ABSTRACT
The aim of this study was to enhance the classical adjuvant functionality of maize starch (Mst) by co-processing with small quantities of carbopol 974P (CaPol). A pre-processed maize starch (MpS) was coated with different concentrations of CaPol corresponding to 0.25, 0.5, 0.75 and 1.0 %w/w and spray-dried at controlled conditions to produce the CaPol-coated motifs (MpS-CaPol). The relevant physicochemical and functional properties of the new CaPol-coated starch, as a direct compression and rapid disintegrating adjuvant were evaluated in the preparation of acetyl salicylic acid (ASA) tablets. The yield of MpS-CaPol produced with the various mix were between 87 to 95% of the weight of the start ingredients. There were no remarkable differences in the relevant physicochemical and functional properties determined for Mst and MpS thus, the results of MpS were not presented. There were no remarkable morphological differences between the particles of Mst and those of the coated motifs. The variants of the new starch motif showed properties that were sensitive to the concentrations of CaPol used in the coating. All the variants of MpS-CaPol generally showed comparative enhanced flow and moisture uptake. The swelling capacity of the new excipients containing different concentrations of CaPol can be ranked thus: 1 > 0.75 > 0.5 > 0.25 > 0% (Mst). The ASA tablets showed relative increased tensile strength and dissolution as the concentrations of CaPol increased (0 to 0.75%). The disintegration time were remarkably modified: The tablets prepared with new excipients containing CaPol of concentrations 0.25 to 0.75 %w/w all disintegrated rapidly within 1 min while that containing 1% w/w disintegrated at 45 ± 0.5 min; ASA tablets without disintegrant did not disintegrate even after 120 min. This study has shown that spray-dried Mst granules coated with small quantities of CaPol produced an excipient with new basic physicochemical properties that enhanced its adjuvant functionality when used for direct compression of rapid release ASA tablets.
Key words: Maize starch, carbopol, film-coating, spray-drying, acetyl salicylic acid tablets.
Pharmaceutical adjuvants are excipients which play a variety of important roles in drug formulation, controlling such properties as mechanical strength, dissolution, bioavailability, efficacy, toxicity and elegance (Ansel et al, 2005). Considering the rapidly expanding field of pharmaceutical technology, the need for excipients with innovative physicochemical and functional properties becomes necessary to aid in the delivery of especially challenging molecules, develop cheaper drug brands and novel drug delivery systems. Novel excipients such as those with multifunctional applications ultimately help reduce production cost by reducing the number of production steps and excipients that will be required for production (Moreton 1996; Tobyn et al 1998; Koo and Squibb, 2011). To produce new excipients, a variety of techniques have been explored, these include complex synthesis of new polymer types using primary monomers and the modification of existing ones to alter or confer new properties on the native materials (Builders et al. 2013a). Co-processing of two or more existing excipients or with other known substances is a popular method of modification of existing materials. This technique has been used to generate new excipients with new functional properties. Important examples include the co-processing of microcrystalline cellulose with other compounds such a silicon dioxide and other polymers (Zhou et al. 2012; Moreton, 2000)
Products such as silicified microcrystaline cellulose is prepared by coating silicon dioxide on the microcrystaline cellulose grains (Moreton, 2000; Rojas and Kumar 2012)
Film coating is a simple process that imparts many advantages to products. Properties such as elegance, dissolution, mechanical strength, protection from moisture, light and other physical factors as well as creating strong recognizable brands are some benefits of film coating operation (Zaid et al. 2013).
Starch is a versatile biopolymer that is widely used in pharmaceutical manufacture especially in solid-oral dosage formulations. It is used in many conventional formulations as binder, diluents and disintegrant (Builders et al., 2013b; Manek et al. 2012). Some good attributes that make starch attractive for drug delivery applications include biocompatibility, biodegradability, low cost, availability and relative easy modification potentials. Though starches from different botanical sources are available, maize starch (Mst) is however, the most commonly used in conventional tablet production (Manek, 2012; Itiola, 1991; Odeku et al, 2005). Starch from different botanical sources has been modified by different techniques to enhance their intrinsic physicochemical and functional properties, thereby expanding their applications (Hauschild and Picker 2004). Etherification, esterification, cross-linking and oxidation are some common techniques used for modifying starches (Odeku, 2013).
The coating of the discrete granules of Mst with carbopol is expected to enhance the functional properties of Mst thereby expanding its applications as a pharmaceutical excipient especially in conventional tablets production.
Carbopol is a group of synthetic polyacrylic polymers that show high swelling and variable viscosities. There are different proprietary brands of carbopols which vary basically in their viscosity/swelling due to varying degrees of crosslinking. The common functional brands include carbopol® 974P NF (CaPol), Carbopol® 971P NF, Carbopol® 71G NF, etc. This group of polymers has demonstrated good performance in oral tablets and capsules production. At high concentrations (3 to 30 %w/w), they retard dissolution of the drug by ensuring the slow release of the active pharmaceutical ingredients when used as matrix retardant; and also enable targeted delivery of drugs to be achieved (Hauschild and Picker, 2004; Cevher et al., 2008). At lower concentrations (0.5 to 3%), carbopol is applied as binder for granule formulation to improve the hardness and friability of conventional rapid release tablets at low compression forces. The use of CaPol to coat Mst implies a novel application to its numerous uses. ASA is only slightly soluble in water and directly compressible, forming hard, non-disintegrating compacts (Luprizol, 2011). ASA was chosen as the prototype drug in this study, as one of the most appropriate to evaluate the tablet properties of the new multifunctional excipient. The objective of this study was to produce a novel multifunctional excipient by coating maize starch granules with carbopol films by the spray drying process.
All the chemicals and reagents used were of analytical grade and they are used as supplied: Mst, ethanol, acetaminophen (Sigma-Aldrich Chemie, Germany), carbopol® 974P (Fluka Biochemica, Ireland); magnesium stearate, sodium chloride, magnesium chloride and potassium dihydrogen phosphate (May & Baker, Dagenham, England); toluene, sodium hydroxide, potassium thiocyanate, potassium chloride and calcium chloride (BDH Chemicals, UK). Acetyl salicylic acid was kindly supplied by Juhel Pharmaceuticals Nig. Limited, Enugu, Nigeria.
Preparation of carbopol 974P film-coated maize starch granules
The film-coated maize starch was prepared by spray-drying dispersions of pre-processed maize starch (MpS) and CaPol using a Buchi Mini Spray Dryer B290 (Buchi Labortechnik AG, Flawil, Switzerland). MpS was prepared by maintaining 200 g of Mst at 60°C in 400 ml of 0.1 N HCl for 30 min with continuous stirring. The starch dispersion was allowed to cool to 27°C (room temperature), sediment and the supernatant decanted. The starch sediment was washed with five different portions of 200 ml distilled water and then dried in a hot air oven at 50°C for 1 h. Dispersions of CaPol corresponding to 0.25, 0.5, 0.75 and 1.0 %w/w of the Mst (used in pre-processing) was prepared in 100 ml of distilled water and appropriate amounts of the MpS were then added with continuous mixing using a magnetic stirrer bar. The resultant dispersion was then spray-dried with a Buchi Mini Spray Dryer B191 (Buchi Labortechnik AG, Flawil, Switzerland): The inlet and outlet air temperatures were 150 and 70°C, respectively and gas pump pressure was 30%. The dry CaPol film-coated particles (MpS-CaPol) were collected and stored in an airtight container.
Characterization of the spray-dried film-coated starch particles
Scanning electron microscopy
The scanning electron micrographs (SEMs) were used to study the morphology of the Mst granules before and after co-processing with CaPol (Zhao and Augsburger, 2005). The samples were prepared by gold-plating, while imaging was carried out on a scanning electron microscope (FEI Quanta 400, FEI Company, Oregun, USA).
Differential scanning calorimetry (DSC) analysis
The thermal properties of Mst, CaPol and MpS-CaPol were studied using a DSC (DSC 204 F1, Phoenix NETZSCH, Germany) equipped with a thermal analysis system. The instrument was calibrated using indium (156.88°C) as the internal standard and dry nitrogen was used as the purge gas (20 ml/min). About 7 mg of the desiccated samples of Mst, CaPol and MpS-CaPol were weighed into the DSC aluminum pan and covered with a perforated lid. The probes were heated within a temperature range of 25 to 400°C at a rate of 10°C/min. The different thermal transitions of the polymers within this temperature range were evaluated using the DSC Proteus analytical software (Builders et al. 2010; Builders et al. 2009)
Flow properties
The flow properties of the Mst and the coated motifs (MpS-CaPol) were determined by the indirect methods: Carr’s compressibility index and angle of repose analysis (Yamashiro et al, 1983). For the Carr’s compressibility index, the bulk and tapped volumes of the modified starch motifs were determined. 50.0 g of the Mst and modified motifs were poured into a 200 ml graduated measuring cylinder. The volume occupied before (V) and after tapping (Vo) with a Stampfvolumeter (STAV 2003 JEF, Germany) were used to evaluate the Carr’s compressibility index according to the following equation (Aulton, 2003). This test was repeated three times for each sample.
Carr’s index = [1- V⁄Vo] ×100 (1)
Angle of repose: Samples of Mst and MpS-CaPol were each carefully poured through a glass funnel clamped to a retort stand, with its tip 2 cm above a graph paper placed on a flat horizontal surface such that the apex of the cone formed by the powder just reached the tip of the funnel. 30 g quantities of each of the powders were allowed to flow through the funnel, under the force of gravity to form a conical heap. The heights (h), of the powder cone and the mean diameter (D), of the base of the powder cones were obtained and applied to obtain the angle of repose (Ø), according to the following equation (Yamashiro et. al. 1983). This test was repeated three times for each sample.
Tan Ø= 2h/D (2)
Moisture uptake
Quantities of Mst and MpS-CaPol motifs were placed in a Petri dish and stored in an activated desiccating chamber at room temperature (corresponding to 27°C) for 48 h to remove residual moisture from the materials. The moisture sorption isotherms of the microparticles were determined by the gravimetric method (Builders et al, 2010). One gram of each dry polymer powder was placed in an aluminum foil and put in a desiccator with a gauze holding tray containing either distilled water or saturated solution of the listed salts to provide the required relative humidity (RH) (water = 100%, potassium chloride = 84%, sodium chloride = 75%, potassium thiocyanate = 47% and calcium chloride = 31%, Lithium chloride = 15%). The powders were weighed at 12 h intervals until equilibrium was attained for each sample [equilibrium was considered to be attained when increase in weight is less than 0.1 g]. This test was repeated three times for each sample.
The equilibrium moisture sorption (EMS) was determined using the equation:
EMS = Me/Md = 100% (3)
Where, Me is the amount of moisture uptake at equilibrium and Md is the dry weight of sample (Builders et al. 2009). The profile of percentage weight gain vs. RH was then evaluated for each material.
Swelling capacity
The tapped volume (V1) occupied by 1 g of each of the powders in a 10 ml measuring cylinder was determined before 2 ml of distilled water was introduced to effect wetting, after which the volume was made up to the 10 ml mark with more water. The measuring cylinder was then allowed to stand for 24 h before the volume of the sediment (V2) corresponding to the volume of the hydrated or swollen material was measured. The swelling capacity (S) was then computed according to the equation (Aulton, 2003).
S = (V2 - V1)/V1 × 100 . . . . (4).
Formulation and characterization of ASA tablets using MpS-CaPol
Preparation of tablets
Tablets containing 200 mg of pure ASA each in combination with 100 mg of Mst or MpS-CaPol motifs were prepared by direct compression. The compacts were prepared by thoroughly mixing the ASA and the excipient (Mst or MpS-CaPol motifs) in a tumbler mixer (JEL, KARL KOLB, Germany) and compressing with a load of 21.25 KN using a single press tableting machine (Shanghai Tiaxiang & Chentai Pharmaceutical Machinery Co. Ltd.) fitted with an 11.5 mm flat-faced punch and die. The tablets were then collected and stored in an air-tight container for 24 h before evaluation tests.
Tensile strength
The tensile strength of each tablet sample was evaluated by first determining the crushing strength using a hardness tester (Erweka ZT2, Germany). The thickness and diameter were also determined using an electronic caliper (Mitutoyo, Japan). The tensile strength was then calculated using the equation:
T = 2 F /π d H (5)
Where, F (Nm-2) is the crushing strength, d and H are the diameter and thickness, respectively (Moreton, 2010). The mean of six tablets was evaluated for each sample.
Weight variation test
The weight variation of each tablet sample was evaluated using the USP method. Twenty tablets from each batch were randomly selected and the individual weight of each tablet was determined using an analytical balance (Mettler Toledo AB54, Switzerland). The average weights were determined. Not more than two of the individual weights should deviate from the average weight by more than ± 5% (The British Pharmacopoeia Commission, 1993).
Tablet friability
The weight of ten ASA tablets (W1) selected at random from each batch were determined before placing on the friabilator (Erweka TAR, Germany) and operated for 4 min at 25 rpm. The tablets were then de-dusted and re-weighed (W2). The percentage friability was determined according to the following equation (Moreton, 2000).
Friability (%) = [(W1 - W2)/W1] ×100 (6)
Disintegration time
The British Pharmacopeia method was used to assess the disintegration properties of the ASA tablets using an Erweka ZT4 (Erweka ZT4, Germany) disintegration apparatus (United States Pharmacopeia, 2003). Distilled water maintained at 37 ± 1°C was used as the disintegration medium. The time taken for each tablet to break up and pass through the mesh screen was considered as the disintegration time.
Dissolution testing
The dissolution profile of the ASA tablets was carried out following the USP method (The British Pharmacopoeia Commission, 1993). The dissolution apparatus (8ST, Caleva Ltd., UK) containing 500 ml of medium was set to a rotation speed of 50 rpm. The medium was a 0.05 M acetate buffer solution (pH 4.5 ± 0.5) maintained at 37 ± 2°C. At regular 10 min intervals, 5 ml samples were removed from the medium and replaced with equal amount of acetate buffer solution maintained at 37 ± 2°C. The drug content of the sample was determined using a spectrophotometer (UV-160A, Shimadzu, Japan) set at a λmax of 265 nm2.
Data analysis
All the measurements/tests were done in triplicates and mean values calculated. The data obtained were analysized by analysis of variance at p < 0.05 level of significance using Excel Microsoft software 2003 version and results expressed as mean ± SD.
Preparation of film-coated starch particles by spray-drying
Film-coating has been used to improve the surface and functional properties of a substrate material by adsorbing a thin film of the coating material on the substrate’s surface (Bruening and Dotzauer, 2009). Co-processing of certain conventional excipients by spray drying, results in the film-coating of the substrate material by adsorbing a thin film of the coating material on the substrate’s surface thereby improving its surface characteristics and other functional properties. Co-processing of cellulose with amorphous silicon dioxide and cassava starch with colloidal silica has resulted in seemly novel product with enhanced physicochemical and functional properties of the substrate material (Missagh and Fassihi 2004; Abdullah and Geldart, 1999; Ebenehi et al. 2013).
Spray-drying the mixture of the colloidal dispersions of CaPol and the modified starch grains was aimed at adsorbing films of the former on the latter. This is expected to modify the physicochemical properties of the starch and impart new functional properties thus, expanding its applications as a pharmaceutical excipient. Coating of the starch granules with small amounts of CaPol constitutes a simple physical modification that has the potential to alter certain important physicochemical and functional properties of Mst as a pharmaceutical excipient. The average yield of the coated starch granules prepared with the different concentrations of CaPol was between 87 and 95% of the weight of the ingredients. This result is an indication of a high throughput and efficient process when precautions are taken to ensure that the collecting cup and transfer joints of the spray drier are well sealed. Important precautions in this process are the setting to an appropriate inlet and outlet temperatures as well as the prevention of escape of the spray-dried particles through the product collector. There were only insignificant differences (p < 0.05) in the yield of the particles obtained with the different concentrations of CaPol. Thus, variation in the concentrations of CaPol dispersions used for the coating did not show any remarkable effect on the yield of the co-processed materials.
Characterization of film-coated Mst particles
Scanning electron microscopy (SEM)
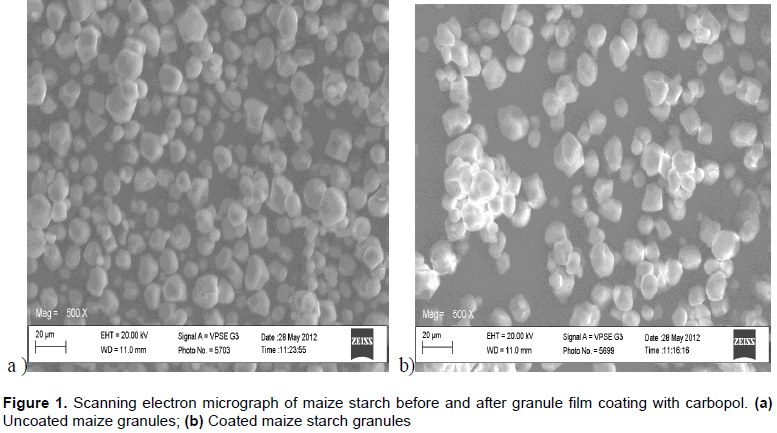
The morphological characteristics of Mst and the MpS-CaPol granules are presented in Figure 1. The SEM images of the Mst and MpS-CaPol showed no basic differences in their morphological features as charac-terized by their round and oval shapes, inter-particulate variability and relative lack of asperity that is the usual characteristic of Mst granules. The motifs produced by coating with the different concentrations of CaPol showed slight increases in size and higher incidence of apparently aggregated clumps of granules. The samples also showed higher incidence of aggregation with increasing concentrations of CaPol. The MpS-CaPol granules showed higher particle sizes compared to Mst which may be explained by way of either one or a combination of these factors: Layering of the CaPol film on the starch granules or swelling of the starch granules during the pre-treatment and moist heating during the spray-drying process.
Differential scanning calorimetry (DSC) analysis
The DSC thermographs of MpS-CaPol, Mst and CaPol are presented as Lines 1, 2 and 3, respectively in Figure 2. The hybrid material, MpS-CaPol showed transitions that are similar to those of Mst and CaPol. The thermographs of Mst, CaPol and MpS-CaPol are characterized by an initial endothermic and exothermic transition curves. However, the various transitions were different for each material. The endothermic curve corresponds to the glass transition (Tg) which is typical of the amorphous portion while the exothermic represents the melting of the materials, a feature which is characteristic of the crystalline portion of the polymers. The thermograph of Mst (Figure 2) is typical of starch being characterized by an endothermic transition and a twin melting peak (Luprizol, 2011). The melting peaks are characteristic of the melting of the amylose and the amylopectin components of the starch. The demarcation in the twin melting in transitions of Mst was less pronounced when compared to that of MpS-CaPol, a feature that may be attributed to the acid pre-treatment of Mst resulting in the removal of part of the amylose portions of the Mst that corresponds to the amorphous portion. The thermograph of CaPol is also typical of a pseudo amorphous polymer as shown by its characteristic glass transition curve and a melting peak at 85.5 and 310°C, respectively (Missagh and Fassihi, 2004). The Tg of MpS-CaPol was lower than that of Mst but higher than the one of CaPol. The onset of melting of MpS-CaPol did not show remarkable difference from that of Mst as compared to that of CaPol. The peak melting temperature of MpS-CaPol showed a shift to higher value compared to those of Mst and CaPol, while the end of this transition was lower than that of Mst but higher than that of CaPol (Table 1). MpS-CaPol thus showed a thermograph that is characteristic. This result thus shows the obvious modification in the thermal properties of Mst when co-processed with small quantities of CaPol.

Particle density and flow properties
The bulk and tapped densities of Mst and the modified motifs are presented in Table 2. While the tapped density of a given powder sample is definite there is however, no unique bulk density for a given powder. This is because the bulk density of a powder changes tremendously depending on the way particles are packed. A powder with a strong structural strength will resist collapse when dispersed in a container and will have a low bulk density, while a structurally weak powder will collapse easily and have a high bulk density 28. High friction between the particles results in a low apparent bulk density. With decreasing friction, the bulk density increases. The bulk densities of the MpS-CaPol motifs were lower than that of Mst which might be related to the technical processing of the polymers by spray-drying of the polymer mixture, resulting in a higher powder bulk volume. The relative higher bulk volumes of the spray-dried powders may also be the reason for their increased porosity (Fazaeli et al. 2012). The bulk and tapped densities also showed sensitivity to the changes in the concentration of CaPol co-processed with Mst (Table 2). The decreasing density due to increasing amount of CaPol might also be related to increased particle aggregation due to adhesive properties of CaPol such that increased consolidation resulted in the particle fines cascading into the inter-particulate spaces between the large particles. The flow characteristics of bulk powders, especially those intended for use as excipients for direct compression application, is important. The flow characteristics of bulk powders and granules can be determined indirectly by manipulating the bulk and tapped densities using certain tested mathematical equations (Aulton, 2003).

The flow characteristics of Mst and the CaPol-coated motifs, as determined by the indirect methods of angle of repose and Carr’s compressibility indices, showed similar results that are corroborative. The results of the flow properties of Mst and the MpS-CaPol assessed by the angle of repose and Carr’s compressibility index are presented in Table 2. The powder flow property as evaluated by this method is based on the inter-particulate cohesion of the powder. As a general guide, angle of repose less than 15° corresponds to excellent flow and less than 25° is good flow, whereas higher values up to 50° is poor (Aulton, 2003).
By assessing flow properties with Carr’s compressibility indices, values below 15% correspond to excellent flow, below 16% is good while values above 25% is poor. The flow quality of the Mst and the motifs produced by coating with different concentrations of CaPol showed similar characteristics when evaluated by Carr’s compressibility index and angle of repose. The motifs showed excellent flow while Mst showed poor flow. The improved flow properties must have been partly imparted by the coating and spray-drying processes.
Swelling capacity
Swelling is one of the fundamental properties of starch. Starch essentially swells in hot water but minimally in cold water (Abdullah and Geldart, 1999; Ebenehi et al., 2013; Rojas and Kumar 2012; Fazaeli et al. 2012; Rawlings, 1982). Though swelling is not the principal mechanism by which starch functions as a disintegrant nevertheless, when cold water is added to it, the granules absorb some quantities of water. The wicking of water and the elastic recovery that is initiated by the water ingress as well as the slight swelling of the starch granules in cold water are among the factors responsible for the classical use of Mst as a disintegrant (Srichuwong et al. 2005).
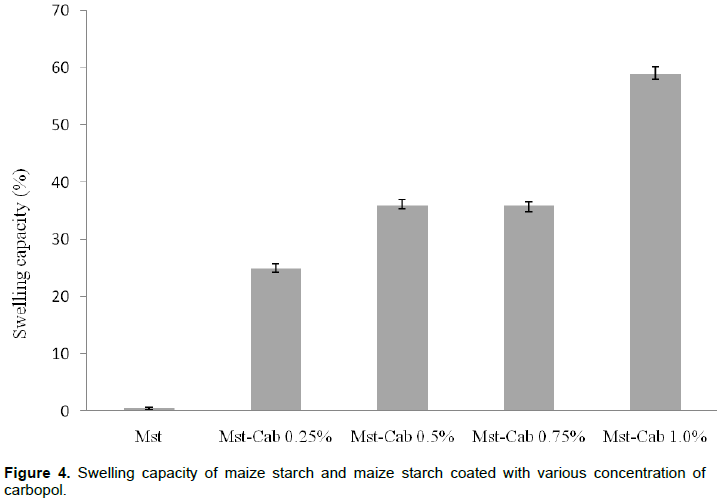
The swelling capacity of Mst and the MpS-CaPol motifs are presented in Figure 3. The swelling of the MpS-CaPol motifs were sensitive to the concentration of CaPol co-processed with Mst. Figure 3 shows a positive shift in swelling capacity when the concentration of CaPol was increased. The increased swelling of MpS-CaPol is attributable to the CaPol film coat on the Mst granules nevertheless, the high swelling potential of carbopol is well known (Brown-Myrie et al. 2008).

Though swelling is an essential characteristic of many disintigrants, those of starch are however, essentially the function of the combinations of capillary activity, slight swelling, and breakdown of the inter-particulate hydrogen bonds resulting in elastic recovery of the compact (Builders and Arhewoh 2016). Some polymers such as carbopol have not been effective as disintegrants so are not commonly used for this purpose. This is perhaps, because of their intrinsic adhesiveness that impart cohesive properties to the particles in the intact tablets, such that when hydrated the colloidal gel serves as an effective bridge and cross-link between particles thus enhancing inter-particulate cohesiveness instead of repulsion and disintegration (Tang et al. 2007). The swelling capacity of Mst and the coated motifs containing different concentrations of CaPol can be ranked thus: 1 > 0.75 > 0.5 > 0.25% > Mst.
Moisture uptake
The moisture sorption characteristics of any excipient are among the critical factors that control the design and optimization of many processes such as drying, packaging and stability (Builders et al. 2009). In many pharmaceutical production processes, the moisture content of the excipients and the active pharmaceutical ingredients critically control such properties as granule/ powder flow, compaction, packaging and stabilities of the intermediates and finished poducts (Builders et al, 2013). In a hermetically sealed system the water activity of a material in a saturated solution at an equilibrium temperature and vapour pressure will correspond to the RH of the sealed system. Thus, the water activity of starch and the various coated motifs in this study will correspond to the RH simulated by the saturated salt solutions (Andrade et al. 2011).
The moisture sorption isotherm of Mst and the CaPol-coated motifs are presented in Figure 4. All the graphs in Figure 4 showed characteristic sigmoidal curves that denote the existence of multilayers at the internal surface of the materials (Andrade et al. 2011). The equilibrium moisture sorption of Mst and the CaPol-coated motifs generally showed sensitivity to the RHs and the concentration of CaPol. Generally, the moisture sorption increased with increasing RH and concentrations of CaPol in the samples. Considering evaluation at RH equal and above 47%, the equilibrium moisture sorption of Mst and the coated motifs containing different concentrations of CaPol can be ranked thus: 1> 0.75 > 0.5 > 0.25% > Mst.
The motifs containing CaPol at 0.75 and 1% showed higher sensitivity as the moisture uptake increased remarkably at a RH above 47% as compared to those of the Mst and the motifs containing CaPol below 0.5% which did not show any remarkable sensitivity until 80% RH. The sensitivity of Mst and the coated motifs to RH as shown by the moisture uptake characteristics corroborates the fact that the new excipients are typically hygroscopic (Andrade et al. 2011). And the amorphousity increased with increasing CaPol concentration (Builders et al. 2009; Okubayashi et al. 2004). Though native starches are typically hydrophilic however, coating Mst with small quantities of CaPol has further increased the hydrophilic potential of the starch as shown by their increased moisture uptake.
Evaluation of the tablet properties
For a robust conventional rapid release tablets, the mechanical strength and the disintegration time are crucial and often interrelated. The physico-mechanical requirements for a good tablet are optimal mechanical strength (non-friable and/or high crushing strength) and rapid disintegration (Abdullah and Geldart 1999). Robust ASA tablets were prepared using the MpS-CaPol as diluent and disintegrant. The ASA tablets produced with Mst and the coated motifs showed remarkable differences in their crushing strength, friability, disintegration time and drug release profile (Figure 5 and Table 3).
Tensile strength
The tensile strengths of the ASA tablets are presented in Table 3. The tablets prepared without adjuvant presented the highest crushing strength. This could be related to the directly compressible and non-disintegrating properties of ASA powder in forming hard compacts when compressed without adjuvant (Cevher et al., 2008). The crushing strength of ASA tablets formulated with the MpS-CaPol increased with higher amounts of CaPol. The low crushing strength of tablets prepared with Mst as diluents may be attributed to the low elasticity and slow plastic deformation of starch which constituted the primary component of the excipients (Ali and Langley 2010). The crushing strength of the tablets prepared with the CaPol increased with increase in concentration. This may be attributed to the good compactibility of CaPol and its ability to impart this onto the composite (Kedar, 2009). The CaPol coat impacted ductility to Mst resulting in a high degree of plastic deformation thereby increasing inter particulate cohesive forces. Also, other factors such as increased particle aggregation resulting in increased irregular particles surface structure as compared to the smooth surface of the Mst granules could be contributory to the relative higher crushing strength of the ASA tablets due to increase in bonding points (Alderborn and Nyström 1984).
Friability
Friability is an official quality parameter that evaluates the mechanical strength of a tablet based on the ability to withstand the stress of abrasion and shock, such as occurs during handling: Packaging, shipping, dispensing and use (Alderborn and Nyström, 1984). The results of friability test of the ASA tablets are presented in Table 3. The limit value for acceptance for a robust conventional tablet is loss not greater than 1.0%w/w of the tablets after the standard abrasive test (The British Pharmacopoeia Commission 1993).
Apart from the ASA tablets formulated with Mst all the other tablets prepared with variants of CaPol-coated Mst met the standard criteria for acceptance (Table 2). The poor friability of the ASA tablets prepared with Mst corroborate the low crushing strength of the tablets. Unmodified starch is not a favored direct compressible excipient because of its poor flow, compactibility, moisture and lubricant sensitivity (Manek et al. 2012). The relative better compactibility impacted by CaPoL may be the reason for the higher crushing strength and non-friable nature of the ASA tablets compared to that of Mst. Generally, the mechanical strength of the ASA tablets was responsive to the concentration of CaPol.
Disintegration time
Fast disintegration is one of the essential attributes of rapid release tablets. Disintegration controls the availability of the drug for dissolution and absorption from the gastrointestinal tract (Block, 2007). Mst remains the most commonly used disintegrant for the formulation of conventional rapid release tablets. This is essentially because of its functional efficiency, low cost, availability, biodegradability and biocompatibility. The disintegration characteristics of the ASA tablets prepared with Mst and MpS-CaPol is presented in Table 3. The tablets prepared with Mst and MpS-CaPol of concentrations 0.25 to 0.75%w/w showed comparable disintegration times, all disintegrating rapidly within one minute. However, the ASA tablets containing starch coated with 1% CaPol and used as disintigrant disintegrated at 45 ± 0.5 min while ASA tablets containing no disintegrant did not disintegrate even after 120 min. Although the tablets prepared with Mst and the different variants of MpS-CaPol showed comparable disintegration times however, there were remarkable differences in their mechanical strength which is an essential attribute of acceptable robust rapid release tablet. The ASA tablets formulated with Mst showed low tensile strength and unacceptable friability. The long disintegration time shown by the ASA tablets prepared with MpS-CaPol coated with 1% CaPol indicates that there is a critical range of concentrations of CaPol that is required for optimal performance in a direct compressible rapid release tablets. The rapid disinte-gration of the ASA tablets prepared with the CaPol-coated Mst granules as disintegrant, despite its high tensile strength, can be related to the hydrophilic property of CaPol which resulted in rapid ingress of water and swelling of the polymer film surrounding the particles. The enhanced swelling due to the CaPol coat on the Mst granules may be responsible for the enhanced disintegration of the ASA tablets by destruction of the inter-particulate cohesive forces within the intact tablet thus, annihilating the hydrogen bonds (Guyot-Hermann and Ringard 1981).
Dissolution rate
Dissolution rate is an official in vitro quality control parameter used to characterize and correlate the bioavailability of solid dosage forms by quantifying the amount and extent of drug released into a bio-simulated solution from the dosage form. The dissolution profiles of ASA from the different tablet samples are shown in Figure 5. The ASA tablets containing Mst showed release profile that were different from those of the MpS-CaPol variants. Tablets containing Mst disintegrated most rapidly when compared to those of ASA tablets containing no disintigrant and the variants of MpS-CaPol. This may be due to the poor mechanical strength and rapid disintigration properties of the tablets containing Mst. However, the ASA tablets containing the various MpS-CaPol [CaPol: 0.25 to 0.75 %w/w] achieved complete (≈ 100) within 10 min. However, tablets containing 1% w/w CaPol showed relatively prolonged release profile as complete release was achieved only after 126 min. The disintegration times for all the tablets were shorter than the time required for achieving maximum dissolution. This corroborates the fact that disintegration does not imply absolute dissolution especially for poorly soluble drugs (Panchagnula and Thomas, 2000; Sachan et al. 2009). A tablet can have a rapid disintegration time while showing divergent biological availability due to low solubility and poor dissolution (Sachan et al. 2009). For a poorly soluble drug such ASA that has dissolution as a rate limiting step to its absorption, the determination of its dissolution rate becomes imperative as this parameter can be used to predict its absorption and probable bioavailability.
Coating of the maize starch granules with carbopol constitutes a simple modification technique that has enhanced the flow, compaction and disintegrant properties of the native starch. Though, the modification showed only minimal changes in the external morphology of the maize starch granules, nevertheless important functional properties were enhanced. Thus, the novel excipient, produced by coating the maize starch granules with certain concentrations of carbopol by the spray drying process and used for formulating aspirin tablets improved the basic physico-technical properties of the tablets. This study has shown that coating maize starch granules with small quantities of carbopol, markedly enhanced the adjuvant functionality of the starch. The high throughput, minimal chemical alteration, simplicity, potential low cost and multifunctional adjuvant properties of this new excipient particularly in the production of aspirin tablets has further expanded the modification potential and applications of its primary constituents: Maize starch and carbopol. This new excipient may also find uses in the formulation of other active pharmaceutical ingredients with similar phyiscochemical properties as aspirin.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdullah EC, Geldart D (1999). The use of bulk density measurements as flowability indicators. Powder Technol. 102:151-165.
Crossref
|
|
|
|
Alderborn G, Nyström C (1984). Radial and axial tensile strength and strength variability of paracetamol tablets. Acta Pharm. Suec 21:1-8.
|
|
|
|
Ali S, Langley N (2010). Formulation of excipients: Discovery, development, validation. Pharmaceutical formulation and quality 12:2.
View
|
|
|
|
Andrade RDP, Lemus RM, Pérez, CEC (2011). Sorption isotherms for food: Uses and limitations. Vitae, Revista De La Facultad De Quõmica Farmaceut 18(3):325-134.
View
|
|
|
|
Ansel HC, Allen LV, Popovich NG (2005). Pharmaceutical dosage forms and drug delivery systems. 8th ed. Lippincott: Williams & Wilkins.
|
|
|
|
Aulton ME (2003). The science of dosage form design. 2nd ed. Toronto: Churchill Livingstone.
|
|
|
|
Block LH (2007). Pharmaceutical principles and drug dosage forms. Comprehensive Pharmacy Review, 6th ed. Lippincott Williams and Wilkins, New York, 68 p.
|
|
|
|
Brown-Myrie E, Itiola OA, Adebayo SA (2008). Comparative disintegrant activities of breadfruit starch and official corn starch. Powder Tech 181(2):98-103.
Crossref
|
|
|
|
Bruening M, Dotzauer D (2009). Polymer films: Just spray it. Nat. Mat. pp. 449-450.
Crossref
|
|
|
|
Builders PF, Anwunobi AP, Mbah CC, Adikwu MU (2013a). New direct compression excipient from tigernut starch: physicochemical and functional properties. AAPS Pharm. Sci. Tech. 14(2):818-827.
Crossref
|
|
|
|
Builders PF, Arhewoh MI (2016). Pharmaceutical applications of native starch in conventional drug delivery. Starch/Stärke 68(9-10):864-873.
Crossref
|
|
|
|
Builders PF, Bonaventure AM, Adelakun T, Okpako LC, Attama AA (2010). Novel multifunctional pharmaceutical excipients derived from microcrystalline cellulose–starch microparticulate composites prepared by compatibilized reactive polymer blending. Int. J. Pharm. 388(1):159-67.
Crossref
|
|
|
|
Builders PF, Ibekwe N, Okpako LC, Attama AA, Kunle OO (2009). Preparation and characterization of mucinated cellulose microparticles for therapeutic and drug delivery purposes. Euro. J. Pharm. Biopharm. 72(1):34-41.
Crossref
|
|
|
|
Builders PF, Kabele-Toge B, Pongri AW, Abolude OD, Mbah CC, Anwunobi PA, Isimi YC (2013b). Impact of co-processing on some fundamental physicochemical and functional properties of microcrystalline cellulose. IOSR J. Pharm. Biol. Sci. 5(2):55-67.
View
|
|
|
|
Cevher E, Taha MAM, Orlu MA (2008). Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv. 15(1):57-67.
Crossref
|
|
|
|
Ebenehi ID, Mohammed BB, Nock SI, Apeji Y (2013). Tableting Performance of Silicified Cassava Starch as a Directly Compressible Excipient. Afr. J. Pharm. Res. Dev. 5(1):52-60.
|
|
|
|
Fazaeli M, Emam-Djomeh Z, Ashtari KA, Omid M (2012). Processing of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food and bioproducts processing. Food Biopro 90:667-675.
Crossref
|
|
|
|
Guyot-Hermann AM, Ringard DJ (1981). Disintegration mechanisms of tablets containing starches: hypothesis about the particle-particle repulsive force. Drug Dev. Ind. Pharm. 7(2):155-177.
Crossref
|
|
|
|
Hauschild K, Picker KM (2004). Evaluation of a new coprocessed compound based on lactose and maize starch for tablet formulation. AAPS Pharm. Sci. 6(2):27-38.
Crossref
|
|
|
|
Itiola OA (1991). Compression characteristics of three starches and the mechanical properties of their tablets. Pharm. World J. 8:91-94.
|
|
|
|
Kedar C (2009). Processing of carbopol polymers in oral solid dose formulations. Pharm. Technol Rev.
View
|
|
|
|
Koo OMY, Squibb B (2011). Application challenges and examples of new excipients in advanced drug delivery systems. Am. Pharm. Rev.
View
|
|
|
|
Luprizol (2011). Formulating controlled release tablets and capsules with carbopol polymers. Pharm Bull 30.
|
|
|
|
Manek RV, Builders PF, Kolling WM, Emeje M, Kunle OO (2012). Physicochemical and binder properties of starch obtained from cyperus esculentus. AAPS Pharm. Sci. Tech. 13(2):379-388.
Crossref
|
|
|
|
Missagh S, Fassihi RA (2004). Novel approach in the assessment of polymeric film formation and film adhesion on different pharmaceutical solid substrates. AAPS Pharm Sci. Tech. 5(2):32-39.
Crossref
|
|
|
|
Moreton RC (1996). Tablet excipients to the year 2001: A look into the crystal ball. Drug Dev Ind Pharm 22(1):11-23.
Crossref
|
|
|
|
Moreton RC (2000). Silicified microcrystalline cellulose. In: The handbook of pharmaceutical excipients. 3rd ed. Kibbe AH editor. Washington: Am. Pharm. Assoc. pp. 685-689.
|
|
|
|
Odeku OA (2013). Potentials of tropical starches as pharmaceutical excipients: Starch/Starke 65:89-106.
Crossref
|
|
|
|
Odeku OA, Awe OO, Popoola B, Odeniyi MA, Itiola OA (2005). Compression and mechanical properties of tablet formulations containing corn, sweet potato and cocoyam starches as binders. Pharm. Technol. 29(4):82-90.
|
|
|
|
Okubayashi S, Griesser UJ, Bechtold TA (2004). Kinetic study of moisture sorption and desorption on lyocell fibers. Carbohydr. Polym. 58(3):293-299.
Crossref
|
|
|
|
Panchagnula R, Thomas NS (2000). Biopharmaceutics and pharmacokinetics in drug research. Int. J. Pharm. 201(2):131-150.
Crossref
|
|
|
|
Rawlings EA (1982). Mechanisms of disintegrants action. In: Bentley's Textbook of Pharmaceutics. 8th ed. London: Balliere and Tindall.
|
|
|
|
Rojas J, Kumar V (2012). Coprocessing of cellulose II with amorphous silicon dioxide: Effect of silicification on the powder and tableting properties. Chemical & pharmaceutical bulletin. Drug Dev. Ind. Pharm. 38(2):209-226.
Crossref
|
|
|
|
Rojas J, Kumar V (2012). Co processing of cellulose II with amorphous silicon dioxide: Effect of silicification on the powder and tableting properties. Drug Dev. Ind. Pharm 38(2):209-226.
Crossref
|
|
|
|
Sachan NK, Bhattacharya A, Pushkar S, Mishra A (2009). Biopharmaceutical classification system: A strategic tool for oral drug delivery technology. Asian J. Pharm 3(2):76-81.
Crossref
|
|
|
|
Seitz JA, Flessland GM (1965). Evaluation of the physical properties of compressed tablets I: Tablet hardness and friability. J. Pharm. Sci. 54(9):1353-1357.
Crossref
|
|
|
|
Srichuwong S, Candra ST, Mishima T, Isono N, Hisamatsu M (2005). Starches from different botanical sources II: Contribution of starch structure to swelling and pasting properties. Carbohydr. Polym. 60(4):529-538.
Crossref
|
|
|
|
Tang C, Yin L, Yu J, Yin C, Pei Y (2007). Swelling behaviour and biocompatibility of carbopol containing superporous hydrogel composites. J. Appl. Polym. Sci. 104(5):2785-2791.
Crossref
|
|
|
|
The British Pharmacopoeia Commission, Appendix XIIA, UK: HMSO; 1993.
|
|
|
|
Tobyn MJ, McCarthy GP, Staniforth JM, Edge S (1998). Physicochemical comparison between microcrystalline cellulose and silicified microcrystalline cellulose. Int. J. Pharm. 269:182-194.
Crossref
|
|
|
|
United States Pharmacopeia (USP, 26), (2003). United States Pharmacopeial Convention Inc., Asian Ed. Canada: Webcom Limited.
|
|
|
|
Yamashiro M, Yuasa Y, Kawakita K (1983). An experimental study on the relationships between compressibility, fluidity and cohesion of powder solids at small tapping numbers. Powder Technol. 34(2):225-231.
Crossref
|
|
|
|
Zaid AN, Natour S, Qaddomi A, Ghoush AA (2013). Formulation and in vitro and in vivo evaluation of film-coated montelukast sodium tablets using an industrial scale. Drug Design Dev. Ther. 7:83-91.
Crossref
|
|
|
|
Zhao N, Augsburger LL (2005). Functional comparison of three classes of superdisintegrants in promoting aspirin tablets disintegration and dissolution. AAPS Pharm. Sci. Tech 6(4):E634-E640.
Crossref
|
|
|
|
Zhou Q, Shi L, Chattoraj S, Sun CC (2012). Preparation and characterization of surface-engineered coarse microcrystalline cellulose through dry coating with silica nanoparticles. J. Pharm. Sci. 101(11):4258-4266.
Crossref
|