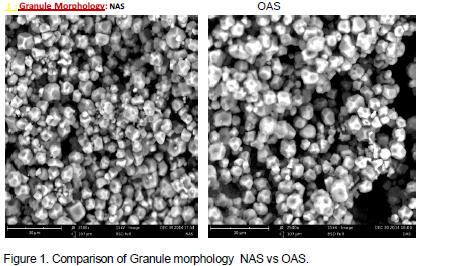
Starch utilization as a food or feed relate to its physical and chemical properties. The solubility of starch starts with swelling in the gel or amorphous region leaving the crystalline structure unaffected. But with higher temperature, the crystalline region melts or dissolves and the entire crystalline structure are destroyed. With increased crystallinity from cross-linked and acid treated modifications, less solubility and improved stability are expected from these modified samples.
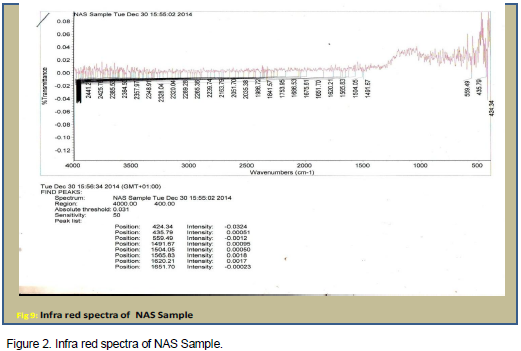
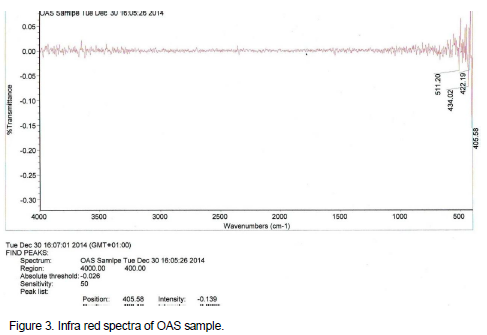
Infra red (IR) spectra
The infra red (IR) spectra of NAS and modified derivative are presented on Figures 2 and 3 respectively. The infra red spectra have similar peaks, except for the additional peak at 3600 cm-1 on Figure 3 which is ascribed to the –OH stretching vibration of the carboxylic acid groups. These bands confirm that modifications of NAS were successfully carried out.
Oxidation of NAS was successfully carried out. The physicochemical properties of NAS and modified derivative were determined. Chemical modifications enhanced emulsion capacity, water absorption capacities but reduced the paste viscosity, oil absorption and foam capacity. Oxidation improved NAS stability and less retrogradation. Potential applications of modified acha starch include good emulsifying agent, starch thickened sauces, soups, paper binding and pharmaceutical drug carriers and disintegrants including paper surface sizing and adhensives.
The authors have not declared any conflict of interest.
REFERENCES
|
Coda R, Cagno RD, Edema MO, Nioeli L, Gobbetti M (2010). Exploitation Of Acha (Digitaria exillis) And Iburu (Digitaria Iburua) Flours: Chemical characterization and their use for sourdough fermentation. Food microbial J. 27:1043-1053. |
|
|
|
Emeje M, Kaita R, Isimi C, Buragohain A, Kunle O, Ofoefule S (2012). Synthesis, physicochemical characterization, and functional properties of an esterified starch. Afr. J. Food Agric. Nutr. Dev. 65:369-374. |
|
|
Ihegwuagu EN, Omojola OM, Emeje MO, Kunle OO (2009). Isolation and evaluation of some physicochemical properties of Parkia Biglobosa starch. Pure Appl. Chem. 81(1):97-104.
Crossref |
|
|
Jideani AI, Akingbala JO (1993). Some physicochemical properties of acha (Digitaria exilis Stapf) and iburu (Digitaria iburua Stapf) grains. J. Sci. Food Agric. 65(4):465-476.
Crossref |
|
|
Olu-Owolabi BI, Olayinka OO, Adegbemile AA, Adebowale KO (2014). Comparison of functional properties between native and chemically modified starches from acha (Digitaria stapf) grains. J. Food Nutr. Sci. 5:222-230
Crossref |
|
|
Sathe SK, Salunkhe DK (1981). Isolation, partial characterization and modification of the great Northern bean (Phaseolus vulgaris L.) Starch. J. Food Sci. 46:617-621.
Crossref |