ABSTRACT
The genus, Calliandra is popularly used for renal pain, cystitis, prostate inflammation, fever and toothache. This study aimed at investigating the antinociceptive activity of methanolic crude extract (MCE) of Calliandra umbellifera, regarding chemical (acetic acid, formalin and glutamate tests) of nociception (in vivo) and the methanolic extract and hidrobutanolic phase (HBF) antioxidant activity by the 2,2-diphenyl-1-picril-hidrazil (DPPH) method, besides determination of the content of phenolic compounds and flavonoids (in vitro). The pre-treatment with the MCE (100, 200 and 300 mg/kg, p.o) was able to reduce the number of abdominal contortions (p<0.01 or p<0.001), the licking times in the formalin (p<0.001) and the glutamate tests (p<0.01), respectively. In the antioxidant assay, the extract showed optimum EC50 and higher flavonoid content as compared to the hydrobutanolic fraction; however, the content of the obtained phenolic compounds were higher in HBF as compared to MCE. The experimental data showed that C. umbellifera has an antinociceptive activity, a good antioxidant activity and high levels of phenolic compounds, which confirms the popular use of Calliandra, contributing to the scientific knowledge of the species.
Key words: Calliandra umbellifera, antinociceptive activity, antioxidant, total phenol, flavonoids, 2,2-diphenyl-1-picril-hidrazil (DPPH).
Herbal medicine has been used for the treatment of many diseases for a long period of time (Asadbeigi et al., 2014). The drugs commonly used to treat pain have significant adverse effects such as induction of complications such as gastric lesions, addiction, tolerance and sedation, besides presenting limited effectiveness. Thus, it is necessary to seek new therapeutic strategies for the treatment of pain and the reduction of side effects. Preclinical studies using test nociception against mechanical, thermal and chemical stimuli, as well as models of inflammatory pain and neuropathic origin in rodents are critical for assessment and development of potential new drugs (Cavendish, 2014).
The chronic oxidative stress is responsible for many degenerative diseases, such as: asthma, autoimmune diseases, gastrointestinal and cardiac diseases and Alzheimer (Lushchak, 2014; Sies, 2015). Hence, it is necessary to assess some mechanisms, as antioxidants, to counteract the damages of oxidation (Wojtunik-Kulesza et al., 2016) and in that respect a large source of antioxidants, such as vegetables have been studied in order to evaluate their antioxidant activity (Boudet, 2007; Rice-Evans et al., 1997).
The genus, Calliandra belong to the family Fabaceae, consisting of approximately 200 species, being spread in Tropical America, India and Madagascar and known as “esponjinha” in Brazil (Mattagajasingh et al., 2006; Lewis and Rico, 2005). The species of Calliandra are used by population for renal pain, cystitis, urethritis, prostate inflammation, fever and toothache (Adesina, 1976; Dimayuga et al., 2006) and some studies showed they are used as anticonvulsivants, analgesic, antidiarrhetic, antispasmodic, antibacterial and antioxidant (Orishadipe et al., 2010; Agunu et al., 2005; Aguwa and Lawal, 1988). The principal secondary metabolites find in Calliandra are cassane diterpens, saponins, flavonoids and tannins (Dimayuga et al., 2006; Barbosa et al., 2008; Murillo et al., 2008).
Due to the popular users and the absence of studies on species of Calliandra, this work aims to evaluate the antinociceptive activity of the methanolic extract of the aerial parts of Calliandra umbellifera through nociception induced by chemical stimuli, besides evaluating the total phenolic and flavonoid contents and the antioxidant activity of methanolic extract and hydrobutanolic fraction by DPPH method.
Animals
Male Swiss mice (Mus musculus) (30 to 40 g), were kept under controlled temperature conditions at 21± 2°C subject to a 12 h light/dark cycle, free access to food and drinking water. The animals were divided into 5 groups (n = 8), being used only once. The experimental procedures were reviewed and approved previously by the CEPA-The Ethics Committee for the Use of Animals CBiotec/UFPB, under certificate No. 0809/12.
Drugs
The chemical substances used in this antinociceptive study were: glacial acetic acid (Synth – U.S.A.), morphine hydrochloride (Merck – U.S.A.), formaldehyde 37% (Vetec – Brazil), DMSO (Sigma – U.S.A) and glutamic acid (Sigma – U.S.A.). The MCE was dissolved in distilled water and DMSO and volume of 0.1 mL/10 g mouse weight was administrated orally (p.o.). For antioxidant study, gallic acid (Sigma-Aldrich), ascorbic acid (Sigma-Aldrich), quercetin (Sigma-Aldrich), as standards, and Folin-Ciocalteu (Sigma-Aldrich), aluminium chloride (Sigma-Aldrich), 1,1-difenil-2-picril-hidrazil – DPPH (Sigma-Aldrich) and sodium carbonate as reagents were used.
Plant
The aerial parts of C. umbellifera, were collected in Matureia - PB, Brazil, identified by the Dra. Maria de Fátima Agra from the Pharmaceutical Technology Laboratory at the Federal University of Paraiba state and catalogued in the Herbarium Professor Lauro Pires Xavier JPB/UFPB, under de code AGRA, 7430.
Extraction
The botanical materials passed through the drying process, followed by a spray in mechanical mill, obtaining 5.0 kg of stem powder which was thoroughly macerate with methanol (MeOH) during 72 h. The extraction solution was concentrated in rot vapor under reduced pressure at a temperature of 35°C by obtaining the methanolic crude extract - MCE (318 g). The MCE was solubilized in methanol : water (1:1) and subjected to liquid-liquid partition hidrobutanol, by obtaining the hidrobuthanolic fraction (HBF).
Acute toxicity
Groups of mice (n = 8) were treated randomly through oral route in doses of 500 and 1000 mg/kg, while the control group received only the vehicle. After the treatment, the pharmacological and/or toxic effects were observed during 4 h. Then, the animals were fed and the number of deaths was observed during 14 days for LD50 determination.
Acetic acid-induced writhing test
This method is based on the fact that the intraperitoneal administration of acetic acid solution at 0.85% in mice causes peritoneal irritation, characterized by abdominal contortions followed by extensions of the hind limbs (Koster et al., 1959). This nociceptive behavior was quantified for 15 min after the administration of the stimulus. The animals were divided into 5 groups (n=8) and it was pre-tread with drug standard (morphine 6 mg/kg, i.p), vehicle and MCE (100, 200 and 300 mg/kg, p.o.). After 30 min of the initial treatments, the animals were treated with acetic acid at 0.85% (0.1 mL/10 g) via i.p., and subsequently placed into a polyethylene box, in order to observe the number of abdominal contortions presented by each animal.
Formalin test
In the formalin test, mice (N = 8) were pre-treated with the vehicle, MCE (100, 200 and 300 mg/kg, p.o.) and morphine (10 mg/kg, i.p.). After 30 min, 20 μL (2.5% formalin solution) was injected into the sub-plantar region of the hind right paw of the mice. After the administration of formalin, the animals were placed into observation boxes. The indication of nociception was the total time of paw licking that was counted in 2 phases. The first phase, usually occurs in the first 5 min after the formalin administration (neurogenic response), then there is an interface of approximately 10 min characterized by inhibitory mechanisms of pain. The second phase (15 to 30 min) is known primarily for an inflammatory response (Hunskaar and Hole, 1987).
Glutamate test
The injection of glutamate induces the direct stimulation of nociceptives neurons, causing the liberation of many inflammatory mediators and neuropeptides involved in the pain transmission (Buzzi et al., 2009). For this test, the animals (n = 6–8) were treated with MCE (100, 200 and 300 mg/kg p.o.) or vehicle, 60 min before the intra-plantar (i.pl.) glutamate (20 μmol/paw) injection. The time of licking or biting the paw that received the stimulation for 15 min was quantified. MK 801 (0.15 mg/kg i.p.) was administered 30 min before stimulation and used as a positive control.
Determination of phenolic content
The total phenolic content was determined by Folin-Ciocalteu method, which is one of the most used tests for this kind of analysis. For this test, gallic acid was used as standard compound, as described by Gulcin et al. (2004), with some adaptations. 0.5 mL of Folin-Ciocalteu reagent was added to 120 µL of sample (concentration of extract = 450 µg/mL). The reaction was maintained at rest for 5 min and then 400 µL of sodium carbonate (7.5%) was added to neutralize the mixture. Samples were maintained at room temperature, in the dark for 120 min, and then were transferred to 96 well plates and the absorbance was measured at 765 nm on UV-Visible Spectrometer (UV-2550, Shimadzu). Experiments were made in triplicate and phenolic content was determined by linear regression equation from the calibration curve constructed with gallic acid (7.5; 15; 75; 100 and 150 µg/ml). The results were expressed in mg EGA/g of sample.
Determination of flavonoid content
Flavonoid content was determined by spectrophotometric method proposed by Schmidt and Ortega (1983), with some modification, using aluminum chloride (AlCl3) as reagent and quercetin as standard. The aluminum chloride has the characteristic of forming stable and colorful complexes with flavonoids when dissolved in methanol; it causes a shunt to higher wave lengths and an increasing of absorption (Souza and Giovani, 2005; Petry et al., 1998; Woisky et al., 1998). For this test, 0.1 mL of the sample (1mg / mL) was treated with 0.1 mL of aluminum chloride solution (2.5%) in 96-well plates and this reaction was maintained away from light for 30 min and then, the absorbance was measured at 410 nm on UV-Visible spectrometer (UV-2550, Shimadzu) (Marques et al., 2012). The analysis were performed in triplicate and the flavonoid content was determined by the linear regression equation from the calibration curve constructed with quercetin solution (25, 50, 100, 150 and 200 μg/mL) and the results were expressed by μg of quercetin/mg of sample.
Antioxidant assay in vitro: DPPH method
Antioxidant activity was determined by the 2,2-diphenyl-1-picril-hidrazil (DPPH) method, using the methodology described by Garcez et al. (2009), with some adaptations. The DPPH method is based on the reduction of this organic radical, which presents its higher absorption at 515 to 520 nm, showing a violet color. After the abstraction of radical hydrogen from the antioxidant used in the study, it is possible to observe a decrease in absorption and staining solutions, moving from violet to yellow. For this test, 100 µL DPPH solution were added to 100 µL of sample at different concentrations (1.625, 3.125, 6.25, 12.5 and 25 µg/mL) which were determined by initial screening. The mixture was kept at rest and in the dark for 30 min and then the absorbance was measured at 518 nm on UV-Visible spectrometer (UV-2550, Shimadzu). The percentage of scavenging activity (% SA) was calculated according to the equation:
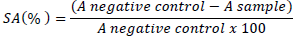
Where, A negative control = DPPH and methanolic solution absorbance; A sample = Radical absorbance in presence of extract or standard.
The antioxidant activity was expressed as EC50 (half maximal effective concentration), which means the concentration of the sample that gives half-maximal response, and as positive control was used ascorbic acid. The experiment was performed in triplicate.
Statistical analysis
The data were analyzed through variance analysis (ANOVA) to calculate if there was a significant difference among groups, followed by the Dunnett or Tukey tests. The results were expressed as means ± S.E.M and the p values were considered as significant when less than 0.05. The LD50 was determined using non-linear regression and the EC50 was determined using linear regression.
Acute toxicity of the MCE
The determination of the acute toxicity and the lethal dose 50 (LD50) allows investigating the possible toxic effects of substances and extracts, determining the dose responsible for the death of 50% of the animals (Litchfield and Wilcoxon, 1949), allowing the achievement of pharmacological tests using safe doses. The methanolic extract of C. umbellifera showed no death.
Effects of MCE on the acetic acid-induced writhing test
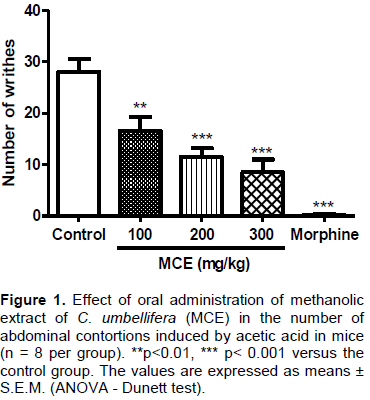
The writhing test is characterized by its high sensibility, although it does not present any selectivity, being also sensitive to sedative drugs, muscle relaxant, analgesics non-steroidal anti-inflammatory drugs and narcotics (Collier et al., 1968). The nociceptive response to acetic acid may involve a direct stimulation of the nociceptive afferent fibers, due to a reduction of the pH or a synthesis of the inflammatory mediators, such as the arachidonic acid metabolism via COX, with consequent biosynthesis of prostaglandins (Duarte et al., 1988; Franzotti et al., 2000). In the study, the number of contortions induced by acetic acid was recorded as a significant reduction (p<0.01 or p<0.001) in the experimental doses of 100, 200 and 300 mg/kg p.o. as compared to the control group which was similar to the morphine (positive control) (Figure 1). From these results, it can be seen that increasing the dose caused a reduction in the number of contortions, demonstrating the effectiveness of the MCE in the writhing test. This proposes that this substance showed antinociceptive activity and/or would be inhibiting the release of inflammatory mediators and cytokines.
Effect of the MCE on the nociception induced by formalin
In order to better evaluate the activity of extract of C. umbellifera, the formalin test was used, which constitutes a valid and safe nociception model and sensitive to several classes of analgesic drugs. Formalin produces a distinct biphasic response where analgesic drugs may act differently in the first and second phases of the test (Morteza-Semnani et al., 2002).
The mice treated with MCE (100, 200 and 300 mg/kg, p. o.) demonstrated no significant reduction in the time of paw licking in the 1st phase, regarding the control group (Figure 2). However, according to the result shown in Figure 3, in the 2nd phase of the test, the animals treated with doses of 100, 200 and 300 mg/kg p.o. of MCE decreased significantly (p < 0.001) the paw licking time, as compared to the control group. Morphine (10 mg/kg, i.p.) reduced the time of licking in both phases. Knowing that drugs which act at central level, such as the analgesic opioids, inhibit both phases of the formalin test; however, drugs of peripheral action like anti-inflammatory are only effective in the second phase. These results indicate a possible peripheral analgesic effect by inhibiting the release of chemical mediators (Adeyemi et al., 2004; Bastos et al., 2006; Ferreira et al., 2006).
Effect of the MCE on the glutamate test
The glutamate test was used as evidence for the interaction of the MCE with the glutamate test. The glutamate promotes the activation of NMDA receptors, causing an increased influx of calcium with the activation of neuronal NO synthase and nitric oxide formation. Thus, glutamate participates in the processes involved in the perception of and central sensitization to pain (Bleakman et al., 2006; Beirith et al., 2002).
From the results presented in Figure 4, it was observed that the extract of C. umbellifera reduce paw licking when compared with the control group, which means that the MCE could inhibit directly the glutamate action through the antagonism of its receptors or inhibit the liberation of other inflammatory mediators, like the nitric oxide.
Determination of phenolic content
Phenolic compounds are a group of natural substances found in vegetable and are present in roots, leaves, flowers and fruits of many plants (Gilaberte and González, 2010). As examples of secondary metabolites of this group, there are the simple phenols, coumarins, tannins, lignins, naphthoquinones and flavonoids (Cunha, 2009). They exhibit antioxidant properties, anti-inflammatory and immunomodulatory. Studies have demonstrated the effectiveness of natural polyphenols in combating inflammation, oxidative stress, DNA damage and suppression of the immune response induced by UV radiation. These protective effects contribute to anti photo carcinogenic action (Ferdinando et al., 2014; Gregoris et al., 2011).
Plant extracts that are rich in phenolic compounds demonstrate its action in scavenging free radicals. In addition, the plant extracts may also have biological properties such as UV protection, skin hydration, restoring the barrier function and the stimulation of collagen synthesis, which increases the skin's defenses, but also favors the recovery of the same (Mercurio et al., 2015; Ferdinando et al., 2014). Thus, admitting the importance of phenolic compounds, their concentration was measured, using the method of Folin-Ciocalteu. To perform their quantification, the linear regression equation and the r² of the standard (gallic acid) was obtained and the results are demonstrated in Table 1.
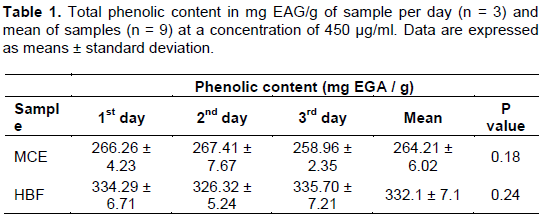
Through the analyzed data in Table 1, it was observed that there was no differences among test days in the 0.05 level of significance being reproducible in the method used in the concentration of 450 µg/ml. It was also observed through this data, that HBF of C. umbellifera presents a higher concentration of phenolic compounds (332.1 ± 7.1 mg EGA/g of sample), when compared with MCE (264.21 ± 6.02 mg EGA/g of sample).
Determination of flavonoid content
Flavonoids can count on a variable number of hydroxyl groups in its structure and protect the body against damage caused by oxidants, including UV radiation, environmental pollution, and substances in some foods (Gilaberte and González, 2010; Martínez-Flórez et al., 2002). They act as antioxidants, acting in reducing inflammation, and against the harmful effects on the DNA generated by ultraviolet radiation (Nichols and Katiyar, 2010). Because of that, to perform the quantification of the phenolic content of the tested sample, spectro-photometric method was used with aluminum chloride. The linear regression equation and the r² of the standard, quercetin were made for later interpolation of the data and to calculate the flavonoid content in µg quercetin/mg of sample (Table 2).
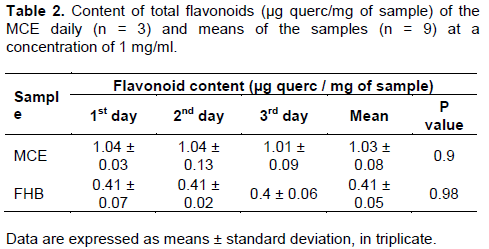
As shown in Table 2, it was observed that there was no difference among test days in the 0.05 significance level, and the method is reproducible in the used concentration (1 mg/mL). It was also possible to observe that the extract presents a higher concentration of flavonoids (1.03 ± 0.08 µg querc/mg of sample), as compared to HBF (0.41 ± 0.05).
Antioxidant assay in vitro: DPPH method
Free radicals are physiologic formed in the organism as part of its respiration or metabolism processes and this even includes processes as phagocytosis, prostaglandin synthesis and cytochrome P450 (Tegeli et al., 2014). Although, the presence of many antioxidant mechanisms in the body to neutralize those reactive species when there is an overproduction of them; sometimes these mechanisms are not enough to eliminate them from the body. This overproduction leading to an imbalance between the production and elimination of free radicals, which is called oxidative stress can cause disturbs in many physiological processes (Cervellati et al., 2014; Kumar, 2011; Lushchak, 2014; Sies, 2015). Because of that, many studies have shown that consuming food rich in antioxidants is very important to reduce the damages caused for reactive species (Rezaire et al., 2014), and many studies with natural products have shown various compounds with antioxidant activity (Almeida et al., 2016), such as phenolic compounds like flavonoids, phenolic acids and anthocyanins (Broinizi et al., 2007).
The DPPH method was the chosen method because it is a simple, fast and sensible technic based on the reduction of this organic radical that allows identifying the antioxidant activity. Through analyzed data presented in Table 3, it was observed that there is no differences among the test days in the 0.05 significance level.
According the results presented in Figure 5, it was possible to observe that the extract of C. umbellífera showed the lowest EC50 (17.64 ± 0.43), presenting the optimum antioxidant activity as compared to hydro-butanolic fraction (EC50 21.47 ± 0.32), though none of them have been effective as the standard (EC50 = 9.39 ± 0.05).
Despite this fact, both samples presented important antioxidant activity, mainly when they are compared with several other studies where the samples showed EC50 > 50 µg/ml. Through all the analyzed data, it was also observed that HBF shows the highest phenolic content, but the antioxidant activity comes from MCE; this fact can
be related to the extract presenting the highest flavonoid content, which is one of the most important compounds with this property (Sá et al., 2012; Phang et al., 2011; Degáspari and Waszczynskyj, 2004; Pietta, 2000).
Calliandra umbellifera has an antinociceptive activity, a good antioxidant activity and high levels of phenolic compounds, which confirms the popular use of plants of Calliandra, contributing to the scientific knowledge of the species.
The authors have not declared any conflicts of interests.
REFERENCES
|
Acu-a UM, Jancovski N, Kennelly EJ (2009). Polyisoprenylated benzophenones from Clusiaceae: Potential drugs and lead. Curr. Top. Med. Chem. 9(16):1560-1580.
Crossref
|
|
|
|
Adeyemi OO, Okpo SO, Okpaka O (2004). The analgesic effect of the methanolic extract of Acanthus montanus. J. Ethnopharmacol. 90(1):45-48.
Crossref
|
|
|
|
|
Adesina GA (1976). Personal Communication. Chemistry Department, University of Ibadan, Ibadan Nigeria.
|
|
|
|
|
Agunu A, Abdurahman E, Shok M, Yusuf SA (2005). Analgesic activity of the roots and leaves extracts of Calliandra portoricensis. Fitoterapia 76(5):442-445.
Crossref
|
|
|
|
|
Aguwa CN, Lawal AM (1988). Pharmacologic studies on the active principles of Calliandra portoricensis leaf extracts. J. Ethnopharmacol. 22(1):63-71.
Crossref
|
|
|
|
|
Almeida MLB, Freitas WE, Morais PL, Sarmento JD, Alves RE (2016). Bioactive compounds and antioxidant potential fruit of Ximenia americana L. Food Chem. 192:1078-1082.
Crossref
|
|
|
|
|
Asadbeigi M, Mohammadi T, Rafieian-Kopaei M, Saki K, Bahmani M, Delfan M (2014). Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: an ethnobotanical study in the Urmia. Asian Pac. J. Trop. Med. 7(Suppl. 1): S364-S368.
Crossref
|
|
|
|
|
Barbosa AP, Da Silva BP, Parente JP (2008). Brevifoliasaponin with Adjuvant Activity from Calliandra brevifolia. Z. Naturforsch 63b:894- 902.
|
|
|
|
|
Bastos GNT, Santos ARS, Ferreira VMM, Costa AMR, Bispo CI, Silveira AJA, Do Nascimento JLM (2006). Antinociceptive effect of the aqueous extract obtained from roots of Physalis angulata L. in mice. J. Ethnopharmacol. 103(2):241-245.
Crossref
|
|
|
|
|
Beirith A, Santos ARS, Calixto JB (2002). Mechanisms underlying the nociception and paw edema caused by injection of glutamate into the mouse paw. Brain Res. 924(2):219-228.
Crossref
|
|
|
|
|
Bleakman D, Alt A, Nisenbaum ES (2006). Glutamate receptors and pain. Semin Cell Dev. Biol. 17:592-604.
Crossref
|
|
|
|
|
Boudet AM (2007). Evolution and current status of research in phenolic compounds. Phytochemistry 68(22-24):2722-2735.
Crossref
|
|
|
|
|
Broizini PRB, Andrade-Wartha ERS, Oliveira AM, Silva AM, Novoa AJV, Torres RP, Azeredo HMC, Alves RE, Mancini-Filho J (2007). Avaliação da atividade antioxidante dos compostos fenólicos naturalmente presentes em subprodutos do pseudofruto de caju (Anacardium occidentale L.). Cienc. Tecnol. Aliment. 27(4):902-908.
Crossref
|
|
|
|
|
Buzzi C, Franzoi FCL, Antonini G, Fracasso M, Cechinel Filho V, Yunes RA, Niero R (2009). Antinociceptive properties of caffeic acid derivatives in mice. Eur. J. Med. Chem. 44(11):4596-602.
Crossref
|
|
|
|
|
Cavendish RL (2014). Atividade antinociceptiva do extrato hidroetanólico de própolis vermelha. Dissertação de Mestrado. Universidade Tiradentes – Aracaju.
|
|
|
|
|
Cervellati C, Romani A, Seripa D, Cremonini E, Bosi C, Magon S, Bergamini CM, Valacchi G, Pilotto A, Zuliani G (2014). Systemic oxidative stress and conversion to dementia of elderly patients with Mild Cognitive Impairment, BioMed. Res. Int. pp. 1-7.
Crossref
|
|
|
|
|
Collier HOJ, Dinnen LC, Jonsnson CA, Schneider C (1968). The abdominal constriction response and its suppression by analgesic drugs in mouse. Br. J. Pharmacol. Chemother. 32(2):295-310.
Crossref
|
|
|
|
|
Cunha WR, Silva MLA, Turatti ICC, Ferreira DS, Batarello HL (2003). Avaliação da atividade analgésica de Miconia ligustroides (Melastomataceae) utilizando o teste de contorção abdominal em camundongos. Rev. Bras. Farm. 84(2):47-49.
|
|
|
|
|
Degáspari CH, Waszczynskyj N (2004). Propriedades antioxidantes de compostos fenólicos. Visão Acadêmica 5(1):33-40.
Crossref
|
|
|
|
|
Dimayuga RE, Espinoza JA, Garcia A, Delgado G, Molina-Salinas GM, Said-Fernandez S (2006). Two new cassane-type diterpenes from Calliandra californica with antituberculosis and cytotoxic activities. Planta Medica 72(8):757-761.
Crossref
|
|
|
|
|
Duarte IDG, Nakamura M, Ferreira SH (1988). Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res. 21:341-343.
|
|
|
|
|
Ferdinando MD, Brunettia C, Agatib G, Tattinic M (2014). Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 103:107-116.
Crossref
|
|
|
|
|
Ferreira AA, Amaral FA, Duarte IDG, Oliveira PM, Alves RB, Silveira D, Azevedo AO, Raslan DS, Castro MAS (2006). Antinociceptive effect from Ipomoea cairica extract. J. Ethnopharmacol. 105:148-153.
Crossref
|
|
|
|
|
Franzotti EM, Santos CVF, Rodrigues HMSL, Mourão RHV, Andrade MR, Antoniolli AR (2000). Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J. Ethnopharmacol. 72(1-2):273-277.
Crossref
|
|
|
|
|
Garcez FR, Garcez WS, Hamerski L, Miguita CH (2009). Fenilpropanóides e outros constituintes bioativos de Nectandra megapotamica. Quim. Nova 32(2):407-411.
Crossref
|
|
|
|
|
Gilaberte Y, González S (2010). Novedades en photoprotection. Actas Dermo-Sifiliogr 1:659-672.
Crossref
|
|
|
|
|
Gregoris E, Fabrisa S, Bertelle M, Grassato L, Stevanato R (2011). Propolis as potential cosmeceutical sunscreen agent for its combined photoprotective and antioxidant properties. Int. J. Pharm. 405:97-101.
Crossref
|
|
|
|
|
Gulcin I, Sat IG, Beydemir S, Elmastas M, Kufrevioglu OI (2004). Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 87(3):393-400.
Crossref
|
|
|
|
|
Hunskaar S, Hole K (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30: 103-104.
Crossref
|
|
|
|
|
Koster R, Anderson M, DeBeer EJ (1959). Acetic acid for analgesic screening. Fed. Proc. 18:412-418.
|
|
|
|
|
Kumar S (2011). Free Radicals and Antioxidants: Human and Food System. Adv. Appl. Sci. Res. 2(1):129.
|
|
|
|
|
Litchfield JT, Wilcoxon FA (1949). A simplified method of evaluation dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99-113.
|
|
|
|
|
Lewis G, Rico L (2005). Legumes of the World. In: Lewis G, Schride B, Mackinder B, Lock M. (Eds.). Royal Bot. Gardens, Kew, UK, P 577.
|
|
|
|
|
Lushchak VI (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 224:164-175.
Crossref
|
|
|
|
|
Marques GS, Monteiro RPM, Leão WF, Lyra MAM, Peixoto MS, Rolim-Neto PJ, Xavier HS, Soares LAL (2012). Avaliação de procedimentos para quantificação espectrofotométrica de flavonoides totais em folhas de Bauhinia forficata LINK. Quim. Nova 35(3):517-522.
Crossref
|
|
|
|
|
Martínez-Flórez S, González-Gallego J, Culebras JM, Tu-ón MJ (2002). Flavonoids: properties and anti-oxidizing action. Natr Hosp. 17(6):271-278.
|
|
|
|
|
Mattagajasingh I, Acharya L, Mukherjee AK, Panda PC, Das P (2006). Genetic relationships among nine cultivated taxa of Calliandra Benth. (Leguminosae: Mimosoidaee) using random amplified polymorphic DNA (RAPD) markers. Sci. Hortic. 110:98-103.
Crossref
|
|
|
|
|
Mercurio DG, Wagemaker TAL, Alves VM, Benevenuto CG, Gaspar LR, Maia PMBG (2015). In vivo photoprotective effects of cosmetic formulations containing UV filters, vitamins, Ginkgo biloba and red algae extracts, J. Photoch. Photobio. B. 153:121-126.
Crossref
|
|
|
|
|
Morteza-Semnani K, Saeedi M, Hamidian M, Vafamehr H, Dehpour AR (2002). Anti-inflammatory, analgesic activity and acute toxicity of Glaucium grandiflorum extract. J. Ethnopharmacol. 80:181-186.
Crossref
|
|
|
|
|
Murillo BM, Sánchez A, Quevedo R, Pabón ML, Carulla JEF (2008). Rev. Colomb. Quim. 37(3):287-295.
|
|
|
|
|
Nichols JA, Katiyar SK (2010). Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 302:71-83.
Crossref
|
|
|
|
|
Orishadipe A, Okogun J, Mishelia E (2010). Gas chromatography–mass spectrometry analysis of the hexane extract of Calliandra portoricensis and its antimicrobial activity. Afr. J. Pure. Appl. Chem. 4(7):131-134.
|
|
|
|
|
Petry RD, Souza KCB, Bassani VL, Petrovick PR, González-Ortega G (1998). Doseamento do teor de flavonoides totais em extratos hidroalcoólicos de Passiflora alata Dryander (maracujá). Rev. Bras. Farmacogn. 79:7-10.
|
|
|
|
|
Pietta PG (2000). Flavonoids as Antioxidants. J. Nat. Prod. 63:1035-1042.
Crossref
|
|
|
|
|
Phang CW, Malek SNA, Ibrahim H, Wahab NA (2011). Antioxidant properties of crude and fractionated extracts of Alpinia mutica rhizomes and their total phenolic content. Afr. J. Pharm. Pharmacol. 5(7):842-852.
|
|
|
|
|
Rezaire A, Robinson JC, Bereau D, Verbaere A, Sommerer N, Khan MK, Durand P, Prost E, Fils-Lycaon B (2014). Amazonian palm Oenocarpus bataua ("patawa"): chemical and biological antioxidant activity--phytochemical composition. Food Chem. 149:62-70.
Crossref
|
|
|
|
|
Rice-Evans CA, Miller NJ, Paganga G (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:152-159.
Crossref
|
|
|
|
|
Sá PGS, Guimarães AL, Oliveira AP, Filho JAS, Fontana AP, Damasceno PKF, Branco CRC, Branco A, Almeida JRGS (2012). Fenóis totais, flavonoides totais e atividade antioxidante de Selaginella convoluta (Arn.) Spring (Selaginellaceae). Rev. Cienc. Farm. Basica. Apl. 33(4):561-566.
|
|
|
|
|
Schmidt PC, Ortega GG (1983). Passionsblumenkraut. Bestimmung des Gesamtflavonoidgehaltes von Passiflorae herba. Deutsche Apotheker Zeitung, 133:17-26.
|
|
|
|
|
Sies H (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4:180-183.
Crossref
|
|
|
|
|
Souza RFV, Giovani WF (2005). Synthesis, spectral and electrochemical properties of Al (III) and Zn (II) complexes with flavonoids. Spectrochim Acta A 61:1985-1990.
Crossref
|
|
|
|
|
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K (1992). The formalin test: an evaluation of the method. Pain 51:5-17.
Crossref
|
|
|
|
|
Tegeli V, Karpe P, Katve V (2014). Importance of free radical and antioxidant on human health. IJPCBS 4(4):1038-1050.
|
|
|
|
|
Woisky RG, Marcucci MC, Salatino A (1998). Uso de cloreto de alumínio na quantificação de flavonoides em amostras de própolis. Mensagem Doce 46:3-8.
|
|
|
|
|
Wojtunik-Kulesza KA, Oniszczuk A, Oniszczuk T, Waksmundzka-Hajnos M (2016). The influence of common free radicals and antioxidants on development of Alzheimer's Disease. Biomed Pharmacother. 78:39-49.
Crossref
|
|