The study was carried out to assess the antioxidant capacity and phytochemical constituents of acetone and water extracts of the vegetables irrigated with fresh and waste water such as Raphanus sativus (Radish), Brassica oleracea capitata (Cabbage), Brassica rapa (Turnip), Coriandrum sativum (Coriander) and Spinacia oleracea (Spinach). A concurrent quantitative investigation of total phenolics, flavonols and flavonoids was also made and according to the results, the higher antioxidant capacity was shown by aqueous extracts in all antioxidant methods than acetone extracts.
Phytochemicals are chief bioreactive non-nutrient in plants (Factor et al., 2000). Phenolic group have capability to extinguish free radicals because of acidity and delocalized á´«-electrons (Brown, 1995). Reactive oxygen and many other free radicals have ability to cause damage to biological macromolecules such as proteins, DNA and also cause many diseases like cardiovascular diseases, diabetes, and cancer (Bhattacharya et al., 2011; Liochev, 2013; Kryston et al., 2011). During the last era, research for determining natural sources of antioxidants has been increased and various studies have been reported for the prevention of diseases as a result of oxidative anxiety by consuming vegetables and fruits (Gulcin, 2012; Ivan et al., 2016). It has been reported that many vegetables are rich in flavonoids, phenolics, flavonol contents and also rich in carotenoids (Ranilla et al., 2010; Kumar et al., 2012). Foods flourishing with flavonoids lessen many diseases, increase the power of vitamin C, protect the blood vessels from leakage, secure cell due to oxygen damage and prevent inflammation of body. Different diseases such as, gout, allergy, cataracts, asthma, diabetes, candida infection, hemorrhoids and stomach ulcer are putt off by flavonoids. Hemorrhoids, nose bleeds, meager resistant function and infection after injury are caused by deficiency of flavonoids. The contents of flavonoids are affected by many factors like acidity level,
extent of processing and heating. By heating flavonoid contents are removed, for example, 50% of total flavonoid contents are removed by boiling fresh spinach and boiling of onion exclude 30% of total flavonoid content.
Lots of nutrients are removed by overcooking of vegetables. Owing to diverse properties of flavonoids like anti-allergic, antioxidant (Cazarolli et al., 2008), anti-inflammatory, antiviral (Friedman, 2007), antimutagenic, antibacterial (Cushnie and Lamb, 2011), antithrombotic and antineoplastic, they have many pharmacological and health reimbursement effects (Middleton et al., 2000).
Reagents
Folin-ciocalteu, gallic acid, aluminium chloride, sodium carbonate, sodium nitrite, sodium hydroxide, rutin, sodium acetate, DPPH (2, 2-diphenyl-1-picrylhydrazyl), methanol, 1, 10-phenanthroline, phosphate buffer, ferrous sulphate, hydrogen peroxide and acetone were used.
Extraction of the samples
The edible parts of the fresh and waste water irrigated samples were washed
with deionized water and dried in shade. For the determination of antioxidant activity and phytochemical constituents, two steps extraction was done with acetone and water.
About one gram of vegetable sample was mixed with 10 mL of distilled water and supernatant was transferred in a beaker after centrifugation at 6000 rpm.
The solid residue which was left after water extraction was then additionally extracted with acetone and its supernatant was also collected. At -10°C, acetone and water extracts were stored for the analysis of flavonoids, ascorbic acid and phenolics.
Determination of phenolic contents
The examination of phenolic contents in extracts was examined with help of Linlin scheme. 5 mL diluted Folin-ciocalteu and 4 mL of 7.5% Na2CO3 was added in the 1 mL water and acetone extract. The mixture was kept at 25°C for ninety minutes before measuring at 760 nm. The results were obtained according to gallic acid equivalents used as standard using the calibration curve equation, y = 1.8929x - 2.4286 (where y = absorbance and x= concentration, GAE in μgmL-1).
Determination of flavonoids contents
Calorimetric method modified by Linlin was used for the detection of flavonoids. Into 5 mL of water or acetone extracts, 0.3 mL of 5% NaNO
2 was added for five minutes. Ten percent aluminium chloride (0.3 mL) was added in the given mixture.
To stop the reaction, 2 mL of 1 M solution of sodium hydroxide was added. To dilute the mixture, 10 mL distilled water was added. The absorbance at 510 nm was measured immediately.
Rutin was used as standard for the detection of flavonoids using calibration curve equation y = 0.0006x + 0.3593 (where y = absorbance and x = concentration of rutin).
Determination of total flavonols
The method given
by Kumaran and Karunakaran (Kumaran and Karunakaran, 2006) is used for the analysis of total flavonols. 2 mL of two percent aluminium chloride solution and 2 mL of sodium acetate (50 g/L) solutions are added in the 2 mL of water or acetone extract. The mixture was allowed to stand at 25°C for two and half hour and then absorbance was measured at 440 nm.
Scavenging activity of DPPH
For the detection of scavenging activity of 2, 2-diphenyl-1-picrylhydrazyl (DPPH), the method described by Yu and Aoshima (Yu et al., 2002; Aoshima et al., 2004)
was used. Took 2 mL of sample extracts and added DPPH 2.5 mL (0.1
mM MeOH ) and the mixture was incubated in dark for half an
hour at room temperature. Vanishing of color of mixture was investigated against blank at 517
nm and % inhibition was determined as:
Evaluation of scavenging activity of OHâ—¦
The scavenging activity of OH
â—¦ in acetone and water extracts of vegetables was determined by the procedure used by Yu et al. (2004). 1 mL of 1, 10-phenanthroline (0.04 M), 2
mL phosphate buffer (0.2 M) and 0.04 mL of Fe
2SO
4 solution (0.02 M) were mixed into 3.0 mL of extracts. To start the reaction 0.1 mL of
seven mM hydrogen peroxide was added in the mixture. Before measuring the absorbance at 560 nm, the mixture was incubated for five minutes at 25°C. The scavenging activity of OH
â—¦ was calculated as:
Because of their hydroxyl groups flavonoids are used in metal chelation and they can act as free radical scavengers and reducing agents as
well (Agati et al., 2012). The phytochemical study (Table 1) revealed the quantifiable analysis of phenolic, flavonoids and flavonol contents in fresh water irrigated vegetables. These results revealed that fresh water irrigated
Brassica rapa has high phenolic and flavonoid contents in water extract followed by
Raphanus sativus, Coriandrum sativum, Spinacia oleracea . The phenolic contents in various plants may vary due to different processing steps such as growing, harvesting, storage and technical method used. Because of their presence in different plants, phenolic compounds have received great attention due to their antioxidant properties and play an important role in antimicrobial, anti-inflammatory and anticancer activities as they can potentially interact with biological systems (Abu-Reidah et al., 2013; Wang et al., 2003). Table 2 reveals the photochemical analysis of wastewater irrigated vegetables. Phenolic contents were found higher in water extract of
B. rapa (961.8 mg/g) followed by
R. sativus (468.3 mg/g) in fresh water vegetables (Figure 1). Wastewater irrigated vegetables have higher phenolic contents in water extract
of C. sativum(693.5 mg/g) (Figure 2). Flavonoid contents were higher in water extract of fresh water irrigated
B. oleracea capitata (371.5 mg/g, Figure 3) and acetone and water extracts of wastewater irrigated
R. sativus (349.8 and 458 mg/g) (Figure 4). Flavonol contents were higher in water extract
of S. oleracea (446.28 mg/g) followed by acetone extract of
R. sativus (413.16 mg/g) (Figure 5)
. B. rapa showed higher flavonol contents (493.16 mg/g) in acetone extract of waste vegetables (Figure 6). Great attention was received by phenolic compounds present in plants due to its antioxidant properties. Phenolic contents play an important role in biological system due to anticancer, antimicrobial and anti-
inflammatory activity (Wang et al., 2003; Abu-Reidah et al., 2013).




Assay of anti-oxidant capacity of vegetables using DPPH and OHo
DPPH is widely used to determine antioxidant activity of plants due to its stable organic radical. At 517 nm, fall in absorbance of 2, 2-diphenyl-1-picrylhydrazyl solution in a spectrophotometer determined the antioxidant capacity, in which the purple colour of DPPH radical is reduced to yellow colour of DPPH
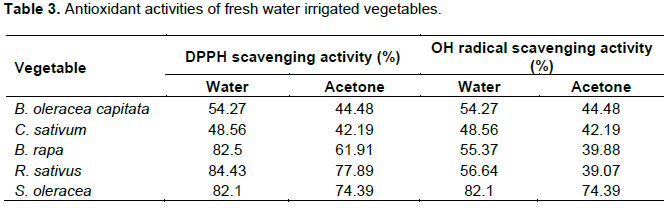
2 (Mishra et al., 2012). Tables 3, 4 and Figures 7, 8 revealed the antioxidant activities of fresh and waste water irrigated vegetables. Highest DPPH scavenging activity is shown by water and acetone extracts of
R. sativus (84.43 and 77.89%). Fresh water
B. rapa and
S. oleracea exhibited the same scavenging activity in water extract (82%) (Table 3). Waste water
irrigated B. rapa showed highest DPPH scavenging activity in water extract (90.06%) (Table 4). Hydroxyl radicals are the more reactive among the reactive organic species, because they can react with biomolecules and cause mutation and severe cell damage, aging, carcinogenesis and cell death (Li et al., 2013). Highest OH radial scavenging activity is shown by acetone and water extracts of fresh water i
rrigated S. olerace a (74.39 and 82.1%, Figure 9, Table 3) and wastewater
irrigated R. sativus (69.94 and 78.81%, Figure 10, Table 4). The activities were found to be significant in those samples which showed percent inhibition greater than 50% (p<0.05).


The authors have not declared any conflict of interests.






