ABSTRACT
Ulcerative colitis is a serious premalignant condition with a confusing multifactorial pathogenesis. It should warrant all attention by researchers, for exploration of new prophylactic or therapeutic drugs targeting its pathophysiology. The current study was conducted to study the possible role of tadalafil and its potential actions in ulcerative colitis rat model, induced by acetic acid. Forty eight male Wistar albino rats were classified into 6 groups: control group, acetic acid (AA)-ulcerated group, AA-ulcerated + tadalafil (1 mg/kg/day), AA-ulcerated + tadalafil (5 mg/kg/day), AA-ulcerated + tadalafil (10 mg/kg/day) and AA-ulcerated + sulfasalazine 100 mg/kg/day groups. Tissue malondialdehyde (MDA), superoxide dismutase (SOD) and myeloperoxidase (MPO) were determined. Also, caspase-3 gene expression was measured as an indicator of apoptosis. Histopathological examination of the colonic tissue was also done. In addition, serum levels of interleukin (IL)-1β and tumour necrosis factor (TNF)-α were measured. In AA-ulcerated group, there was significant elevation in tissue MDA levels and MPO activity with upregulation of caspase 3 gene expression. Meanwhile, AA caused decreases in the SOD activities. Also, AA induced elevation in the serum IL-1β and TNF- α levels. Pretreatment with tadalafil in doses of 1, 5 and 10 mg/kg/days guarded against changes in these parameters. Its effects were dose-dependent. According to the results, pretreatment with tadalafil in doses of 1, 5 and 10 mg/kg/day exerted dose-dependent beneficial effects against AA-induced damage of the colon, possibly by exerting anti-inflammatory and antioxidant effects. Also, reduction of apoptosis proved to be one of the contributing protective mechanisms of actions.
Key words: Tadalafil, ulcerative colitis, rats, acetic acid, anti-apoptotic.
Inflammatory bowel disease (IBD) is a debilitating and life threatening disease affecting the colon and primarily including ulcerative colitis (UC) and Crohn's disease (CD). UC is a premalignant disease, with both inflammatory and oxidative factors sharing fundamentally in its pathogenesis (Karakoyun et al. 2011). Transforming growth factor beta 1 (TGF-B1) is a corner stone mediator in UC, which may induce production of proinflammatory cytokines such as tumour necrosis factor alpha (TNF-α). The pathogenesis of IBD also involves activated T-cells, which release various cytokines responsible for production of free radicals and destructive enzymes, with injurious effect on the gastrointestinal tract (Wan et al. 2014). These T cells are abnormal and contribute to the pathogenesis of IBD through apoptosis and can possibly cause the progression to colon cancer (Salari-Sharif and Abdollahi 2010). Defective apoptotic functions occur at the sites of inflammation in UC (Sturm et al. 2008). There is augmentation in intestinal epithelial cell apoptosis with reduction in inflammatory cell apoptosis. Caspase-3, a key enzyme in apoptosis, is activated in apoptotic cells through both extrinsic and intrinsic pathway. This causes colonic destruction with disturbed functions (Qiu et al. 2011).
Almost all drugs used in treatment of UC, aim at reducing symptoms or maintaining remission. These are aminosalicylates, corticosteroids, immunosuppressants and biologic medications, but still they can have side effects as hepatitis, nephritis, fluid retention, immunosuppression and others (Head and Jurenka 2003). Ongoing researches aim at exploring new remedies which may be of further benefit in ameliorating the disease or have additive or synergistic effects to the already used therapies for better disease prognosis.
In studying the anti-inflammatory drugs, phosphodiesterase inhibitors (PDEIs) were found to be effective in different inflammatory disorders and not just restricted to treating erectile dysfunction. Some of the PDE4I showed potential effects in animal studies. Salari-Sharif and Abdollahi (2010) showed their effectiveness in IBD. Their study was the most reliable study in this field. They concluded that PDE4Is benefited IBD by several mechanisms including reduction of inflammation, fibrosis and depression. Parallel to the discovery of PDE4Is and their anti-inflammatory properties, there are ongoing studies on other PDE isoenzymes in immune and proinflammatory cell (Salari and Abdollahi 2012). Thus, more attention is being paid to the effects and further mechanisms of action of PDE4 and PDE5 and their specific inhibitors that affect intestine.
The PDE enzymes are grouped into 11 subfamilies according to their properties, their amino acid sequence and susceptibility to pharmacological therapies (Anwar and Alchter, 2013). They metabolize cAMP and cGMP to 5′-GMP and 5′-AMP (Chung, 2006). PDEs as PDE4 and PDE7 are mainly selective on hydrolysis of cAMP whereas, PDE5, PDE6 and PDE9 are selective on cGMP. Since cGMP accumulation inhibits inflammation, the selective PDE5 inhibitors as sildenafil, tadalafil and vardenafil may seem good candidates for targeting UC diseases in which inflammation plays a central role (Keravis and Lugnier, 2012; Titus et al. 2014).
Tadalafil is a strong long-acting, highly selective inhibitor of PDE5, which targets the enzyme PDE5 and causes smooth muscle relaxation and increases vascular blood supply (La Vignera et al. 2011). Moreover, tadalafil is superior to other well-known selective PDE5 inhibitors as sildenafil or vardenafil in its pharmacokinetic properties which allow for its sustained actions. Also, it has greater selectivity on PDE5 and is slowly metabolized thus could probably be used at lower doses for long-term management of patients. Tadalafil is the only PDE5I which is not affected by food, and therefore it could be given less frequently during long term therapies and it has a relatively rapid onset of action (16 to 17 min) (Kuan and Brock, 2002; Kouvelas et al. 2009).
The aim of this study was to extend the work of other researchers on PDE5I role in colitis and investigate the potential effects and mechanisms of action of tadalafil in a rat model of AA-induced UC.
Drugs, chemicals and kits
Sulfasalazine (Pfizer, Australia) and Tadalafil (Pfizer, NY, USA) were used. Drugs were stored at 2 to 4°C and kept away from exposure to light. Acetic acid glacial (CID pharmaceutical Co, Egypt) 4% (volume/volume), myeloperoxidase (MPO) assay kit (Ray Bio, USA), interleukin (IL)-1β and TNF-α enzyme-linked immunosorbent assay (ELISA) kits, malondialdehyde (MDA) and superoxide dismutase (SOD) assay kits (Nanjing jiancheng Bioengineering, China) were used for analyses.
Animals
A total of 48 adult healthy male Wistar-albino rats weighing 150 to 200 g were used in this study. Animals were harbored on a 12-h light/dark cycle (lights on from 08:00 am) at a constant temperature (24±1°C) and humidity with normal rat chow and water was available ad libitum. The study followed the guidelines for animal welfare and was approved by the Institutional Reviewer Board of Faculty of Medicine, Cairo University.
Induction of colitis
According to Mascolo (Mascolo et al. 1995), after fasting overnight, rats under ether anaesthesia were intrarectally infused with 2 ml AA (4%) using a lubricated paediatric catheter inserted 8 cm proximal to the anus. Rats were kept in a horizontal position for 30 s to avoid AA leakage. Rats in the normal control group received an equal volume of 0.9% saline instead of AA.
Experimental design
Six groups of animals were studied (eight animals in each). Group I: Normal control group that received only distilled water; Group II: AA-ulcerated, non-treated group, animals that received AA for UC induction; Group III: Tadalafil (1 mg/kg/day)- treated group + AA; Group IV: Tadalafil (5 mg/kg/day)-treated group + AA; Group V: Tadalafil (10 mg/kg/day)-treated group + AA; Group VI: sulfasalazine (100 mg/kg/day)-treated group + AA (Sener et al. 2014; Thippeswamy et al. 2011).
From previous studies, the dose of tadalafil which achieved therapeutic effects varied from 0.5 to 10 mg/kg/day in different experimental works (Oh et al. 2008; Ko et al. 2009; Sawamura et al. 2009). It was found that doses of 0.5 and 10 mg/kg tadalafil in rats simulate human doses of 2.5 and 40 mg/day, respectively (Sawamura et al. 2009). Therefore, the doses of 1, 5 and 10 mg/kg per day of tadalafil were chosen in the study.
The pretreated groups received tadalafil or sulfasalazine orally daily for 7 days before induction of colitis and for another 3 days following colitis-induction. Blood samples were collected from the tail vein. Serum was separated by centrifugation and stored at -80°C until used for measuring serum IL-1β and TNF-α. Then, the animals were sacrificed by cervical dislocation under deep anesthesia (Kannan and Jain, 2000). After dissection, the colonic specimens were kept in 10% formalin for histopathological examination. The remaining colonic tissues were maintained at -80°C (ultra-low freezer, Environmental Equipment, Ohio, USA) till homogenized and used for assessment of MDA levels, MPO, SOD activities and caspase 3 gene expression.
Assessment of colitis
Macroscopic colonic damage scoring
According to the scoring system of Millar (Millar et al. 1996), mucosal damage was assessed using the microscope. Inflammation scores were assigned using a scale ranging from 0 to 4: 0 indicates no macroscopic changes, 1 indicates mucosal erythema only, 2 indicates mild mucosal edema, slight bleeding, or small erosions, 3 indicates moderate edema, bleeding ulcers or erosions, and 4 indicates severe ulceration, erosions, edema and tissue necrosis.
Histopathological study
Cross sections of colonic tissues were fixed in 10% formaldehyde, embedded in paraffin blocks, and cut into fine sections. Samples were collected on glass slides, stained with hematoxylin and eosin (H&E) and examined under the microscope by a pathologist in a blinded manner. Additional sections from the paraffin wax blocks were stained with Alcian blue dye. Histopathological slides were examined for destruction of the epithelium and glands, dilatation of glandular crypts, depletion and loss of goblet cells, inflammatory cells infiltration, edema, hemorrhagic mucosa and crypt abscesses using parameters scored from 0 to 3. The colitis score of each rat was assessed by the sum of the subscores of different parameters (Gaudio et al. 1999).
Biochemical studies
Sera stored at -80°C was used to determine TNF-α and IL-1β levels. Homogenized tissue samples were used for the measurement of MDA, MPO and SOD and caspase 3 gene expression.
Measurement of tissue MDA
According to Balasubramanian (Balasubramanian et al. 1988), the colonic content of MDA was measured using the assay kit of TBARS. The results were expressed as nmol/g.
Measurement of tissue MPO activity
MPO activity was measured as described by Krawisz et al. (1984). The results were expressed in ng/g.
Measurement of tissue SOD activity
The enzymatic activity of SOD was measured as described by Kono (1978). The results were expressed in u/g.
Gene expression of caspase 3
According to the manufacturer instructions, isolation of RNA from 100 mg of tissue was done by the aid of an RNA extraction kit (Qiagene, USA). After synthesis of first-strand complementary DNA from 2 μg total RNA (Invitrogen Inc., Carlsbad, California, USA) and denaturing the template RNA and primers (25 pmol of each reverse oligonucleotide primer) at 70°C for 10 min, 40 U reverse transcriptase was added in the presence of RT buffer, 4 μl dNTP mix (250 μmol/l each), 40 U RNase inhibitor, and RNase-free water to achieve the final volume. Incubation of the mixture (50 μl) was done for 1 h at 43°C, then stopped at 4°C, and used on the spot for polymerase chain reaction (PCR) or kept at -80°C until use. Reactions were carried out in triplicate. Conditions of the reaction were: an initial 15 min at 95°C, followed by 40 cycles of 15 s at 94°C, 30 s at 55 to 60°C, and 30 s at 72°C. Real-time PCR was carried out in an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, California, USA). Calculation of relative gene expression was done using the comparative threshold cycle (Ct) method (Livak and Schmittgen, 2001).
Measurement of serum levels of TNF-α and IL-1β
Serum levels of IL-1β and TNF-α were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Values were expressed as pg/ml.
Statistical methods
Data were coded and entered using the statistical package SPSS version 24. Data was described using mean and standard deviation for quantitative variables. Comparisons between groups were done using analysis of variance (ANOVA) with multiple comparisons post hoc test. P-values less than 0.05 were considered as statistically significant.
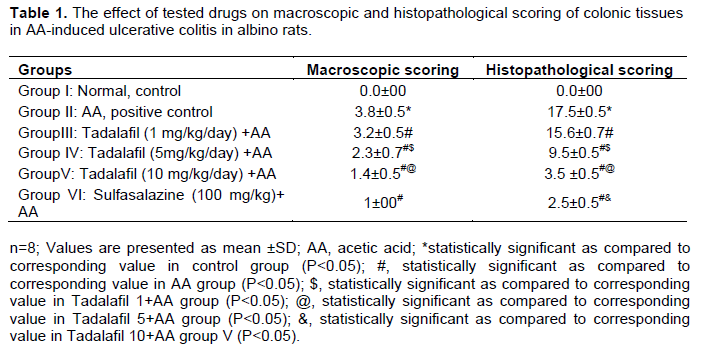
Macroscopic examination
AA caused severe edematous inflammation in the colon, with a high macroscopic scoring of colonic damage as compared to the control group (p<0.05). Sulfasalazine (100 mg/kg/day) significantly reduced the severity of gross lesion scores as compared to the AA group (p<0.05). There was an improvement in colonic damage score by tadalafil (1, 5 and 10 mg/kg/day). The improvement occurred in a dose-related manner (Table 1). Significant difference was found between tadalafil 5 and 10 mg/kg. The effect of sulfasalazine was comparable to that of tadalafil 10 mg/kg.
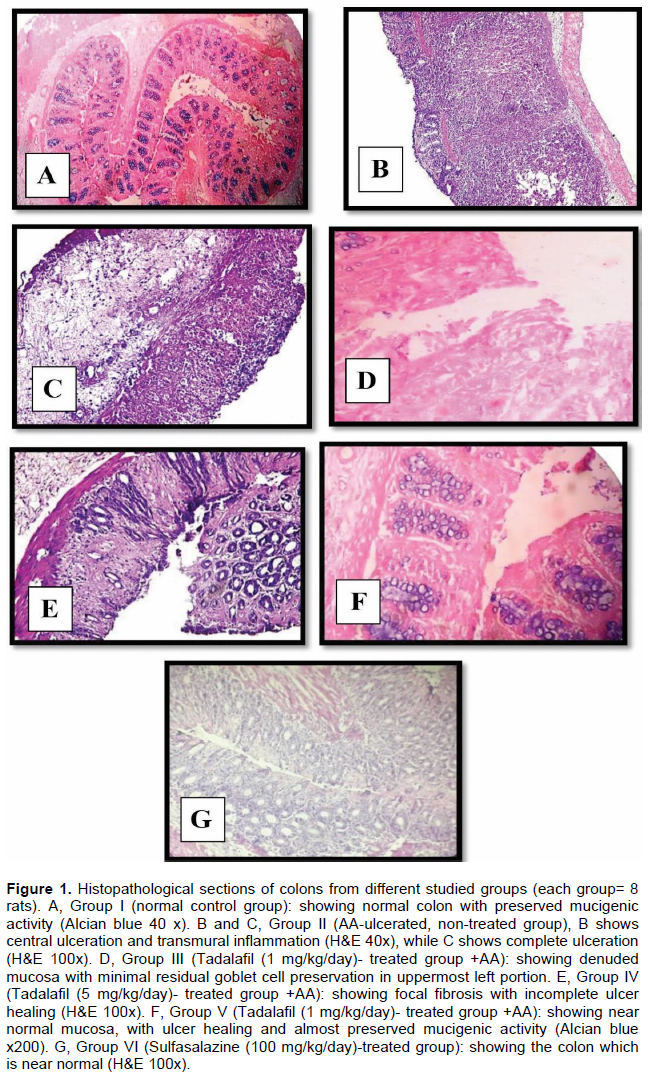
Histopathological changes
The histopathological examination is shown in Figure 1. The normal control group showed normal colonic mucosa with preserved mucigenic activity. AA group showed ulceration of the colonic mucosa with inflammation. Rats pretreated with tadalafil 1 mg/kg showed mucosa with minimal residual goblet cell preservation. Tadalafil 5 mg/kg showed focal fibrosis with incomplete ulcer healing whereas, tadalafil 10 mg/kg showed near normal mucosa, with ulcer healing and preserved mucigenic activity. The drug showed a dose-dependent protective effect against AA-colitis, whereas, sulfasalazine treated group showed near normal results. The histopathological scoring of all groups is illustrated in Table 1. The scoring of AA group was significantly increased as compared to the control group (p<0.05), whereas, tadalafil 1, 5 and 10 mg/kg and sulfasalazine significantly decreased as compared to AA (p<0.05). The effects of tadalafil were dose-dependent and those of sulfasalazine were significant as compared to tadalafil 10 mg/kg.
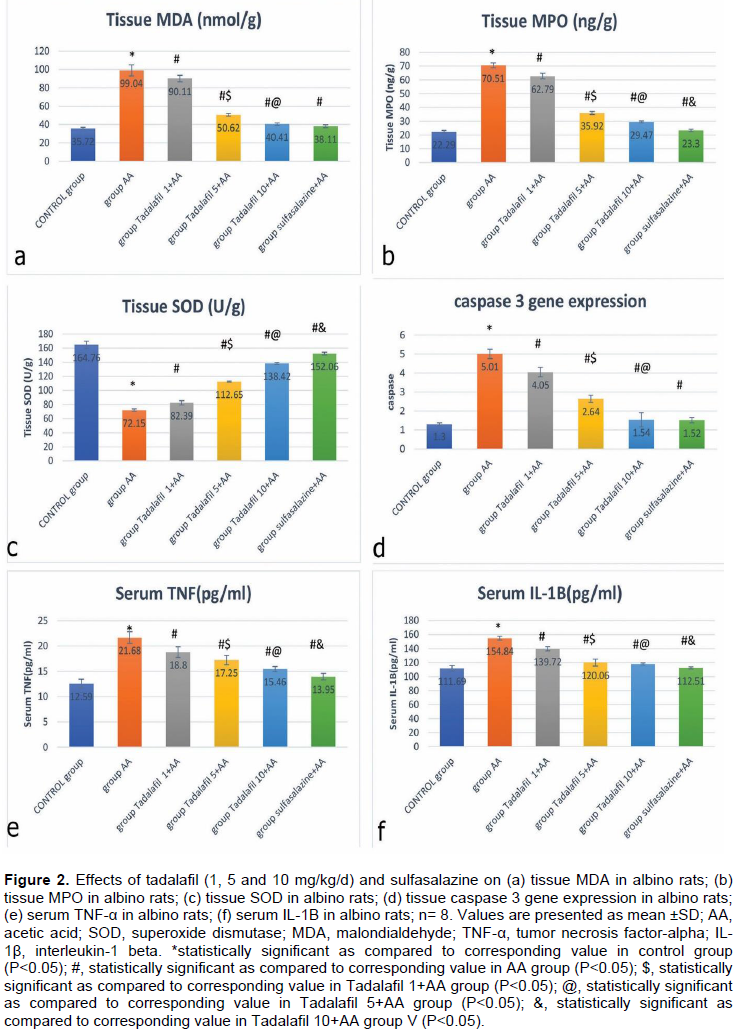
Tissue MDA concentration
In the disease model of UC (AA-ulcerated group), the colonic MDA levels showed significant elevation as compared to the control group (p<0.05). Pretreatment of rats with tadalafil 1, 5 and 10 mg/kg/day showed dose-dependent improvements in each level. Sulfasalazine significantly inhibited elevation of MDA as compared to AA group (p<0.05) meanwhile, no significant difference was found between treatment by sulfasalazine and treatment by tadalafil 10 mg/kg/day (p>0.05) (Figure 2a).
Tissue MPO concentrations
In the AA group, colonic MPO measurements proved to be significantly elevated as compared to control group
(p<0.05). Tadalafil (1, 5 and 10 mg/kg/d) pre-treatment and sulfasalazine significantly prevented the increase in MPO activity as compared to the AA group (p<0.05). The effects of tadalafil were dose-dependent meanwhile, the effect of sulfasalazine was significant as compared to tadalafil 10 mg/kg (Figure 2b).
Colonic SOD activity
Activity of colonic SOD was significantly decreased in AA group as compared to control animals (p<0.05). Three doses of tadalafil and sulfasalazine used showed significant increase in SOD activity as compared to AA group (p<0.05). In addition, the effect of sulfasalazine was significant as compared to tadalafil 10 mg/kg (Figure 2c).
Caspase 3 gene expression
Caspase 3 proteins were highly upregulated in the AA group when compared with the control group (P<0.05). Tadalafil pretreatment by the 3 doses: 1, 5 and 10 mg/kg showed normal levels of caspase 3. In the sulfasalazine-treated group, results were significantly lower as compared to the AA group (P<0.05). Meanwhile, no significant difference was found between sulfasalazine and tadalafil 10 mg/kg (p>0.05) (Figure 2d)
Serum level of TNF-α
The levels of IL-1β and TNF-α in the serum of AA group significantly increased as compared to the control group (p<0.05). But they were significantly lower in the tadalafil (1, 5 and 10 mg/kg) and sulfasalazine pretreated groups as compared to AA-induced colitis group, respectively (p<0.05). The effect of sulfasalazine was significant as compared to tadalafil 10 mg/kg (Figure 2e and f).
IBD is a chronic inflammatory disease with highly expressed inflammatory cytokines such as TNF-α, IL-1β, IFN-g and enzymes such as inducible nitric oxide synthase (iNOS) and cyclo-oxygenase 2 (COX-2). Reactive oxygen metabolites (ROM) are responsible in part, for tissue injury in all inflammatory conditions including colitis. Toxic oxidants can cause damage if the capacity of the endogenous antioxidant enzymes as SOD, catalase and glutathione peroxidase cannot cope with their excess production. Thus, increased oxidative stress and impairment of the antioxidant defenses by the deleterious effect of ROMs contributes to the pathogenesis of colitis (Iseri et al. 2009). It was previously found that oxidative stress and its consequent lipid peroxidation results in increased colonic MDA contents. This in part, is responsible for impaired defensive mechanism (Girgin et al. 2000; Ek et al. 2007). The current study showed that colonic MDA levels were significantly increased in the AA control group and this data is in agreement with the study of Cetinkaya (Cetinkaya et al. 2005). Meanwhile, the administration of tadalafil 1, 5 and 10 mg showed a dose-dependent decrease in MDA levels. Similarly, in the study of Wu et al. (2015), the increased MDA levels in testicular tissues, following I/R injury, were reversed with tadalafil treatment.
Furthermore, there is a strong relation between the status of antioxidant enzymes e.g., SOD and the systemic protection against inflammation. SOD is responsible for the conversion of superoxide to peroxide. This guards against lipid peroxidation in colon by eliminating free-radicals. Decreasing SOD activity in the colonic tissue leads to mucosal injury due to decreased ability of scavenging oxidative radicals (Barazzone and White 2000; Krieglstein et al. 2001). The current study showed that SOD activity was significantly decreased in the AA control group and this data is in agreement with others such as Al-Rejaie (Al-Rejaie et al. 2013). The data demonstrated that administration of tadalafil 1, 5 and 10 mg ameliorated alterations induced by AA in SOD levels. This improvement was dose-related. Similarly, Adeneye and Benebo (2016) proved that tadalafil pretreatment succeeded to restore SOD levels near normal.
The deleterious effects of activated neutrophils lie mainly in production of oxygen metabolites and activation of MPO (Kettle and Winterbourn, 1997). The results of this study showed that AA group was associated with an increase in MPO activity. This was previously confirmed by Mannasaheb (Mannasaheb et al. 2015). Pretreatment with tadalafil in the 3 doses effectively reduced this enzyme activity, suggesting that tadalafil could exert anti-inflammatory effects. Tadalafil inhibited tissue neutrophil accumulation and the associated MPO activity. Similarly, tadalafil succeeded to reverse the increase in renal MPO activity following I/R injury (Küçük et al. 2012). A study by Santos et al. (2005) showed that Sildenafil, another prototype of PDE5I, provided effective protection against indomethacin-induced gastropathy in rats by decreasing the MPO activity.
Serum TNF-α and IL-1β levels significantly increased in the AA control group. Tadalafil limited the up-regulation of pro-inflammatory cytokines, TNF-α and IL-1β which are believed to play a significant role in the pathogenesis of IBD. These authors provided evidence that tadalafil therapy decreased the levels of circulating inflammatory cytokines in the studied animals (Varma et al. 2012). In accordance with the current findings, another study demonstrated that pretreatment with PDE5I, zaprinast, inhibited the increase in serum TNF-a level in mice (Iric et al. 2001). Similarly, Sildenafil, a prototype of PDE5I has shown good effects in experimental colitis by balancing oxidant-antioxidant status and inhibiting ROM production and release of cytokines (Salari-Sharif and Abdollahi, 2010). In a rat model of colitis, Sildenafil prevented lipid peroxidation, cytokine production and neutrophil accumulation, Sildenafil reversed TNF-α and IL-1β in the colitis back to the control value (Iseri et al. 2009). A recent study confirmed the anti-inflammatory effect of a PDE5I in a colitis model in rats (Margonis et al.2015). Also, Sildenafil was found to be beneficial in AA-induced colitis in rats by preventing lipid peroxidation, cytokine release and maintaining oxidant anti-oxidant status (Ahmed et al. 2012).
In the AA group, caspase 3 protein expression was upregulated as previously described by Kaushal et al. (2001). The current study results showed that tadalafil pretreatment by either 1, 5 or 10 mg/kg reduced caspase 3 protein expression. Similarly, Tavukçu et al. (2014) in their study found that tadalafil is able to reduce caspase 3 activity, as an index of apoptosis.
Furthermore, histological data also match the biochemical changes. Microscopical examination confirmed the previous results, where AA group showed ulceration of the colonic mucosa with inflammation. Rats pretreated with tadalafil 1, 5 and 10 mg/kg/day showed a dose-dependent amelioration of AA-colitis. The scoring of AA group significantly increased as compared to the control group, whereas that of tadalafil 1, 5 and 10 mg/kg and sulfasalazine significantly decreased as compared to AA (p<0.05). The effect of sulfasalazine was significant as compared to tadalafil 10 mg/kg.
Overall, the findings of the present study demonstrated that tadalafil pretreatment in doses of 1, 5 and 10 mg/kg exerted significant ameliorating effect against AA-induced UC model. This is most probably attributed to its anti-oxidant, anti-inflammatory and anti-apoptotic effects. The improvement was dose-dependent. Patients treated with tadalafil will benefit from its additional colo-protective effect. A further comparative study on the effects of tadalafil and other selective PDE5I such as sildenafil and vardenafil in UC is recommended. It would be of benefit to point out the one that is most potent and can relatively achieve the best results.
The authors declare that there is no conflict of interest.
REFERENCES
|
Adeneye AA, Benebo AS (2016). Chemopreventive effect of tadalafil in cisplatin-induced nephrotoxicity in rats. Nig. J. Physiol. Sci. 31(1):1-10.
|
|
|
|
Ahmed OM, Afifi A, Ali TM, Ramadan SA, Mahmoud AM (2012). Ameliorative effects of sildenafil in acetic acid-induced chronic colitis in rats. Life Sci. J. 9(1):354-361.
|
|
|
|
|
Al-Rejaie SS, Abuohashish HM, Al-Enazi, MM, Al-Assaf AH, Parmar MY, Ahmed MM (2013). Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 19(34):5633-5644.
Crossref
|
|
|
|
|
Anwar S, Alchter MH (2013). Cardiovascular and other pharmacological approaches of phosphodiesterase enzyme inhibitors. Int. J. Adv. Pharm. Ned. Bioallied Sci. 1:35-39.
|
|
|
|
|
Balasubramanian KA, Manohar M, Mathan VI (1988). An unidentified inhibitor of lipid peroxidation in intestinal mucosa. Biochimica Biophysica Acta (BBA)-Lipids Lipid Metab. 962:51-58.
Crossref
|
|
|
|
|
Barazzone C White CW (2000). Mechanisms of cell injury and death in hyperoxia. Role of cytokines and Bcl-2 family proteins. Am. J. Respir. Cell Mol. Biol. 22:517.
Crossref
|
|
|
|
|
Cetinkaya A, Bulbuloglu E, Kurutas EB, Ciralik H, Kantarceken B, Buyukbese MA. (2005). Beneficial effects of N-acetylcysteine on acetic acid induced colitis in rats. Tohoku J. Exp. Med. 206:131-139.
Crossref
|
|
|
|
|
Chung KF (2006). Phosphodiesterase inhibitors in airways disease. Euro. J. Pharmacol. 533:110-172.
Crossref
|
|
|
|
|
Ek RO, Serter M, Ergin K, Yildiz Y, Cecen S, Kavak T (2007). The Effects of caffeic acid phenethyl ester (CAPE) on TNBS-induced colitis in ovariectomized rats. Dig Dis. Sci. 1007:1609-1617.
|
|
|
|
|
Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R (1999). Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis. Sci. 44(7):1458-1533.
Crossref
|
|
|
|
|
Girgin F, Karaoglu O, Erkus M, Tuzun S, Ozutemiz O, Dincer C (2000). Effects of trimetazidine on oxidant/ antioxidant status in trinitro benzene sulfonic acid-induced chronic colitis. J. Toxicol. Environ. Health Part A. 59:641-52.
Crossref
|
|
|
|
|
Head KA, Jurenka JS (2003). Inflammatory bowel disease, part 1: ulcerative colitis-pathophysiology, conventional and alternative treatment options. Altern. Med. Rev. 8:247-283.
|
|
|
|
|
Iric K, Fujii E, Ishida H, Wada K, Suganuma T, Nishikori T, Yoshioka T, Muraki T (2001). Inhibitory effects of cyclic AMP elevating agents on lipopolysaccharide (LPS)-induced microvascular permeability change in mouse skin. Br. J. Pharmacol. 133:237-342.
Crossref
|
|
|
|
|
Iseri, SO, Ersoy Y, Ercan F, Yuksel M, Atukeren P, Gumustas K, Alican I (2009). The effect of sildenafil, a phosphodiesterase-5inhibitor, on acetic acid-induced colonic inflammation in the rat. J. Gastroenterol. Hepatol. 24:1142-1148.
Crossref
|
|
|
|
|
Kannan K, Jain SK (2000). Oxidative stress and apoptosis. Pathophysiology 7:153-163
Crossref
|
|
|
|
|
Karakoyun B, Uslu U, Ercan F, Aydin MS, Yuksel M, Ogunc AV, Alican I (2011). The effect of phosphodiesterase-5 inhibition by sildenafil citrate on inflammation and apoptosis in rat experimental colitis. Life Sci. 89:402-407.
Crossref
|
|
|
|
|
Kaushal GP, Kaushal V, Hong X, Shah SV (2001). Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 60:1726-1736
Crossref
|
|
|
|
|
Keravis T, Lugnier C (2012). Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 5:1288-305.
Crossref
|
|
|
|
|
Kettle AJ, Winterbourn CC (1997). Myeloperoxidase a key regulator of neutrophil oxidant production. Redox Report 3:3-15.
Crossref
|
|
|
|
|
Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, Shin MC, Cho HJ, Kim SC, Kim SH, Kim KH (2009). Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis in hippocampal neuronal cells in gerbils. Pharmacol. Biochem. Behav. 91: 629-635
Crossref
|
|
|
|
|
Kono Y (1978). Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys.186:189-195.
Crossref
|
|
|
|
|
Kouvelas D, Goulas A, Papazisis G, Sardeli C, Pourzitaki C (2009). PDE5 inhibitors: in vitro and in vivo pharmacological profile. Curr. Pharm. Des. 15: 3464-3475.
Crossref
|
|
|
|
|
Krawisz JE, Sharon P, Stenson WF (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344-1350.
|
|
|
|
|
Krieglstein CF, Cerwinka WH, Laroux FS, Salter JW, Russell JM, Schuermann G (2001). Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J. Exp. Med. 194:1207e18.
|
|
|
|
|
Kuan J, Brock G (2002). Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert Opin. Investig. Drugs 11: 1605-1613
Crossref
|
|
|
|
|
Küçük A, Yucel M, Erkasap N, Tosun M, Koken T, Ozkurt M, Erkasap S (2012). The effects of PDE5 inhibitory drugs on renal ischemia/reperfusion injury in rats. Mol. Biol. Rep. 39:9775-9782.
Crossref
|
|
|
|
|
La Vignera, S, Condorelli, RA, Vicari E, D'agata R, Calogero, A.E (2011). Endothelial apoptosis decrease following tadalafil administration in patients with arterial ED does not last after its discontinuation. Int. J. Impot. Res. 23(5): 200-205.
Crossref
|
|
|
|
|
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402-408.
Crossref
|
|
|
|
|
Mannasaheb BA, Kulkarni PV, Sangreskopp MA, Savant C, Mohan A (2015). Protective effect of Agave americana Linn. leaf extract in acetic acid-induced ulcerative colitis in rats. Ayu. 36(1):101.
Crossref
|
|
|
|
|
Margonis GA, Christoloukas N, Antoniou E, Arkadopoulos N, Theodoropoulos G, Agrogiannis G, Pikoulis E, Patsouris ES, Zografos GC, Papalois AE (2015). Effectiveness of sildenafil and U-74389G in a rat model of colitis. J. Surg. Res. 193:667-674.
Crossref
|
|
|
|
|
Mascolo N, Izzo AA, Autore G, Maiello FM, Di Carlo G, Capasso F (1995). Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J. Pharmacol. Exp. Ther. 272:469-475.
|
|
|
|
|
Millar AD, Rampton DS, Chander CL, Claxson AWD, Blake DR (1996). Evaluating the antioxidant potential of new treatments for inflammatory bowel disease in a rat model of colitis. Gut. 39:407-415
Crossref
|
|
|
|
|
Oh M, Chang H, Minn KW (2008). The effects of tadalafil on axial-pattern skin flap survival in rats. Dermatol. Surg. 34:626-623.
|
|
|
|
|
Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L (2011). PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J. Clin. Investig. 121(5):1722.
Crossref
|
|
|
|
|
Salari P, Abdollahi M (2012). Phosphodiesterase inhibitors in inflammatory bowel disease. Expert Opin. Investig. Drugs 21(3):261-264.
Crossref
|
|
|
|
|
Salari-Sharif P, Abdollahi M (2010). Phosphodiesterase 4 inhibitors in inflammatory bowel disease: a comprehensive review. Curr. Pharm. Design. 16(33):3661-3667.
Crossref
|
|
|
|
|
Santos CL, Souza MHLP, Gomes AS (2005). Sildenafil prevents indomethacin-induced gastropathy in rats: role of leukocyte adherence and gastric blood flow. Br. J. Pharmacol. 146:481-486.
Crossref
|
|
|
|
|
Sawamura F, Kato M, Fujita K, Nakazawa T, Beardsworth A (2009). Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J. Pharmacol. Sci. 111:235-43.
Crossref
|
|
|
|
|
Sener T E, Tinay I, Akbal C, Ersahin M, Cevik O, Cadirci S, Cilingir OT, Cetinel S, Sener G (2014). Tadalafil attenuates spinal cord injury induced oxidative organ damage in rats. Marmara Pharm. 18:49-55.
Crossref
|
|
|
|
|
Sturm A, de Souza HS, Fiocchi, C (2008). Mucosal T cell proliferation and apoptosis in inflammatory bowel disease. Current Drug Targets 9(5):381-387.
Crossref
|
|
|
|
|
Tavukçu HH., Åžener TE., Tinay I, Akbal C, ErÅŸahin M, Çevik, Ö, Åžener G (2014). Melatonin and tadalafil treatment improves erectile dysfunction after spinal cord injury in rats. Clin. Exp. Pharmacol. Physiol. 41(4):309-316.
Crossref
|
|
|
|
|
Thippeswamy BS, Mahendran S, Biradar MI, Raj P, Srivastava K, Badami S, & Veerapur, VP (2011). Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Euro. J. Pharmacol. 654(1):100-105.
Crossref
|
|
|
|
|
Titus DJ, Oliva AA, Wilson NM, Atkins CM (2014). Phosphodiesterase inhibitors as therapeutics for traumatic brain injury. Curr. Pharm. Des. 21:332-342.
Crossref
|
|
|
|
|
Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC (2012). Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. 7:e45243.
|
|
|
|
|
Wan P, Chen H, Guo Y, Bai AP (2014). Advances in treatment of ulcerative colitis with herbs: from bench to bedside. World J. Gastroenterol. 20(39):14099.
Crossref
|
|
|
|
|
Wu ZG, Wang GB, Xiao YB, Chen TK, Cai J, Li CD (2015). Protective effect of tadalafil against ischemia-reperfusion injury in rats. Natl. J. Androl. 21(3):214-218
|
|