ABSTRACT
This study was carried out to determine acute oral toxicity and histopathological effects associated with consumption of Euphorbia heterophylla ethanolic extract using 9 to 10 weeks old Albino mice randomized in six groups. The five groups were orally administered with single graded doses of plant extract at 1500, 2000, 2500, 3500 and 4000 mg/kg body weight while the sixth group was administered 1 ml of physiological saline and the animals were observed for toxicity signs and death. Viscera organs were obtained after cervical dislocation for histopathological assessment. The graded extracts induced dose-dependent toxicity signs with major clinical manifestation prior to death including: polyurea, circling, paralysis, thirst, loss of appetite and gait, tachypnea, dehydration and stupor. The major dose- dependent histopathological lesions included: Hemorrhages, congestion, peri-vascular degeneration and necrosis in viscera organs in the groups that received 2000 to 4000 mg/kg body weight. The 24 h median lethal dose was 2831 mg/kg body weight and the 95% confidence interval of median lethal dose was 2490 to 3218 mg/kg body weight and R2 is 0.96 indicating E. heterophylla is of low toxicity. The study demonstrated the toxicity potential associated with uncontrolled use of this plant by the communities. Toxicological studies of sub chronic and chronic toxicity, as well as in vitro mutagenicity and genotoxicity need to be conducted considering the well claimed prolonged use of the plant extract to assess the effect prolonged use on animals.
Key words: Acute oral toxicity, histopathological lessions, Euphorbia heterophylla.
Medicinal plants play a significant role in the livelihoods of rural communities of developing countries especially where health and veterinary services are limited. About 80% of the world population depends partially or whole on medicinal plants for primary healthcare (WHO, 2010). The medicinal plants are used in humans and livestock to control a multiplicity of diseases and conditions. For instance, Euphorbia heterophylla Linn belong to the family Euphorbiaceae, has been used for treatment of several diseases and conditions of man and livestock. Although most members of Euphorbiaceae family are reportedly poisonous, some are of economic and medicinal value (Burkill, 1994). The leaves and stem of Euphorbiaceae species exude a milky sap when injured and contain latex in all parts of the plant (Parsons and Cuthbertson, 1992).
In Uganda, the E. heterophylla is used to treat a number of diseases and conditions like helminthes and constipation in man and livestock in addition to feed the plant to animals like pigs and rabbits (Nalule et al., 2011). In Africa and India, E. heterophylla is used to treat constipation, bacterial and inflammatory disease conditions such, arthritis and rheumatism, migraine and wart cures (Falodun et al., 2008; Anilkumar, 2010; Karimi et al., 2010). The plant lattices are used as fish poison, repulsive, insecticide and ordeal poisons (Rodriguez et al., 1976; Falodun et al., 2003) and medically for fungal infection and gonorrheal treatment (Moshi et al., 2007). The plant is also reported to have diuretic and purgative action in addition to treating asthma by causing bronchial relaxation (Johnson et al., 1999, Edeoga and Okafor, 2005). In 2010, Oluduro and Olumide demonstrated anti-typhoid activity of aqueous and methanolic leaf extracts. According to Edeoga et al. (2005), the plant vegetable and latex are used in insect bites, treatment of erysipelas, cough, bronchial paroxymal asthma by causing bronchial relaxation hay fever and catarrh. E. heterophylla was reported useful in rheumatism, dropsy, gout, neuropathy, deafness and cough (Kirtikar and Basu, 1975). A study by Okoli et al. (2009) revealed alcoholic extract of E. heterophylla, was effective against Streptococcus sp. In Nigeria, the plant extract is used to treat ear pain, and induces milk flow in addition to increasing sperm quality (Okoli et al., 2009). A study of anthelmintic activity of E. heterophylla used by Ugandan pastoralists revealed the plant is very effective and potent (Nalule et al., 2013). In the same study, phytochemical screening revealed presence of tannins, alkaloid, saponins, flavonoids, steroids glycosides, triterpenes, coumarin derivatives, anthocyanocides, anthracenocides and reducing sugars whose intensity varied with solvent used for extraction.
However, despite the several uses of medicinal plants, herbal medicine popularity has raised concerns over the quality and safety with adverse effects resulting from taking herbal medicines having been reported (Chan, 2003; Fennell et al., 2004). For instance, toxicity has been reported in most members of genus Euphorbia where individuals sensitive to latex show strong reactions, including dermatitis and anaphylaxis to the sap exuded by this plant (Wilken and Schempp, 2005). Milkweed has been recorded as toxic to stock (Parsons and Cuthbertson, 1992) despite reports that root bark is used as medicines. There has been a tendency of generalized reports of euphorbia species latex toxicity to animals and humans (Burkill, 1935; Wattman and Breyer-Brandwijk, 1962). Considering the medicinal importance of this plant, the current study was undertaken to determine acute oral toxicity and histopathological effects of single graded doses of ethanolic extract of whole plant over a twenty four hour period.
Plant collection and preparation of plant extract
The plant material was collected from Nakasongola district and identified in Makerere University herbarium where a sample specimen was deposited. The whole aerial plant part material was oven dried and milled into fine powder. Two hundred fifty grams (250 g) was macerated in 2 L of 70% ethanol for 72 h in a dark room with intermittent shaking. Filtration through cotton wool was done to remove coarse particles (residues) and finely through filter paper 12.5 mm (Whatman®, England). This was followed by concentration on Rota-vapor type Buchi-R, Switzerland under reduced pressure at 50°C. The extracts was then put into an oven to dry completely at 50°C and finally packed into universal bottles and kept at 4°C till needed for toxicity studies.
Experimental animals
Thirty six 9-10 week old mice of either sex, of an average body weight of 19.4 g were randomly divided into six animals per group and put in a cage for easy observation. The animals were kept at 24 to 28°C and at a relative humidity of 60 to 70% for one week to acclimatize to experimental laboratory conditions. During this period, the mice were fed on a standard diet and given ordinary tap water ad libitum. After one week of acclimatization, the animals were starved for 18 h but water provided ad libtum prior to administration of extracts. The individual body weights were taken shortly before drug administration to guide dose and drug volume for each animal.
Acute oral toxicity and determination of median lethal dose (LD50)
The studies were carried out according to Good Laboratory Practice (GLP) Regulations of Organization for Economic Cooperation and Development (OECD) (UNDP/World Bank/WHO, 2001). Acute oral toxicity of ethanolic Euphorbia heterophylla extract was determined orally in mice following the procedure used by Ghosh (1984) and Toma et al. (2009). The five animal groups were orally administered with single graded doses of plant ethanolic extract at 1500, 2000, 2500, 3500, and 4000 mg/kg body weight as treatments 1 to 5. The mice in the control group (group 6) were each orally administered 1ml of physiological saline. After applying the treatments, the animals were observed for six hours continuously and thereafter at six hour intervals up to 24 h. The animals were observed for signs of toxicity: motor activity, tremors, convulsions, posture and spasticity, ataxia, righting reflex, lacrymation, salivation, urination, urine output, diarrhoea, sensation, respiratory rates and mortality. The LD50 of the ethanolic extract was determined using Graph pad prism version 5.01 computer programme and also calculated using the arithmetic method of Karber as described by Ghosh (1984) using the formula;
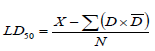
Where, LD
50, is the median effective dose; X, is the least dose that killed all the animals; D, is the dose difference;

, is the mean dose and N, is the number of animals in a group.
Histopathological examination
Collection of organs samples
After the experimental regimen (24 h) post oral administration of the extract, the animals were sacrificed by cervical dislocation under mild chloroform anesthesia and their viscera organs including intestine, liver, brain, lung and kidneys were collected and preserved in 10% formalin immediately after removal from the animal.
Tissue processing for histopathology
The tissue processing was conducted in an automatic processor model Leica TP 1020 in the college of veterinary medicine animal resources and Bio security, Pathology laboratory. The procedures described by Drury and Wallington (1980) and Prophet et al. (1994) were followed with slight modification. The tissues were placed in 10% formalin (10 parts of formalin and 90 parts of distilled water) for 1 h to rectify shrinkage due to high concentration of formalin. The tissues were dehydrated by ascending grades of isopropyl alcohol by immersing in a series of isopropyl alcohol including 70 and 80% for an hour each, then through 90 and 96% for 1.5 h each to avoid shrinkage of cells then in three 100% each for 1½ h each to ensure no traces of water in the cells. The dehydrated tissues were cleared in two changes of xylene for 1½ h each. The tissues were impregnated in two changes of molten paraffin wax for two hours each to provide an internal support. The wax impregnated tissues were embedded in paraffin wax using the same grade wax. The paraffin wax were mounted and cut with rotary microtome machine model Leica RM 2235 at 5µ thickness. The sections were floated on a tissue floatation water bath model Leica HI 1210 at 44°C and taken on plain glass slides. The cut sections were then transferred to an incubator at 53°C for overnight, and thereafter cooled and ready for staining.
Tissue staining
The staining procedures described by Prophet et al. (1994) were followed. The sections were deparaffinised by immersing in two changes of xylene for 2 min each in horizontal staining jar. The deparaffinised sections were washed in two changes of 100% isopropyl alcohol, then in 95% alcohol thereafter dipped in cold water and stained in Meyers hematoxylin for 8 to 12 min in horizontal staining jar. After staining in Meyer’s hematoxylin, the sections were blued in tap water for 10 to 15 min to wash out excess hematoxylin in others structures and make the nuclei distinct. The sections were counter stained with eosin for 5 min (the cytoplasm and other organelles stain varying shades of pink to red and nucleus stain blue). The sections were counter stained in 1% aqueous eosin (1 g in 100 ml tap water) for 5 min and the excess stain was washed in tap water. Complete dehydration of stained sections was ensured by placing the sections in graded alcohols including 95, 100 and 100% for 5 min each. The sections were then mounted in DPX having the optical index of glass (the sections were wetted in xylene and inverted on to the mount and placed on the cover slip). The slides were observed at low power objective under microscope. The cell injury and over aspects were observed under high power dry objective (Dunn, 1974). After mounting the slides were left on table to air dry for microscopic observation and evaluation. The slide imaging was carried out using Carl Zeiss photographic microscope. Model: German-176045 and Canon camera PC 1200. The haematoxylin and Eosin stained slides images were read and the pathological lesions described at magnification x5.
Data analysis
The Graph pad prism computer programme version 5.01, software was used to transform the dataand determinate LD50 and the 95% confidence interval of median lethal dose and the slope.
Toxicity signs in mice dosed with whole plant ethanolic Euphorbia heterophylla crude extract
The animals to which the different doses of ethanolic crude extract of E. heterophylla were administered displayed graded clinical signs whose severity increased with increasing dose before death (Table 1).
Median lethal dose (LD50)
The survival and mortality results of mice dosed with ethanolic extract of E. heterophylla are given in Table 2. The 24 h post treatment LD50 by graph pad prism computer programme was determined to be 2831 mg/kg body weight and the 95% confidence interval of median lethal dose (LD50) was 2490 to 3218 mg/kg body weight and R2 was 0.96 indicating the plant is of low toxicity. Table 2 also provides data used in calculation of median lethal dose using arithmetic method of Karber for comparison. The LD50 of E. heterophylla was 2958 mg/kg when the Karber arithmetic method (Ghosh, 1984) was used. The calculated LD50 still indicate the plant is relatively of low toxicity.
Gross and histopathological findings
The histopathological examination was conducted to determine the damages caused by ethanolic extract to the tissues. At gross examination, the animals in groups treated with 4000 mg/kg of extracts looked dehydrated and weak. There were hemorrhages on the brain meninges and peritoneum, while their liver looked congested. However, the kidneys apparently appeared to be of normal colour and consistence. The organs from the both groups administered 2500 and 4000 mg/kg body weight, showed histopathological lesions or alterations in histology including congestion of blood vessels, hemorrhages, degenerative changes and cell damage (Table 3). The effects obtained from both doses were the same although varied in magnitude with 4000 mg/kg causing severe injuries compared with 2500 mg/kg. However, the kidney, liver, brain, intestine and lungs of mice from the control groups showed normal architecture and normal cells. The stomach and intestines from treated groups revealed severe inflammation of the walls of the stomach and intestine, ulcers, erosive necrosis and sloughing off of mucosa though the basement remained intact. The histopathological representations of light micrograms of intestine, liver, kidney, brain and lungs lesions are illustrated in Figures 1 to 5 respectively.





This study has established that the ethanolic extract of E. heterophylla is slightly toxic with LD50 of 2831 mg/kg according to Matsumura (1975) and Ghosh (1984) classification of toxicity. This is in agreement with the report by Moshi et al. (2007) who also established an LC50 of 80.2 µg/ml using brine shrimp model; and a study by Ologe and Sogbesan (2007) that established an LC50 of 1.8 g/L of the aqueous extract on Barbus occidentalis fingerlings. The difference in the LC50 could be attributed to the source of the plant, maturity, species of the animals on which it is tested and solvent of extraction. Kuppusamy and Murugan (2008) reported an ovicidal and pupicidal activity of ethanolic extract of E. heterophylla on mosquito development stages with LC50 ranging 14.98 to 25.72 ppm. Adedapo et al. (2004) reported macrocytic normochromic anaemia and a total white blood cell count resulting from 14 day’s administration of aqueous fresh extract at 1 g/100 g in rats.
The study further indicated that the ethanolic extract has potential to cause severe clinical signs in the mice (Table 1) and damage to viscera organs exhibiting as severe haemorrhgaes, cell degeneration, ulcerations and necrosis (Figures 1 to 5). The severe haemorrhages observed indicate that animals feeding on the plant or unregulated use of the plant may cause anaemia and reduced immunity. These results agree with the findings of Adedapo et al. (2004) who reported anaemia and reduction in total white cell count. The haemorrhage could be due to a plant lectin, phytohaemagglutinins reported by Nsimba-Lubaki et al. (1983). The observed damages in the viscera organs of animals is agreement with earlier observations that euphorbia species induces inflammation on mucous membranes in animals (Karimi et al., 2010). The clinical manifestations observed in the mice may be linked to presence of polyphenols such as tannins and triterpenes such as saponins present in the plant (Nalule et al., 2013). Damage to the brain and kidney could have caused excessive urination probably due to the effect on the anti-diuretic hormone (ADH). Alldredge (1993) and Kongvongxay et al. (2011) attributed reduced feed intake in animals to tannin containing diets that have strong astringent property and cause induction of internal malaise in mammals, which may contribute to reduce feed intake.
Haemorrhages, cell infiltration and cell degeneration present in all organs assess could be attributed to presence saponins and tannins which damage cells. A plant lectin (phyto-haemagglutinins) has been isolated from this plant (Nsimba-Lubaki et al., 1983). The authors reported that E. heterophylla agglutinins agglutinate the human and animals erythrocytes in a non blood group specific. The observed mucosal erosion and ulceration (Figure 1) in the stomach and intestine of the test mice administered high doses are consistent with a website report that a post-mortem examination of people killed by Euphorbia latex have severe inflammation of the walls of the stomach and intestine while in some patients, the wall of the stomach are perforated (http://www.the amateur.digest.com?epoisons.htm, accessed April, 2011).
The excitement and increased urination observed in the mice could be due to the damage caused to the brain and kidney that were characterized by severe haemorrhages and cell degeneration. Thirst was observed in all animals and it could also be associated with the excessive urination that directs to the diuretic effect of the plant extract on the anti-diuretic hormone. The diuretic activity observed in this study agrees with report by Johnson et al. (1999) who reported that euphorbia species have diuretic activity. Similar observations were reported by community who said that humans taking the aqueous plant extract need to drink a lot of water due to the excessive thirst the extract cause to the patient (Nalule et al., 2011).
The histopathological lesions in the viscera organs suggest the level of toxicity and the affected body systems targeted by the E. heterophylla extract. Beside these perilous effects, Euphorbia species have numerous therapeutic beneficial effects including anti-inflammatory and purgative, antioxidant and hepato-protective activity (Jyothi et al., 2008). These effects may be attributed to the rich phytochemical composition present in this plant which include but not limited to alkalloids saponins, glycocides, flavonoids, tannins (Falodun et al., 2006; Karimi et al., 2010). The many secondary metabolites are said to have a positive or negative effect on animals and
as parasites/disease causing agent.
Tannins and anthraquinones are said to have both proxidant and antioxidant effects on the animal. While antioxidant act as an anti-inflammatory (Ogueke et al., 2007; Anilkumar, 2010; Karimi et al., 2010) and protect body, the proxidants cause damage to animal tissues and organs. The tannins and saponins are responsible for the antibacterial activity of the plant extracts (Gloor, 1997). Saponins have been used in veterinary vaccines such foot-and-mouth disease vaccines as adjuvant and helping to enhance immune response. The presence of anthraquinones in the extracts could be contributing to the damage observed. Huang et al. (1992) reported that anthraquinones produce free radicals and hydrogen peroxide during its oxidation to semiquinone in the body that damages the body cells. The terpen ester composition in the plant determines how caustic and irritating to body tissues such as the skin and mucous membranes (Karimi et al., 2010). Karimi et al. (2010) reported the sap from euphorbia species cause keratoconjuctivities and that the latex (milky sap) of spurges act as deterrent for the herbiovores as well as wound healer. A study by Adedapo et al. (2004) showed slight reduction in packed cell volume (PCV) level of the test mice but a significant decrease in the levels of red blood cells (RBC) and haemoglobin concentration, indicating that the leaves of this plant could also cause anaemia in animals.
Flavonoids are well known for ant-oxidant activity and in this study they may have acted to reduce some of the inflammatory reactions in the animal. Flavonoids have also been reported to show anti-inflammatory, anti-allergic, antimicrobical and anticancer activity (Balch and Balch, 2000). The nitrogen containing alkaloids are physiologically active with sedative and analgesic properties and are reported to relieve pains, anxiety and depression (Jisika et al., 1992). However, alkaloids are toxic due to their stimulatory effects that lead to excitation of cells and neurological dysfunction (Ekam and Ebong, 2007; Malu et al., 2009).
The excitatory behavour is consistent with previous findings of Adedapo et al. (2004) who reported that the aqueous crude extracts of E. heterophylla, caused an excitatory effect followed by dullness in addition to anaemia and leucocytosis. These effects could be explained by the severe haemorrhage that was observed in all internal organs in the current study. Adedapo et al (2004) study also reported increase in the level of albumin, ALT and AST. Although the E. heterophylla lower doses, caused no observable effects, the hemorrhages caused indicate that prolonged consumption of this plant could also cause anaemia in animals that feed on it like the pigs and rabbits. Gross and histopathological findings, which included, increased urine output, hemorrhages, depression, cellular degenerative loss of appetite among others, were indicative of poisoning by this plant that were also consistent with findings of Adedapo et al. (2004).
The significant change in the architecture of the viscera organs observed in this study is a clear indication of toxicity potential associated with this plant. Adedapo et al. (2004) reported increase in the levels of aspartate aminotransferase (AST) and alanine associated with the liver damage observed in this study. It therefore evident that with continuous administration of this plant extracts to animal, the principal function of vital organs would be compromised with consequent failures of a number of body systems. The toxicity of the plant, especially of the root and latex, was also recognized in East Africa (Burkill, 1985).
CONCLUSIONS AND RECOMMENDATIONS
It should be concluded that although E. heterophylla possess medicinal benefits to humans and their livestock, this study has shown that using the extract at a high dose can cause fatal vital organs’ damage. It is imperative to note that continuous exposure of livestock to these plants may lead to morbidity and/or mortality leading to reduced livestock productivity. Caution should therefore be exercised in the use of E. heterophylla for medicinal purposes and feed. Patients, whether human or animal taking the extract should equally be availed with enough fluids due to severe loss of fluids. Understanding the toxic principle and its mechanism of action would greatly guide on the protection of the patients. Toxicological studies of sub chronic and chronic toxicity, as well as in vitro mutagenicity and genotoxicity need to conduct considering the well claimed prolonged use of the plant extract to assess the effect prolonged use on animals.
The authors have not declared any conflict of interests.
REFERENCES
|
Adedapo AA, Abatan MO, Olorunsogo OO (2004). Toxic effects of some plants in the genus Euphorbia on haematological and biochemical parameters of rats. Vet. Arhiv. 74(1):53-62.
|
|
|
|
Alldredge J (1993). The effect of condensed tannins on browsers and grazers: Quantitative and Qualitative defense? Colorado State University, Fort Collins. Colorado P 7.
|
|
|
|
|
Anilkumar M (2010). Ethnomedicinal plants as anti-inflammatory and analgesic agents. In: Chattopadhyay D (ed). Ethnomedicine: A Source of Complementary Therapeutics, pp. 267-293.
|
|
|
|
|
Burkill HM (1994). The useful plants of West Tropical Africa. Royal Botanical Garden, Ken. 2:12-150.
|
|
|
|
|
Burkill IH (1935). A dictionary of the economic products of the Malay Peninsula. Crown Agents for the Colonies, London.
|
|
|
|
|
Chan K (2003). Some aspects of toxic contaminants in herbal medicines. Chemosphere 52(9):1361-1371.
Crossref
|
|
|
|
|
Drury RAB, Wallington EA (1980). Carleton's histological techniques. 5th Ed. Oxford University press. Oxford, New York, Toronto pp. 188-189, 237-240, 290-291.
|
|
|
|
|
Dunn WL (1974). Hand book of histopathological and histochemical techniques. 3rd Ed. Redwood, Burn, Ltd., Trowbridge and Esher.
|
|
|
|
|
Edeoga HO, Okwu DE, Mbaebie BO (2005). Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 4(7): 685-688.
Crossref
|
|
|
|
|
Ekam VS, Ebong PE (2007). Serum protein and enzyme levels in rats following administration of antioxidant vitamins during caffeinated and non-caffeinated paracetamol induced hepatotoxicity. Niger. J. Physiol. Sci. 22(1-2):65-68
|
|
|
|
|
Falodun A, Sajjad A, Irfan MQ, Iqbal MIC (2008). Phytochemical and biological investigation of chloroform and ethylacetate fractions of Euphorbia heterophylla leaf (Euphorbiaceae). J. Med. Plants Res. 2(12): 365-369.
|
|
|
|
|
Falodun A, Agbakwuru EOP, Ukoh GC (2003). Antibacterial Activity of Euphorbia heterophylla Linn (Family Euphorbiaceae). Pak. J. Biol. Sci. Res. 46(6):471- 472.
|
|
|
|
|
Falodun A, Okunrobo LO, Uzoamaka N (2006). Phytochemical screening and anti-inflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae). Afr. J. Biotech. 5:529-531.
|
|
|
|
|
Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, Grace OM, van Staden J (2004). Review Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J. Ethnopharmacol. 94:205-217.
Crossref
|
|
|
|
|
Ghosh MN (1984). Fundamentals of experimental pharmacology. 2nd edition.
|
|
|
|
|
Gloor SM (1997). Relevance of Na+-K+-ATPase to local extracellular potassium homeostasis and modulation of synaptic transmission. Federation of European Biochemical Societies Letter, 412: 1-4.
Crossref
|
|
|
|
|
Huang KC, Chang JH, Tung SF, Wu RT, Foegh ML (1992). Immunosupressive effect of emodin a free radical generator. Euro. J. Pharm. 211:359-364.
Crossref
|
|
|
|
|
Johnson P, Abduraham EM, Tiam EA, Abduaguye I, Hussaini IN (1999). E. hirta extracts increase urine output and electrolytes in rats. J. Ethnopharmacol. 65:63-69.
Crossref
|
|
|
|
|
Jyothi TM, Prabhu K, Jayachandran E, Lakshminarasu S, Ramachandra SS (2008). Hepatoprotective and antioxidant activity of Euphorbia antiquorum. Pharmacogn. Mag. 4:127-133.
|
|
|
|
|
Karimi I, Yousefi J, Ghashghaei A (2010). Ocular Toxicity Caused by Euphorbia Sap: A Case Report. Iranian of Pharmacol. Therapeut. 9(1): 37-39.
|
|
|
|
|
Kirtikar KR, Basu BD (1975). Indian medicinal plants. M/s Bishen singh Mahendrapal singh, Dehardun. 3:2204-2216.
|
|
|
|
|
Kongvongxay S, Preston TR, Leng RA, Khang DN (2011). Effect of a tannin-rich foliage (Mimosa pigra) on feed intake, digestibility, N retention and methane production in goats fed a basal diet of Muntingia calabura. Livestock Research for Rural Development. Volume 23.
|
|
|
|
|
Kuppusamy C, Muugan K (2008). Mosquitocidal effect of Euphorbia heterophylla Linn. Against the Bancroftian filariasis vector, Culex quinguefasciatus say (Diptera: Culicidae). Inter. J. Integr. Biol. 4(1):34.
|
|
|
|
|
Malu SP, Obochi GO, Edem CA, Nyong BE (2009). Effect of methods of extraction on phytochemical constituents and antibacterial properties of tetracarpidium conophorum seeds. Glob. J. Pure Appl. Sci. 15(3):373-376.
|
|
|
|
|
Matsumura F (1975). Toxicology of Insecticides 2nd Edition Plenum Press N.Y. pp. 4-6.
Crossref
|
|
|
|
|
Moshi MJ, van den Beukel CJP, Hamza OJM, Mbwambo ZH, Nondo ROS, Masimba PJ, Matee MIN, Kapingu MC, Mikx F, Verweij PE, van der Ven AJAM (2007). Brine shrimp toxicity evaluation of some potential and phytochemical composition of ethanolic and water crude extracts of Euphorbia heterophylla Linn. J. Med. Plants Res. 7(43):3202-3210.
|
|
|
|
|
Tanzanian plants used traditionally for the treatment of fungal infections. Afr. J. Tradit. Complement Altern . Med. 4(2):219-225.
|
|
|
|
|
Nalule AS, Mbaria JM, Kimenju JW (2013). In vitro anthelmintic Nalule AS, Mbaria JM, Olila D, Kimenju JW (2011). Ethnopharmacological practices in management of livestock helminthes by pastoral communities in the drylands of Uganda. Livestock Res. Rural Dev. 23 p.
|
|
|
|
|
Nsimba-Lubaki M, Peumans WJ, Carlier AR (1983). Isolation and partial characterization of a lectin from Euphorbia heterophylla seeds. Biochem. J. 215(1):141-145.
Crossref
|
|
|
|
|
Ogueke CC, Ogbulie JN, Okoli IC, Anyanwu BN (2007). Activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta antibacterial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta. Am. J. Sci. 3:11-16.
|
|
|
|
|
Okoli RI, Turay AA, Mensah JK, Aigbe AO (2009). Phytochemical and Antimicrobial Properties of Four Herbs from Edo State, Nigeria. Report and Opinion 1(5):67-73.
|
|
|
|
|
Ologe IA, Sogbesan OA (2007). Pscicidal potential of dried E. heterophylla (L.) stem water extract on Barbus occidentalis (Pisce: Cyprinidae) Boulenger 1920) fingerings. Res. J. Environ. Toxicol. 1(4):191-197.
Crossref
|
|
|
|
|
Oluduro A, Olumide O (2010). In vitro antibacterial potential and synergistic effect of south western Nigerian plant parts used in folkore remedy against salmonella typhi infection. Nature and Science 8:9.
|
|
|
|
|
Parsons W, Cuthbertson E (1992). Noxious Weeds of Australia. pp. 420-422.
|
|
|
|
|
Prophet EB, Mills B, Arrington JB, Sobin LHMD (Eds) (1994). Laboratory methods in Histotechnology. Armed Forces Institute of Pathology. Published by the American Registry of Pathology Washington, D.C. ISBN: 1-881041-00-X
|
|
|
|
|
Rodriguez E, Twers GHN, Mitchell JC (1976). Biological activities of sesquiterpene Lactones. Phytochemistry, 15:1573.
Crossref
|
|
|
|
|
Toma I, Karumi Y, Geidam MA (2009). Phytochemical screening and toxicity studies of the aqueous extract of the pods pulp of Cassia sieberiana DC. (Cassia Kotchiyana Oliv.). Afr. J. Pure Appl. Chem. 3(2):26-30.
|
|
|
|
|
UNDP/World Bank/WHO (2001). Introduction of the OECD principles of GLP. Special Programme for Research and Training in Tropical Diseases (TDR)-Good Laboratory Practice Training Manual for the Trainee. pp. 3-19.
|
|
|
|
|
Wattman JM, Breyer-Brandiwijk MG (1962). Medicinal and Poisonous Plants of Southern and Eastern Africa. E and Livingstone Ltd 2nd Edition, London: E and S Livingstone; pp. 554-555.
|
|
|
|
|
Wilken K, Schempp CM (2005). Toxic phytodermatitis caused by Euphorbia helioscopia L. (sun spurge) Hautarzt.56 (10): 955-957.
Crossref
|
|
|
|
|
World Health Organisation (WHO) (2010). Climate change and human health. Retrieved December 2, 2010 from
View
|
|