ABSTRACT
Vanadium may or may not be a cholesterol-lowering agent. It could potentially be used as an additional therapy or alone in therapies for treatment of dyslipidemia. This study aimed to investigate the effect of vanadium on hypercholesterolemia in the presence and absence of statins. Sixty rats were divided into five groups. The first group was kept on a normal diet and the second group was kept on a high fat diet. The three remaining groups of rats were prepared for the treatment; one group received simvastatin, one was given vanadium, and the third group was tested with both. Blood samples from all groups were investigated. Body functions were considered a tool in expressing efficacy and toxicity for the three types of treatment and were compared with the control groups. Vanadium alone causes marked increases in cholesterol levels. When added to statins, all lipid values were negatively affected as compared to the statins-only treated group. Rats with high fat diets showed significant (P≤ 0.05) elevation in the levels of serum triglycerides (TG), total cholesterol (TC), low density lipoprotein concentration (LDL-C) and very low density lipoprotein concentration (VLDL-C) as compared to the control group. All generated data proved that vanadium is impracticable for treating dyslipidemia. Vanadium is not safe or efficient in therapies for lowering cholesterol and other lipid levels.
Key words: Vanadium, statin, body functions, cholesterol, toxicity, rats.
Dyslipidemia is one of the key risk factors for cardiovascular diseases and several studies have shown that there is a strong causal relationship between dyslipidemia and cardiac diseases (Subramanian et al., 2014). Hypercholesterolemia is the presence of high levels of cholesterol in the blood of living organisms. Longstanding elevation of cholesterol can lead to several health complications such as atherosclerosis. The most efficient antihypercholesterolemia therapeutic agents are known as statins. Statins or hyadroxy-methyl-gluteryl- coenzyme-A-CoA reductase inhibitors (HMG-CoA reductase inhibitors), are the highest selling drugs worldwide (IMS Health, 2012; Lecarpentier et al., 2012; Bhatnagar et al., 2008). These drugs revolutionized the treatment of hypercholesterolemia. Yet, a large number of statins in the market continue to cause a plethora of side effects graded from moderate to severe (Mancini et al., 2011).
The Food and Drug Administration issued safety warnings in 2012 about statins. The most popular drug causes several side effects including cognitive problems, such as memory lapses, dementia, and confusion. In addition to its serious neurological side effects, statins can cause liver damage, muscle pain and heart muscle damage, and is found to increase blood glucose (type II diabetes) (Shechter and Shisheva, 1993). For these reasons, searching for a drug agent to replace statins is necessary. Combining other therapeutic agents to statins may be able to reduce these side effects.
Vanadium is an ultra-trace element believed to be important for normal cell function and development (Verma et al., 1998; Badmaev et al., 1999; Meyerovitch et al., 1991). Vanadium deficiency (less than 1 mg/day) is associated with reproduction impairment, changes in red blood cell formation and iron metabolism (Mingxia et al., 2014). Katheriene (2004) have investigated several metal compounds such as vanadium, zinc, cobalt, chromium and molybdenum, to test their potential of being used as therapeutic agent to treat diabetes mellitus. Vanadium produced the highest effect in lowering glucose and lipids in Wistar rats. The metabolic effects of vanadium are known to be dose dependent and require more than 4 weeks for a complete response (Pugazhenti et al., 1991; Battell et al., 1992; Cam et al., 1995; Liu et al., 2012). The main food sources of vanadium are rice, oats, beans, radishes, barley, buckwheat, lettuce, peas, potatoes, dill, parsley, black pepper, shellfish, meat, mushrooms, soy, wheat and olives (Yuen et al., 1995). Vanadium has toxic effect, but believed to have therapeutic uses as well (Yuen et al., 1995). Diabetes, cancer, chlorosis, anemia and tuberculosis are the most known diseases directly or indirectly affected by vanadium. The mechanism by which vanadium restricts elevation of plasma cholesterol appears to involve both inhibition of cholesterol synthesis and accelerated catabolism of cholesterol (Deborah et al., 2008). The treatment for insulin resistance includes the use of vanadium compounds, which have been shown to enhance insulin responsiveness in animal models (Yuen et al., 1995).
Sanchez and co-workers studied the bioavailability of vanadium and its hypoglycemic effect in magnesium-deficient rats (Sanchez et al., 2011). The group generated data proving that vanadium plays an important role as a micronutrient and as an antidiabetic agent. In that study, vanadium was supplied in the form of bis(maltolato)oxovanadium (IV) in rats drinking water for a duration of five weeks. Recently, Soveid et al. (2013) studied long-term efficacy and safety of vanadium in the treatment of type one diabetes. This research team found that vanadium compounds can reduce blood glucose in experimentally-induced diabetic rats and type 2 diabetic patients. They also reported that cholesterol levels declined but the extent was not reported. Between therapy and toxicity, the research question is;” is vanadium a safe and efficient therapy in lowering cholesterol levels or is it solely a toxic agent”? The evaluation of vanadium as a toxic agent or treatment has not been investigated in the presence of communally used cholesterol lowering agents such as statins. It is believed that comparing vanadium and statins individually, and in combination, can guide in a clear description of the heavy metals value.
The purpose of this work was to examine the effect of vanadium on the level of cholesterol and other body functions in the presence and absence of statins. Effect of vanadium on healthy and high cholesterol rates was studied.
Chemicals and diet
Vanadium (III) chloride (purity 97%) was purchased from Acros, Belgium whereas, cholesterol (purity ≥92.5%) powder was purchased from Sigma Aldrich, St. Louis, MO, USA. All other chemicals were of analytical grade unless specified. To prepare high-fat diet, cholesterol (1 %, w/w) powder was thoroughly mixed with crushed rat pellet diet. The pellets mixed with cholesterol were reconstituted with water and dried properly to avoid any fungal contamination.
Animal treatment
Thirty healthy male Wistar albino rats, weighing between 150 and 200 g, were obtained from the Experimental Animal Care Centre, College of Pharmacy, King Saud University, Riyadh, KSA. They were kept at constant temperature (22±2°C), humidity (55%) and 12 h light/dark conditions during the experiment. Animals were randomly divided into five groups and each group comprised of 6 rats.
Group 1: rats fed with a normal pellet diet for 45 days in addition to normal saline for one week (control group); group 2: rats fed with a cholesterol mixed pellet diet for 45 days in addition to normal saline for one week (HFD group); group 3: rats fed with a cholesterol mixed pellet diet for 45 days plus simvastatin (30 mg/kg) orally by gavage for one week (HFD+ S group); group 4: rats fed with a cholesterol mixed pellet diet for 45 days plus vanadium chloride (15 mg/kg) orally by gavage for one week (HFD + V group); group 5: rats fed with a cholesterol mixed pellet diet for 45 days plus vanadium chloride (15 mg/kg) and simvastatin (30 mg/kg) orally by gavage for one week (HFD + SV group).
After one week of treatment, blood was collected from retro-orbital plexus under light ether anaesthesia in tubes. Serum was separated by centrifugation at 2500 ×g for 10 min and transferred to prelabelled Eppendorf tubes for various biochemical parameters. The experiment was carried out according to the guidelines of
animal care and use committee of King Saud University, Riyadh, Saudi Arabia.
Lipid profile
Total cholesterol (Demacker et al., 1980), triglycerides (Lowell et al., 1973) and high-density lipoprotein (HDL) (Burstein et al., 1970) levels were estimated in serum using Roche diagnostic kits (Roche Diagnostics GmbH, Mannheim, Germany).
Liver function enzymes and bilirubin
Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) (Reitman and Frankel, 1957), alkaline phosphatase (ALP) (Kind and King, 1954), gamma-glutamyl transferase (GGT) (Fiala et al., 1972) and bilirubin (Raedsch and Stiehl, 1983) were determined using a Reflotron Plus Analyzer as well as Roche kits (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Results are presented as mean ± SE (standard error). All analyses were carried out using Statistical Package (SPSS, Version16.0). Data were analyze dusing one-way ANOVA to assess differences between groups. Means were statistically compared using Dennett’s multiple comparison tests at 0.05 significance level. Probability values p < 0.05 were considered to be statistically significant.
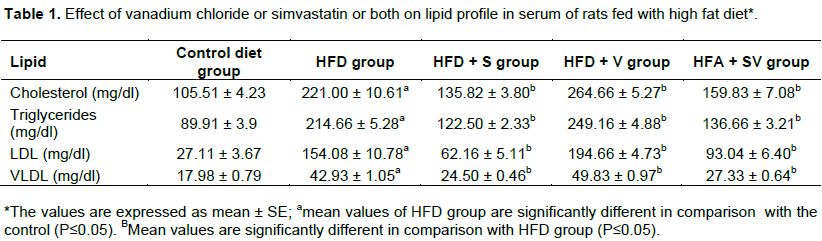
The effect of vanadium chloride alone and with simvastatin on serum TG, TC, LDL-C and VLDL-C of rats fed high fat diet
Rats with high fat diets showed significant (P≤ 0.05) elevation in levels of serum TG, TC, LDL-C and VLDL-C as compared to the control group. Adding simvastatin alone, or with vanadium chloride, to the high fat diet groups 3 and 5, respectively, resulted in a significant (P ≤ 0.05) decrease in the levels of TG, TC, LDL-C and VLDL-C as compared to the HFD-group. On the other hand, HFD-rats with vanadium chloride in group 4 showed further significant increase (P ≤ 0.05) in the plasma levels of TG, TC, LDL-C and VLDL-C when compared with the HFD-group (Table 1).
The effect of vanadium chloride alone and with simvastatin on serum HDL-C of HFD-rats
Significant (P≤ 0.05) decreases in HDL-C levels were noticed in HFD-rats as compared to the control group. Further significant (P ≤ 0.05) reduction in plasma levels in HDL-C was observed with the vanadium chloride treatment in group 4, as compared to the HFD-group. Oral ingestion of simvastatin alone, and with vanadium chloride in groups 3 and 5 caused significant (P ≤ 0.05) elevation in plasma HDL-C concentration as compared to the HFD-group (Figure 1).
The effect of vanadium chloride alone and with simvastatin on integrity and secretory function of HFD-rats
Alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT) activities, the specific biomarkers of hepatic damage, showed significant (P≤ 0.05) increase in HFD-group as compared to the control. The activities of aspartate aminotransferase (AST) and alkaline phosphatase (ALP) and the concentration of total bilirubin were also significantly (P≤ 0.05) elevated in HFD-group as compared to the control. The administration of vanadium chloride with the HFD-rats in group 4, exhibited further significant (P≤ 0.05) increase in all the above hepatic parameters as compared to the HFD-group, while simvastatin alone in group 3 or with vanadium chloride in group 5 exhibited significant improvement in hepatic function. This was indicated by the significant (P ≤ 0.05) decrease in the activities of plasma ALT, AST, GGT and ALP and in the concentration of total bilirubin in both groups 3 and 5 as compared to the HFD-group (Table 2).

The effect of vanadium chloride alone and with simvastatin on synthetic function of liver of HFD-rats
The HFD alone or with vanadium chloride in groups 2 and 4 respectively, affected the synthetic function of the liver as indicated by significant (P ≤ 0.05) reduction in the serum levels of total protein and albumin, while a significant improvement in the synthetic function of liver was observed in groups 3 (simvastatin alone) and 5 (simvastatin combined with vanadium chloride). This was indicated by the significant (P ≤ 0.05) increase in serum total protein and serum albumin concentrations in those groups as compared to the HFD-group (Figures 2 and 3).
Vanadium therapy has been shown to normalize blood glucose levels in diabetic-induced rats and to cure many hyperglycemia-related disorders (Gail et al., 2011). Many other researchers focused on the toxicity of vanadium compounds depending on its oxidation state, administration or exposure route, doses and sensitivity of the organism. Here, the effects of oral administration of 15 mg/kg of vanadium chloride alone and in combination with 30 mg/kg of simvastatin on serum lipids and liver function of rats fed with a high cholesterol diet were investigated. The current study showed that feeding rats with a high cholesterol diet for 4 weeks, results in significant increase in serum concentration of total cholesterol, LDL-cholesterol, VLDL-cholesterol and triglyceride as compared to the control group. However, the HDL-cholesterol decreased in the case of the HFD treated group as compared to the control. These results are in agreement with those emphasized by Zhang and his co-worker (2013) who demonstrated the same pattern of changes in lipid profile in the HFD-induced model of hyperlipidemia. It has been reported that exposure to large quantities of fructose and fats leads to rapid stimulation of lipogenesis with accumulation of triglycerides and contributes to hepatic insulin resistance (Schaalan et al., 2008). High intake of dietary saturated fatty acids and cholesterol also increases the level of plasma cholesterol, particularly that within the LDL fraction. Apo-B is required for assembly and secretion of LDL and VLDL, and increases the plasma’s total cholesterol and triglyceride (TG) levels (Vallim and Salter, 2010).
The HFD-rats’ oral ingestion with 30 mg/kg body weight of simvastatin significantly decreases serum total cholesterol, LDL-C, VLDL-C and triglycerides, while causing significant increase in HDL-C as compared to the HFD-group. The results are in agreement with Corsini et al. (1992) who reported that statins are used for lowering hypercholesterolemia through its inhibitory effect on the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase enzyme that catalyzes the rate-limiting step of the cholesterol synthesis in the liver and other tissue. It has been suggested that lipophilic statins, such as simvastatin, have absorptivity by tissue and are more effective in inhibition of extra-hepatic HMG-CoA reductase than other hydrophilic statins, such as pravastatin (McKenney, 2003). Also, it has been reported that the HDL-C level was higher in simvastatin-treated groups when compared with the control (Yong et al., 2014). The LDL-receptor uptake and clear LDL-C from circulation. This receptor is regulated at the transcriptional or posttranscriptional level (Kong et al., 2006). Simvastatin induced the upregulation of the LDL receptor gene expression. The study showed an increase in the rate of LDL receptor degradation with statin as well. This finding suggests that statins might increase the rate of LDL-C removal from the blood by increasing the rate of its receptor cycling (Ness et al., 1996). Other studies reported that plasma oxidized LDL was significantly reduced in statin-treated rats. The reduction in oxidized LDL is parallel to that of LDL-C (Elisavet et al., 2013).
Unfortunately, vanadium chloride (HFD+S group) did not improve dyslipidemea in rats fed with the high cholesterol diet. Diversely, vanadium chloride worsened the lipid profile as shown by the further significant increase in serum total cholesterol, LDL-C, VLDL-C and triglycerides. In addition, HDL-C unfavourably and significantly decreased as compared to the HFD-group. This change in blood lipids found was most likely generated by disturbance in the hepatocytes lipid metabolism. It is well known that the in vivo effect of various metals, such as vanadium, may results from their interactions with protein-bound essential groups (Mahmoud et al., 2011). These interactions may lead to activation or inactivation of specific enzymes that affect lipid metabolism. It has been reported that vanadium ions may interfere with many metabolic processes at many levels (Garribba et al., 2015). Vanadium-protein interactions are starting points for understanding the effects of vanadium on the biological system (Pessoa, 2015). Phosphate is important because it is involved in a number of biological recognition bio-catalytic systems. Due to the closely and analogously physicochemical properties of vanadate and phosphate, the vanadium ion is able to systematically inhibit many enzymes, such as phosphatase, phosphodiesterases and phosphoglucomutases (Costa Pessoa, 2015). Such enzymes may be involved in acceleration of lipogenic pathways in tissues that lead to lipid accumulation in blood. The co-administration of simvastatin with vanadium chloride significantly decreases the deleterious effects of vanadium and high cholesterol diet. This is explained by the decline of blood total cholesterol, LDL-C, VLDL-C and triglycerides, and the incline in HDL-C levels in the (HFD+SV) group as compared to the HFD-group.
The present study showed that feeding rats with a high cholesterol diet alone (HFD-group), or with oral ingestion of 15 mg/kg of body weight vanadium chloride (HFD+V group), causes structural and functional damage to the liver. High cholesterol diets significantly increased serum activity levels of ALT, AST, GGT and ALP as compared to the control group, which indicates damage in the structural integrity of hepatocytes. In addition, the synthetic and excretory functions of the liver deteriorated in HFD groups as indicated by the significant decrease in the serum total protein and albumin concentration as well as a significant increase in the level of total bilirubin, respectively, as compared with the control group. These results are in accordance with those obtained by Chen et al. (2010). It was reported that rodents fed high fat diets demonstrate visceral adiposity, hyperglycaemia, dyslipidaemia, hyperinsulinemia and hepatic steatosis (Beyegue et al., 2012). These symptoms may be considered a liver dysfunction risk. The oral ingestion of vanadium chloride with a high cholesterol diet in the HFD+V group does not protect the liver as expected. This is indicated by further significant increase in the serum activity of ALT, AST, GGT and ALP and further significant increase in the serum concentration of total bilirubin as compared to the HFD-group. Also, the synthetic dysfunction of liver becomes more significant as indicated by the decreased concentration of total serum protein and serum albumin as compared to the HFD-group. The results are consistent with the findings of Mahmoud et al. (2011). It has been reported that vanadium is a potentially toxic environmental pollutant that can interact and inhibit many enzymes and therefore may have negative effects on the liver (Mahmoud et al., 2011). According to the work done by Stohs and Bagchi (1995), vanadium induces lipid peroxidation and oxidative stress, which may be considered as a potent factor in hepatotoxicity.
On the other hand, the oral ingestion of 30 mg/kg of BW simvastatin alone or with vanadium chloride by rats fed high cholesterol diets in HFD+S- and HFD+SV groups, respectively, significantly amended hepatotoxic effects of both high cholesterol diet and/or vanadium chloride. This is indicated by significant reduction in all of the above assayed biochemical parameters except serum total proteins and serum bilirubin which significantly increased as compared to the HFD-group. It has been reported that simvastatin has anti-inflammatory properties that may reduce the risk of hepatotoxic effects (Vaughan et al., 1996). Other studies showed that simvastatin supresses the production of pro-inflammatory cytokines from hepatocytes and exerted immunomodulatory effects, (Buchwald et al., 1995) which may contribute to the reduction of vanadium and a high fat diet toxicity in liver.
Although, this research was carefully conducted, it still has limitations and shortcomings. First, the research was conducted using treatment for short period of seven days. Seven days is not enough to observe the clear picture of the changes made by vanadate. Second, using a single dose of vanadium does not present the exact level in which vanadium begins to cause negative effects. Since the assessment of the dose, as well as its response, was based on studying one dose level of vanadium, further research is recommended to test longer treatment time of up to, for at least, three weeks as well as test with at least three dosage levels of vanadate.
This study indicated that vanadium did not show reduction of cholesterol levels or other lipid profiles in the experimental animals. The examined vanadium (III) compound was found to cause an elevation of cholesterol, which provides evidence to avoid the use of vanadium as a dyslipidaemia therapy. Since the assessment of the dose and response was based on one dose level of vanadium, further long-term mechanistic study should be conducted to consider at least, three dosage levels in order to confirm these results.
The authors have not declared any conflict of interests.
REFERENCES
|
Badmaev V, Prakash S, Majeed M (1999). Vanadium: a review of its potential role in the fight against diabetes. J. Altern. Complementary Med. 5(3):273-291.
Crossref
|
|
|
|
Battell M, Yuen V, McNeill J (1992). Treatment of BB rats with vanadyl sulfate. Pharmacol. Commun. 1:291-301.
|
|
|
|
|
Beyegue CFN, Ngangoum RMC, Kuate D, Ngondi JL, Oben JE (2012). Effect of Guibourtia tessmannii extracts on blood lipids and oxidative stress markers in triton WR 1339 and high fat diet induced hyperlipidemic rats. Biol. Med. 4(1):1-9.
Crossref
|
|
|
|
|
Bhatnagar D, Soran H, Durrington PN (2008). Hypercholesterolaemia and its management. Bmj. 337:a993.
Crossref
|
|
|
|
|
Buchwald H, Campos CT, Boen JR, Nguyen PA, Williams SE (1995). Disease-free intervals after partial ileal bypass in patients with coronary heart disease and hypercholesterolemia: report from the Program on the Surgical Control of the Hyperlipidemias (POSCH). J. Am. Coll. Cardiol. 26(2):351-357.
Crossref
|
|
|
|
|
Burstein M, Scholnick HF, Morfin R (1970). Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11(6):583-593.
|
|
|
|
|
Cam M, Faun J, McNeill J (1995). Concentration-dependent glucose-lowering effects of oral vanadyl are maintained following treatment withdrawal in streptozotocin-diabetic rats. Metabolism 44:332-339.
Crossref
|
|
|
|
|
Chen WY, Chen CJ, Liu CH, Mao FC (2010). Chromium attenuates high-fat diet-induced non-alcoholic fatty liver disease in KK/HlJ mice. Biochem. Biophy. Res. Commun. 397(3):459-464.
Crossref
|
|
|
|
|
Corsini A, Raiteri M, Soma MR, Gabbiani G, Paoletti R (1992). Simvastatin but not pravastatin has a direct inhibitory effect on rat and human myocyte proliferation. Clin. Biochem. 25(5):399-400.
Crossref
|
|
|
|
|
Costa Pessoa J (2015). Thirty years through vanadium chemistry. J. Inorg. Biochem. 147:4-24.
Crossref
|
|
|
|
|
Deborah A, Smith SM, Winter P, Zhou J, Dou P, Baruah B, Trujillo AM, Levinger NE, Yang X, Barisas BG, Crans DC (2008). Effects of Vanadiumâ€Containing Compounds on Membrane Lipids and on Microdomains Used in Receptorâ€Mediated Signaling. Chem. Biodivers. 5(8):1558-1570.
Crossref
|
|
|
|
|
Demacker PN, Hijmans AG, Vos-Janssen HE, van't Laar A, Jansen AP (1980). A study of the use of polyethylene glycol in estimating cholesterol in high-density lipoprotein. Clin. Chem. 26(13):1775-1779.
|
|
|
|
|
Elisavet M, Evangelos NL, Constantinos CT, Haralambos JM, Alexandros DT, Moses SE (2013). Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis 231(1):8-14.
Crossref
|
|
|
|
|
Fiala S, Fiala AE, Dixon B (1972). γ-Glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and spontaneous mouse hepatomas. J. Nat. Cancer. Inst. 48(5):1393-1401.
|
|
|
|
|
Gail RW, Lai-Har C, Michael G, Paul JK, Jason JS, Alejandro MT, Josephine AA, Wenjin D, Zihua H, Debbie CC (2011). Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 255(19):2258-2269.
|
|
|
|
|
Garribba E, Santos MF, Santos-Silva T (2015). Vanadium and proteins: uptake, transport, structure, activity and function. Coord. Chem. Rev. 301:49-86.
|
|
|
|
|
IMS Health (2012). US Top Ten Products by Prescriptions Source: IMS HEALTH, National Prescription Audit PlusTM. Available at: http://www.imshealth.com/en/about-us/news/top-line-market-data#
|
|
|
|
|
Kind RPN, King EJ (1954). Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Path. 7:332-336.
Crossref
|
|
|
|
|
Kong WJ, Liu J, Jiang JD (2006). Human low-density lipoprotein receptor gene and its regulation. J. Mol. Med. 84:29-36.
Crossref
|
|
|
|
|
Lecarpentier E, Morel O, Fournier T, Elefant E, Chavatte-Palmer P, Tsatsaris V (2012). Statins and pregnancy: Between supposed risks and theoretical benefits. Drugs 72(6):773-788.
Crossref
|
|
|
|
|
Liu J, Cui H, Liu X, Peng X, Deng J, Zuo Z, Wang K (2012). Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol. Trace Elem. Res. 145(2):189-200.
Crossref
|
|
|
|
|
Lowell BF, Ralph TD (1973). Stable reagents for determination of serum triglycerides by a colorimetric hantzsch condensation method. Clin. Chem. 19(3):338-340.
|
|
|
|
|
Mahmoud KE, Shalahmetova T, Deraz S, Umbayev B (2011). Combined effect of vanadium and nickel on lipid peroxidation and selected parameters of antioxidant system in liver and kidney of male rat. Afr. J. Biotechnol. 10(79):18319-18325.
|
|
|
|
|
Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pope J (2011). Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can. J. Cardiol. 27:635-662.
Crossref
|
|
|
|
|
McKenney JM (2003). Pharmacologic characteristics of statins. Clin. Cardiol. 26(S3):32-38.
Crossref
|
|
|
|
|
Meyerovitch J, Rothenberg P, Shecter Y, Bonner-Weir S, Kahn R (1991). Vanadate normalizes hyperglycemia in two mouse models of non-insulin-dependent diabetes mellitus. J. Clin. Invest. 87(4):1286-1294.
Crossref
|
|
|
|
|
Mingxia X, Deliang C, Fang Z, Gail RW, Debbie CC, Wenjun D (2014). Effects of vanadium (III, IV, V)-chlorodipicolinate on glycolysis and antioxidant status in the liver of STZ-induced diabetic rats. J. Inorg. Biochem. 136:47-56.
Crossref
|
|
|
|
|
Ness GC, Zhao Z, Lopez D (1996). Inhibitors of cholesterol biosynthesis increase hepatic low density lipoprotein receptor protein degradation. Arch. Biochem. Biophys. 325:242-248.
Crossref
|
|
|
|
|
Pugazhenti S, Angel J, Khandelwal R (1991). Long-term effects of vanadate treatment on glycogen metabolism and lipogenic enzymes of liver in genetically diabetic (db/db) mice. Metabolism 40(9):941-946.
Crossref
|
|
|
|
|
Raedsch R, Stiehl A, Gundert-Remy U, Walker S, Sieg A, Czygan P, Kommerell B (1983). Hepatic Secretion of Bilirubin and Biliary Lipids in Patients with Alcoholic Cirrhosis of the Liver. Digestion 26(2):80-88.
Crossref
|
|
|
|
|
Reitman S, Frankel S (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28(1):56-63.
Crossref
|
|
|
|
|
Sanchez C, Torres M, Bermudez-Pena MC, Aranada P, Montes-Bayon M, Sanz-Medel A, LIopis J (2011). Bioavailability, tissue distribution and hypoglycemic effect of vanadium in magnesium-deficient rats. Magnes. Res. 24(4):196-208.
|
|
|
|
|
Schaalan M, El-Abhar HS, Barakat M, El-Denshary ES (2008). Westernized-like-diet-fed rats: effect on glucose homeostasis, lipid profile, and adipocyte hormones and their modulation by rosiglitazone and glimepiride. J. Diabet. Complications 23(3):199-208.
Crossref
|
|
|
|
|
Shechter Y, Shisheva A (1993). Vanadium salts and the future treatment of diabetes. Endeavour 17:27-31.
Crossref
|
|
|
|
|
Soveid M, Dehghani GA, Omrani GR (2013). Long-term efficacy and safety of vanadium in the treatment of type 1 diabetes. Arch Iran Med. 16(7):408-4011.
|
|
|
|
|
Stohs SJ, Bagchi D (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18(2):321-326.
Crossref
|
|
|
|
|
Subramanian IP, Sorimuthu PS, Muthusamy K (2014). Antidyslipidemic effect of a novel vanadium-3-hydroxy flavone complex in streptozotocin-induced experimental diabetes in rats. Biomed. Prevent. Nutr. 4(2):189-193.
Crossref
|
|
|
|
|
Vallim T, Salter AM (2010). Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins, Leukot. Essent. Fatty Acids 82(4):211-218.
Crossref
|
|
|
|
|
Vaughan CJ, Murphy MB, Buckley BM (1996). Statins do more than just lower cholesterol. Lancet 348:1079-1082.
Crossref
|
|
|
|
|
Verma S, Cam M, McNeill J (1998). Nutritional factors that can favorably influence the glucose/insulin system: vanadium. J. Am. College. Nutr. 17(1):11-18.
Crossref
|
|
|
|
|
Yong JY, Hyo JK, Sang KJ, Sung GK, Young RS, Wookyung C, Kum HH, Chang HL, Young-Hwan H, Kook-Hwan O (2014). Comparison of the Efficacy and Safety Profile of Morning Administration of Controlled-release Simvastatin Versus Evening Administration of Immediate-release Simvastatin in Chronic Kidney Disease Patients with Dyslipidemia. Clin. Therapeut. 36(8):1182-1190.
Crossref
|
|
|
|
|
Yuen V, Orvig C, Mcneill J (1995). Comparison of the glucose-lowering properties of vanadyl sulfate and bis(maltolato)oxovanadium(IV) following acute and chronic administration. Can. J. Physiol. Pharmacol. 73(1):55-64.
Crossref
|
|
|
|
|
Zhang S, Zheng L, Dong D, Xu L, Yin L, Qi Y, Han X, Lin Y, Liu K, Peng J (2013). Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 141(3):2108-2116.
Crossref
|
|