ABSTRACT
The liberation of propranolol HCl from a controlled release matrix, containing the hydrophilic polymer, sodium carboxymethylcellulose (NaCMC) and the hydrophobic polymer, Eudragit RL 100 (RL 100) as excipients, was studied. The influences of surface active agents on the dissolution rate of the drug were examined. Tablets were made by direct compression methods. The dissolution tests were performed by using the basket method. The incorporation of the cationic surfactants within the matrices increased the drug release until the critical micelle concentration (CMC). While, after the CMC, the increase in drug release was to a lesser extent. The incorporation of the anionic surfactants reduced the release rate of the drug from the matrices. At the CMC, the percent of drug release from the matrices were approximately the same with the matrices without the surfactants. While an increase in drug release was observed above the CMC of the anionic surfactants. The data obtained from in vitro drug release studies were plotted according to three kinetic models to study the release kinetic. These were zero order release, the first order release and the Higuchi equation. Zero order release of the drug was observed in all the formulations. The release mechanism was influenced considerably by the ratio of the excipients.
Key words: Propranolol HCl, controlled release, critical micelle concentration (CMC), dissolution, dissolution rate kinetics, surfactant.
It has been established that excipients can influence or even alter considerably, the release rate of a drug from solid dosage forms. Matrices have been commonly used to enhance drugs dosage forms and consequently manage the drug release by embedding different types of matrices: hydrophobic matrix (Dredan et al., 1998), hydrophilic matrix (Nokhodchi et al., 1999), or a combination of both (Efentakis et al., 1990). The applied
method in the preparation of these matrices is the direct compression technique, which is rapid, cheap and needs less time, personnel and equipments. The effect of various surfactants on the release rate behavior of drug from ethylcellulose based matrices was discussed previously (Bolourtchian et al., 2005).
A controlled release matrix with propranolol hydrochloride as a model drug with a hydrophilic polymer, NaCMC and the hydrophobic polymer, Eudragit RL100 as excipients were prepared and investigated (Al-Hmoud, 2000a; Al-Hmoud, et al., 2014a, b). The effect of surfactants on the dissolution rate of drug from controlled release matrices has also been discussed in several studies (Efentakis et al., 1992; Nokhodki et al., 2002, 2008; Bolourtchian et al., 2005). The results of these studies varied, with many of the surfactants causing an increase in the dissolution of the drug, while others demonstrated a decrease, with some having no effect. This was dependent on several factors such as, their interaction with the other components of the matrix, surfactant charge and solubility, surfactant concentration, their wettability effect and their CMC value.
The purpose of this study was to investigate the effect of several surface active agents on the in vitro drug release above and below their CMC when incorporated in a solid matrix consisting of mixtures of Eudragit RL100 and NaCMC and the propranolole HCl as a model drug. In addition, this study compared the effect of different surfactants at the same concentrations and changes on the drug dissolution when they are incorporated within the matrices at different ratios. Also, how the solubility of each surfactant affects its CMC value is discussed.
The models used to describe the release mechanism of drug release and the kinetic assessment of the release data for all the formulations used in the study are:
A. The zero order release which describes the dissolution of the drug of many controlled release dosage forms such as, matrices, coated forms, and osmotic devices (Nrashimhan et al., 1999).
B. The first order release kinetic used in the dissolution of the pharmaceutical dosage forms which contain water soluble drugs that are released from the pores of the matrices (Nrashimhan et al., 1999).
C. Higuchi model can be used to describe the drug dissolution from the types of modified release pharmaceutical dosage forms such as transdermal and matrix tablets (Shoaib et al., 2006).
For the preparation of the heterogeneous matrices, the following materials were employed: Propranolol HCl was provided by the Arab Pharmaceutical Manufacturing- Jordan (APM), sodium lauryl sulfate and magnesium stearate were purchased from BDH, cetrimide was purchased from Serva, Eudragit RL100 was provided by Rohm Pharma, sodium carboxymethyl cellulose was purchased from FMC, and sodium taurocholate and cetylpyridinium chloride were purchased from Fluka. All chemicals were of reagent grade.
Preparation of tablets
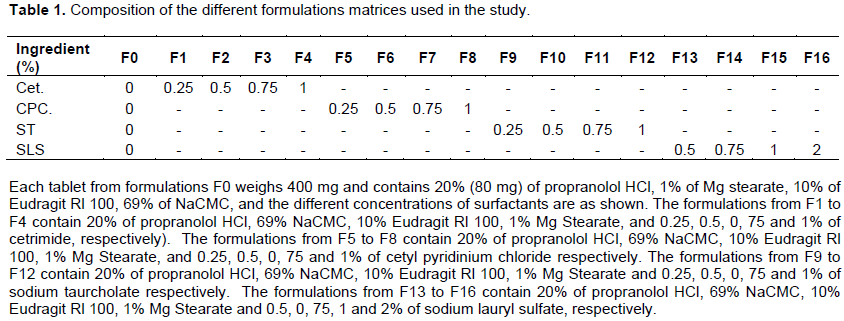
The acrylic resin Eudragit RL100 was powdered in a Ball mil and sieved through a 300-μm sieve and further blended with propranolol HCl and the other additives for 5 min in a blender. The different formulations prepared, containing various amounts of surfactants and their proportions, are shown in Table 1. The powder mixture was compressed to prepare tablets of 400 mg using the direct compression technique, an instrumental single punch tableting machine, (Korch-Erweka). The diameter and the thickness of the cylindrical tablets were 1 and 0.4 cm, respectively.
Tablet hardness
The hardness level of the tablets was about 9 kg and a schleuniger – 2 hardness tester was employed for its determination. A previous study indicated that the hardness of the tablets has no effect on the release rate of the drug (Al- Hmoud, 2002).
In vitro dissolution test
The United States pharmacopoeia (USP) basket method (Erweka, DT 6R, Heusenstamm, Germany) was used for all the in vitro dissolution studies. The test was performed at 37 ± 0.1°C with a rotation speed of 50 rpm using 900 ml of 0.1 N HCl, pH 1.2, as a dissolution medium.
Previous studies revealed that the buffer dissolution medium of a pH ≈ 7.4 increased the release rate of the drug, due to the eroding effect of this medium on the swollen matrices of NaCMC (Bavega et al., 1987; Al-Hmoud et al., 1991). In addition, it was observed from previous studies that an increase in the stirring speed increased the release rate of the drug due to the increase of agitation of the dissolution medium (Al-Hmoud et al., 1991).
Assay
Samples of 5 ml were withdrawn each hour; from the dissolution medium and replaced immediately with an equal volume of the respective dissolution medium maintained at 37 ± 0.1°C. Test samples were filtered through 0.45 μm filter, and assayed spectrophotometrically at 289 nm using a blank solution as a reference with a UV-Vis double-beam spectrophotometer (Systronic 2202). The mean of three determinations was used to calculate the drug release rate from each of the formulations (Al-Hmoud et al., 2014).
Assessment of dissolution data
The release data were assessed for the kinetics of release and dissolution using a suitable computer program
Measurement of the surface tension
The critical micelle concentrations of the surfactants used in the study were measured previously, (Al-Hmoud et al., 2014). These values were approximately as follows: cetrimide 0.5%, cetylpyridiniumchloride 0.75%, sodium taurcholate 0.5% and sodium lauryl sulphate 0.75% by using the DuNouy tensiometer (Kruss).
Kinetic and mechanism of drug release analysis
The data obtained from the in vitro drug release studies were plotted according to two kinetic models to study the release kinetics. The zero order release model (equation 1), describes the concentration independent of drug release rate from the formulation, cumulative amount of drug release plotted versus time (Figures 1 and 2).
C = k˳t (1)
Where ko is the zero-order release constant expressed as units of concentration/time and t is the time in hours. The Higuchi's model (Equation 2) describes the release of drug based on Fickian diffusion as a square root of time-dependent process from swellable insoluble matrix. Cumulative percentage of drug released plotted versus square root of time is shown in Figures 3 and 4.
Q = kᵸ t½ (2)
Where, kᵸ is the constant of Higuchi.
Different concentrations of four surfactants are incorporated in the matrix tablets F0 (Propranolol/RL100/NaCMC/MgO), prepared previously (Al-hmoud, 2002) to investigate the effect that these surfactants on drug release above and below their CMC.
In the current study, four surfactants were used, two cationic: cetrimide (Cet) and cetylpyridinium chloride (CP), and two anionic: sodium lauryl sulfate (SLS) and sodium tauorcholate (ST). The concentrations of these surfactants were above and below the CMC.
The results revealed that, the incorporation of the cationic surfactants within the formulation increased the
release rate of the drug (Figures 1 and 3). The increase was proportional to the incorporated amount of the surfactant until the CMC of the surfactant was reached. The results also revealed that above the CMC, the increase of drug release was in a lesser extent despite doubling of the surfactant concentration within the formula F0. This might be due to 1- The formation of a stagnant layer around the tablets in the dissolution medium. Formula F2 shows that the incorporation of 0.5% (CMC of cetrimide) increased the release of propranolol HCl by 28%, while, doubling the concentration to 1% (formula F4) shows that the percent of drug release increased by 37% only (Figure 1). The formula F7 shows that the incorporation of 0.75% (CMC of CPC) increased the release of propranolol HCl by 26%, while in formula F8, when the concentration is 1%, the percent of drug release increased by 34% only (Figure 1). 2- The decrease in the wetting effect of the surfactant due to the relaxation of the hydrophilic polymer of the matrix which formed a net of gel that captured some of the surfactant inside (Al- Hmoud et al., 2014; Shargel and Yu, 1999).
Comparison of drug release from the matrices when embedding in 0.5% cetrimide and 0.5% cetylpyridinium chloride shows that the increase with cetrimide is 28%, while with cetylpyridinium chloride, it is 18%.
This was studied previously by Nokhodki et al. (2008). This increase could be attributed to the differences in solubility of the two surfactants, which caused wide pores within the matrices and more drug release in the dissolution medium (Effentakis et al., 1991a; Effentakis, 1992b).
Moreover, the CMC of the more soluble surfactant is lower than that of the less soluble one, which may be due to the early formation of micelles. These results were observed in both the cationic and anionic surfactants.
The effect of the anionic surfactants show decrease in propranolol HCl release rate from the matrices until the CMC of the surfactant was reached. Following that, an increase in drug release was observed once the surfactant concentration was above the CMC. This increase in drug release may have been due to the formation of wide pores within the swollen matrices as a result of the high solubility of the surfactants. These pores permit the release of a large quantity of drug in the dissolution medium. It may have also been due to the formation of soluble micelles, which may facilitate the diffusion of the drug from the tablets into the dissolution medium.
The incorporation of the anionic surfactants SLS and ST within the matrices at concentrations below the CMC showed a decrease in the release rate of the drug (Figures 2 and 4), this decrease is 11% by incorporation of 0.25% of ST and about 25% by the incorporation of0.5% SLS. This decrease could be attributed to the formation of weak complexes between the cationic propranolol HCl and the anionic surfactants, and these complexes might form tortuous channels within the tablets (Wells and Parrott, 1992). Above CMC, the results were not the same, and increase in the drug release rate was observed (Figure 2). This increase is about 20% with the incorporation of 1% ST (F11), and 12.5% with the incorporation of 1% SLS. This increase may be due to more than one reason.
1. The high concentration of the very soluble surfactant ST within the tablets (2 g of ST dissolved in 1 ml of water), and the freely soluble SLS in which 1 g was dissolved in 10 ml of water. These surfactants may facilitate the access of the dissolution medium to the formed pores within the tablets, lowering the contact angle of water on the tablets and increase their wettability, which lead to an increase in the release rate of the drug (Effentakis et al., 1991).
2. Formation of soluble micelles with a clear phase around the tablets, which increases the release rate of the drug (Al- Hmoud et al., 2014).
The kinetic assessments of the release data for all the formulations (from F0 to F16) with the different surfactant concentrations, and the estimated values of the correlation coefficient (r²) (Table 2) appears to fit all the models used in the kinetic assessment of the release analysis. All these values (r²) were of 0.980 and more which suggests that the release rate of drug was according to zero order kinetics in the specified time.
The results of the study revealed that the cationic surfactant showed an increase in the release rate of the proprnolol HCl below and above the CMC of the surfactant but in different ratios. The percent of increase below the CMC was more than that above the CMC; these little increase may be due to the formation of a stagnant layer of solution around the tablets in the dissolution medium, which retard the drug diffusion from the matrix.
The anionic surfactants caused decrease of the drug release below the CMC of the surfactant. While, an increase of drug release was observed above the CMC of the surfactant, which might be due to the wide pores which formed within the swollen matrices as a result of the high solubility of the surfactants.
These pores permit the liberation of the drug in the dissolution medium; also, the formation of soluble micelles around the tablets may facilitate more dissolution and drug diffusion from the swellable tablets to the dissolution medium.
The author have not declared any conflict of interests.
REFERENCES
|
Al-Hmoud H, Ibrahim M, El-Hallous E (2014a). Surfactant solubility, concentration and the other formulation effects on the drug release rate from a controlled- release matrix. Afr. J. Pharm. Pharmacol. 8(13):364-371.
|
|
|
|
Al-Hmoud H, Ehab IE, Nasser EI Attia OA, Eldessoky SD (2014b). Formulation of propranolol HCl controlled release tablets: effect of surfactant charge and mechanism of drug release. Afr. J. Pharm. Pharmacol. 8(43):1110-1117.
|
|
|
|
Al-Hmoud H (2002). Preparation of controlled release tablet propranolol Hydrochlorid using Eudragit RL100 and other excipients Dirasat. Med. Biol. Sci. 29:1-2.
|
|
|
|
Al-Hmoud H, Efentakis M, Choulis NH (1991). A controlled release matrix using a mixture of hydrophilic and hydrophobic polymers. Int. J. Pharm. 68(1):R1-R3.
Crossref
|
|
|
|
Bavega SK, Ranga KV, Puri PK (1987). Zero order release hydrophilic matrix tablets of áµ- adrenergicblockers. Int. J. Pharm. 3939-3945.
|
|
|
|
Bolourtchian N, Farrin SJ, Simin D (2005). The effect of various surfactants on the release rate Behavior of prcainamide HCl from ehylcellulose based matrices. Iranian J. Pharm. Res. 13-19.
|
|
|
|
Dredan J, Zelko R, Bihari E, Racz I, Gondfar E (1998). Effect of polysorbates on drug release from wax matrices. Drug Dev. Ind. Pharm. 24(6):573-576.
Crossref
|
|
|
|
Efentakis M, Al-Hmoud H, Buckton G, Rajan Z (1991). The influence of surfactants on drug release from a hydrophobic matrix. Int. J. Pharm. 70(1):153-158.
Crossref
|
|
|
|
Efentakis M, Al-hmoud H, Choulis NH (1990). Effect of additives on Fluorbiprofen controlled release preparation. Acta. Pharm. Technol. 36(4):237-239.
|
|
|
|
Efentakis M, Buckton G, Al-Hmoud H (1992). The effect of surfactant charge on drug release from acrylic matrices. STP Pharm. Sci. 2(4):332-336.
|
|
|
|
Shargel L, Yu ABC (1999). Biopharmaceutic considerations in drug product design. Applied biopharmaceutics & pharmacokinetics., 4th ed., Appleton and Lange, Stamford, Connecticut. pp. 129-167.
|
|
|
|
Nokhodchi A, Khalseh P, ghaforian T, Siahi-shadbad MR (1999). The role of surfactants and fillers in controlling the release rate of theophyllin from HPMC matrices. STP Pharm. Sci. 9(6):555-560.
|
|
|
|
Nokhodki A, Norouzi-Sani S, Siahi- Shadbad MR, Lotfipoor F, Saeedi M (2002). The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC- Eudragit) matrices. Eur. J. Pharm. Biopharm. 54(3):349-356.
Crossref
|
|
|
|
Nokhodki A, Daoud HZ, Farnaz MZ, Nita TZ (2008). The effect of various surfactants and their concentrations on controlled release of captopril from polymeric matrices. Acta Pharm. 58(2):151-162.
Crossref
|
|
|
|
Wells ML, Parrott EL (1992). Effect of surfactants on release of highly water-soluble medicinal compound from an inert, heterogeneous matrix. J. Pharm. Sci. 81:453-457.
Crossref
|