ABSTRACT
This study presents results of ozonation of some different aromatic rings of medicines in aqueous solution. The aim of the research was to investigate the influence of ozone on ring cleavage of drug molecules with an aromatic structure. In this regard, phenobarbital, naphazoline, doxycycline, isoniazid and salbutamol were chosen for exposure to ozone gas overnight. It is proved that ozone can attack the OH groups, opening the aromatic ring to form unsaturated acids, aldehydes and linear alkyl/cycloalkyl groups which then appear as stable products. It is suggested that the first step in these reactions includes penetration of ozone at the C-H bond with the formation of a quinone and a subsequent attack of the aromatic ring T-bond system by the ozone with the formation of ozonides. With this process, ozonation can produce simple, biodegradable and less carcinogenic compounds from complex aromatic ones.
Key words: Ozone, aromatic compound, oxidation, toxicity.
In recent years one of the most significant concerns of the research-based pharmaceutical industry is environ-mental contamination and, more specifically for pharmaceutical agents, safety. Among the organic compounds, soluble aromatics have been especially studied because of their biological stability (Dore et al., 1980). Aromatic rings are found in most drugs and have been studied widely due to their toxicity. Their potent toxicity or carcinogenesis is a major issue for human populations, as are their potential impact as environmental pollutants and in emissions from diesel and gasoline engines. Relative to their importance, the toxicity, carcinogenicity and their metabolic pathways in organisms have been examined (Marie, 2009). They also preferred to be replaced with linear and branched alkyl/cycloalkyl groups.
Ozonation has been known for more than a century. In the 1920s, this phenomenon was studied while ozone-induced cracking was developed during and immediately after World War II; the research led to the discovery of the first chemical antiozonants. Ozone, a triatomic allotrope of oxygen, is a tremendously strong oxidant agent that can easily react with the unsaturated chemical bonds in organics. It also can cleave and oxidize benzene and other aromatic rings (Gong et al., 2008) (Sahu, 2011). The term ‘‘ozonolysis’’ as applied in this study specifically refers to the cleavage of bonds between sp2 or sp carbon atoms. The reaction between ozone and the C=C bond is assumed to follow a bimolecular law, where each C=C bond functions as an independent kinetic unit. The concepts behind this mechanism had been summarized previously (Anachkov et al., 2000; Anachkov et al., 1996; Razumovsky et al., 1986). Moreover, it also has been observed that ozonation can convert large molecules into smaller ones and increase the ratio of hydrophilic organics in water (Gong et al., 2008). Ozone is widely used in Europe for the removal of colors, tastes, odors and pathogens in drinking water.
Two mechanisms were considered for the reaction between the ozone molecule and an organic: (1) Direct O3 molecule oxidization (electrophilic addition) or (2) Indirect oxidization through •OH (nucleophilic addition). Comparatively, the electron- donating groups (-OH, -NH etc.) react faster with O3 than the electron-withdrawing groups (-COOH, -NO2 etc.) when ozone attacks the ortho and para positions primarily. In the other words, O3 reacts easily with electron-donor with aromatic compounds resulting in ortho and para intermediates which can be further oxidized, consequently to quinonoid compounds and later to aliphatic compounds concluding with hydroxide radical and carboxy compounds. The following findings demonstrate the manner in which radicals like •OH may be produced and the manner in which they function (Hui-xiang et al., 2005; Staehelin and Hoigne, 1982).
The literature concerning the kinetics and mechanism of ozonation of such macromolecular compounds is scarce. Most qualitative opinions on the kinetics have been published (Marie, 2009; Sweeney, 1981). The problem is that ozonation is multistage process, and a wide variety of intermediates are formed that are difficult to identify.
Sample preparation
All of the selected drugs were used as model to investigate the ability of the ozonation process to eliminate the aromatic rings of these compounds, with an ultimate goal of reducing their toxicity. Experiments were carried out in a glass reactor with jacket at room temperature, as illustrated in Figure 1. Ozone was produced for the test using an ozone generator, model CD- 0013.5. This unit produces 13.5 g of ozone per hour embedded in a flow of medical oxygen at 3 L min-1. Depending on a solubility of the compounds, 50 mg of phenobarbital, naphazoline, doxycycline, isoniazid and salbutamol in deionized water in a volume of 50 ml were tested. The top of each glass reactor had five connections providing for the collection of materials with the same flow rate of the ozone bubbling. After ozonation, samples were collected, lyophilized and kept under refrigeration until characterization.
Laboratory determinations
Fourier transform infrared spectroscopy (FTIR) analysis using a Perkin-Elmer spectrum GX spectrometer was used to determine the FTIR spectra of samples before and after ozonation. Lyophilized powders of the sample were mixed with spectrophotometric grade KBr at 1:5 ratios and pressed into pellet form. FTIR spectra were recorded in the frequency range of 400 to 4000 cm-1 with 32 scans and a resolution of 4 cm-1. The functional groups in the compounds were identified by comparisons of the resultant IR spectra before and after ozonation. This technique also was deployed for studying various stretching and bending vibrations of the aromatic functional groups. UV/Vis absorption spectra were recorded with a Shimatzo 160A UV/Vis spectrometer. A 1 cm quartz cuvette was used for measuring absorbance and deionized water was used as a blank. The Nuclear Magnetic Resonance (NMR) spectra were recorded in D2O using Bruker Drx-500 Avance spectrometers at 296 K. Approximately 50 mg of each dried sample were added to 0.5 ml D2O in a 10 mm NMR tube. The signal for D2O was used as a reference and set to 4.8 ppm chemical shift. Chemical shifts are given in parts per million (δ-scale).
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of the compounds were prepared, before and after ozonation. All the samples (Figure 2) showed band shifting and reductions in the functional groups of the aromatic rings. For comparison the vibration of the O-H bond in hydroxyl groups in the region of ~3200 to 3500 cm-1 after ozonation for all compounds are shifted to lower frequencies at ~3076 to 3291 cm-1. This may imply that more OH groups are involved in intermolecular bonds (Allen et al., 2001). In all compounds except naphazoline, there is a broad carbonyl peak in aromatic rings at ~1730 cm-1 due to lactone, followed by a maximum at ~1739 cm-1 from the formation of aliphatic esters. In phenobarbital the peak splits to one additional data at 1752 cm-1 that is related to ketones. In doxycycline and isoniazid, the main peak in the region of ~1620 cm-1 is related to carbonyl groups which disappear after ozonation. This suggests that the carbonyl group is changed to a, b-unsaturated aliphatic groups (Sahu, 2011).

The appearance of two absorption bands in phenobarbital and isoniazide at ~1599 to 1523 cm-1 are consistent with the formation of an unusual but novel -C=C- stretch of an enol tautomer of a, b-diketone product. The band at ~1407 cm-1 is synonymous with the formation of methyl ketones or aldehydes. The bands that appeared at ~1174 cm-1 are related with a mixture of aliphatic esters and ethers. The two shoulders at 1050 to1000 cm-1 typically are due to residual ozonides. Mono substituted terminal vinyl groups also appear to be formed at ~900 cm-1. FTIR bands decreasing in phenobarbital and doxycycline are the aromatic in-chain di and tri-substituted vinyl groups absorbing at ~810 cm-1 (Allen et al., 2001).
Some peaks rose due to the treatment of the residue with ozone. In other words, a number of functional groups were apparent in the ozone-treated samples that were elusive before treatment. The application of small doses of ozone may result in polymerization processes (Amy, 1988). It is seen that all the spectra are quite similar. Specifically, the O-H in-plane bending vibration is observed in the region 1398 to 1413 cm-1. The bands centered in the region ~2970 to ~2872 cm-1 in naphazoline, phenobarbital, salbutamol correspond to the stretching mode of asymmetrical CH3 and CH2 vibrations mainly of aliphatic carbonyl-containing functional groups. The peak centered at 2794 cm-1 in salbutamol may correspond to the stretching mode of symmetrical C-H vibrations of cyclic ether. The absorption peaks detected in the region of 1500 to 1800 cm-1 are typical for the overlapping of the (C=C) stretching vibration mode of the aromatic ring, quinone, with the (C=O) absorption peaks of free carboxylic, ester and carbonyl groups. The most remarkable feature of the spectra is the appearance of a broad carbonyl (C=O) peak in the region 1709 to 1712 cm-1 which is asymmetrical to higher frequencies, and displays a shoulder at 1770 cm-1.
Ultraviolet spectrometry (UV visible)
A further literature search on reaction mechanisms shows that most authors are in agreement that the first step consists in hydroxylation of the aromatic ring. The absorption bands for phenol and quinone, and the yields resulting from ring opening, are strong and separate. Some organic impurities also have absorption at 200 to 300 nm. At the same time, there was a split parallel increase in the intensity of absorption at 230 to 240 nm region of the spectrum associated with absorption of the unsaturated carbonyl and carboxylic acids formed in aromatic ring opening. The spectrum of quinone in aqueous solution shows a maximum at 260 nm. The fact that there was no obvious increase in the band at 260 nm ruled out the possibility of the formation of quinone as an intermediate in the interaction of O3 with aromatic ring in aqueous solution. The ozone attacks the O-H bond of the aromatic, giving a mixture of several tautomeric radicals which then break down to form the mixed reaction yields (Razumovskii et al., 1979).
A study of the change in UV spectra after ozonation, suggests the progressive disappearance of absorbance maxima which corresponds to bands of aromatic rings (transition π•+ π*). As for salbutamol, appearance and Disappearance of a band was observed and the absorbance maximum was 245 nm. This band corresponds to the formation of p-quinone.
Spectroscopy
The 1HNMR spectrum for all samples confirmed the presence of seven strong proton signals (1.26, 4.08, 4.69, 4.71, 2.56, 3.93 and 3.26 ppm) (Figure 5). The signals at 5.85 and 6.02 ppm are attributable to aromatic hydrogen, respectively. The twin signals at 4.08 and 4.71 ppm for salbutamol and another signal at 4.69 ppm for phenobarbital indicated the presence of three amino groups (NH). These findings are in complete agreement with data obtained from literature reviews (Fotouhi et al., 2006). A small signal between 8.00 and 9.00 ppm for isoniazid is assigned to amide proton. This cleavage seems to be favored due to the possible resonance stabilized carbanion formed from deprotonation of methine carbon. In doxycycline and salbutamol at 0 to 1.6 ppm, protons on methyl and methylene carbons directly bonded to other carbons. The small signal at 1.6 ppm in salbutamol is due to protons on methyl and methylene carbons in alpha position to aromatic rings and the 3.2 ppm signal in phenobarbital, naphazoline and doxycycline is related to carboxyl and carbonyl groups. At 3.2 to 4.3 ppm, protons on methyl, methylene or methine carbons directly bond to oxygen or nitrogen, including carbohydrate and amino acid protons. The small signals at 8.07, 7.49 and 8.23 ppm in doxycycline, naphazoline and isoniazid are assumed to be from protons attached to unsaturated carbons or aromatic protons (Kim and Yu, 2007). Similar signals in doxycycline and phenobarbital were observed at 0.9 to 1.3 ppm, assigned mainly to saturated CH2 groups. Within the region of 2.8 to 4.4 ppm, an increased signal was noticed, which indicates an increase in oxygen-containing functional groups. The signal at 3.07 ppm in doxycycline and salbutamol is due to H and CH3 groups and the 3.93 ppm in naphazoline and salbutamol is for CH2; it implies the 6.82 to7.74 CH aromatic disappeared. In addition, the 4.65 ppm for all samples is related to CH2 (Grinhut et al., 2011, Ohlenbusch et al., 1998). Thus, the obtained 1HNMR data obtained support the hypothesis that the ozonation of aromatic compounds can be the result of cleavage of the aromatic rings. Comparatively, the protons of the products are much more than aromatics counterparts. However, comparison of the NMR and optical data provides evidence for a complex mechanism of interactions of substrates with ozone, and does allow us to state with certainty the cleavage of aromaticity direct coordination.

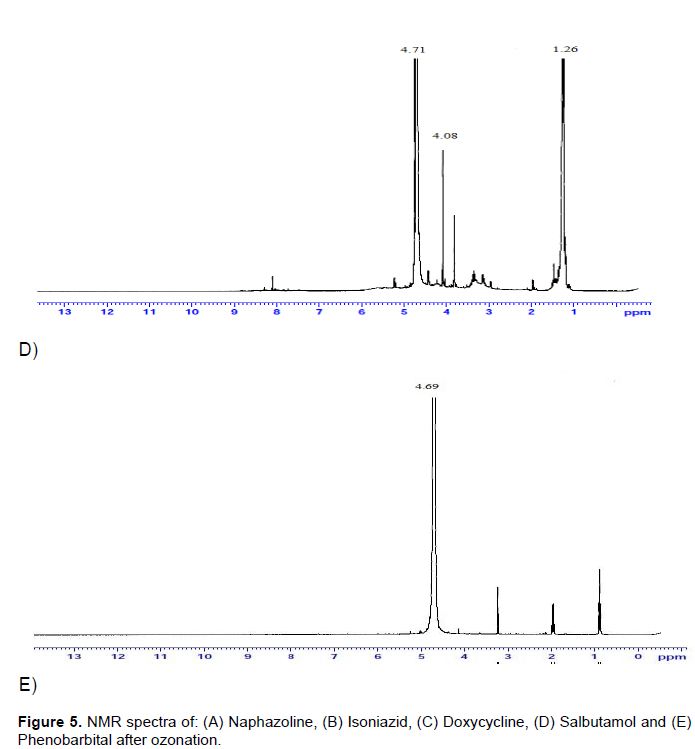
The data demonstrates exposure of some drugs with aromatic rings to ozone gas resulted in the consistent formation of unsaturated yields based on aliphatic esters, ketones, and lactones as well as aromatic cleavage. Results obtained during the ozonation of aqueous solutions of aromatic compounds have shown that the reaction occurs in two steps: A first phase leading to the opening of the aromatic ring and a second phase in which the sub-products, resulting from the first phase, are stable towards further ozonation. They do not, consequently, consume ozone; moreover they are biodegradable (Evison, 1977; Hann, 1956). Since ozone is such a powerful oxidizer, this suggests this process may be used for the destruction of organic substances. It is evident that ozone preferably oxidizes in electron-rich sites (Hoighe and Bader, 1983). As a result, a dramatic decrease in UV absorbance at 254 to 280 nm is observed during ozonation of the aromatic compounds. It is known that during ozonation in water solutions, hydroxyl radicals (OH•) proceed along with direct ozonation. OH• radicals react relatively unselectively (Hoighe, 1982; Staehelin and Hoigne, 1985). In its turn, OH• radicals produce organic radicals as a result of their reactions with organic compounds. The reactions with OH• radicals may bring about considerable structural changes in macromolecules including hydroxylation, decarboxylation, and deploy-merisation of the initial materials, producing oxidized structures that are hydrophobic and less aromatic.
The authors have not declared any conflict of interests.
REFERENCES
|
Allen NS, Edge M, Wilkinson A, Liauw CM, Mourelatou D, Barrio J, Martinez-Zaporta MA (2001). Degradation and stabilisation of styrene-ethylenebutadiene-styrene (SEBS) copolymer. Polym. Degrad. Stab. 7:113-22.
|
|
|
|
Amy GL,Kuo CJ,Sierka RA(1988).Ozonation of humic substances: effects on molecular weight distributions of organic carbon and trihalomethane formation potential. Ozone: Sci. Eng. J.10(1):39-54.
Crossref
|
|
|
|
|
Anachkov MP, Rakovski SK, Stefanova RV (2000). Ozonolysis of 1, 4-cis-polyisoprene and 1, 4-trans-polyisoprene in solution. Polym. Degrad. Stab. 67(2):355-363.
Crossref
|
|
|
|
|
Anachkov MP, Rakovski SK, Stoyanov AK (1996). DSC study of thermal decomposition of partially ozonized diene rubbers. J. Appl. Polym. Sci. 61(4):585-590.
hCrossref
|
|
|
|
|
Dore M, Langlais B, Legube B (1980). Mechanism of the reaction of ozone with soluble aromatic pollutants. Ozone: Sci. Eng. J. 2(1):39-54.
Crossref
|
|
|
|
|
Evison LM (1977). Disinfection of water with ozone: Comparative studies with enteroviruses, phages and bacteria. In 3rd Congress IOI. Paris, Intl. Cleveland, Ohio: Ozone Association.
|
|
|
|
|
Fotouhi L, Mosavi M, Heravi MM, Nematollahi D. (2006). "Efficient electrosynthesis of 1,2,4-triazino[3,4-b]-1,3,4-thiadiazine derivatives." Tetrahedron Lett. 47(48):8553-8557.
Crossref
|
|
|
|
|
Gong J, Liu Y, Sun X (2008). O 3 and UV/O 3 oxidation of organic constituents of biotreated municipal wastewater. Water Res. 42(4):1238-1244.
Crossref
|
|
|
|
|
Grinhut T, Hertkorn N, Schmitt-Kopplin P, Hadar Y, Chen Y (2011). Mechanisms of humic acids degradation by white rot fungi explored using 1H NMR spectroscopy and FTICR mass spectrometry. Environ. Sci. Technol. 45(7):2748-2754.
Crossref
|
|
|
|
|
Hann VA (1956)."Disinfection of drinking water with ozone." J. Am. Water Works Assoc. 48:1316-1320.
|
|
|
|
|
Hoigne J (1982). Mechanisms, rates and selectivities of oxidations of organic compounds initiated by ozonation of water. Handbook of Ozone Technology and Applications, 1.
|
|
|
|
|
Hui-xiang SH, Xian-wen XU, Xin-hua XU, Da-hui WA, Qi-da WA (2005). Mechanistic study of ozonation of p-nitrophenol in aqueous solution. J. Environ. Sci. 17(6):926-929.
|
|
|
|
|
Kim HC, Yu MJ (2007). Characterization of aquatic humic substances to DBPs formation in advanced treatment processes for conventionally treated water. J. Hazard Mater. 143(1):486-493.
Crossref
|
|
|
|
|
Ohlenbusch G, Hesse S, Frimmel FH (1998). Effects of ozone treatment on the soil organic matter on contaminated sites. Chemosphere 37(8):1557-1569.
Crossref
|
|
|
|
|
Razumovskii SD, Ovechkin VS, Konstantinova ML (1979). The kinetics and mechanism of the reaction of ozone with phenol in aqueous solution. Russian Chem. Bull. 28(2):261-264.
Crossref
|
|
|
|
|
Razumovsky SD, Podmasteriyev VV, Zaikov G (1986). Kinetics of the growth of cracks on polyisoprene vulcanizates in ozone. Polym. Degrad. Stab. 16(4):317-324.
Crossref
|
|
|
|
|
Sahu MK, Tewari K, Sinha ASK (2011). Oxidation of vacuum residue by ozone and nitrous oxide: FTIR analysis. Indian J. Chem. Technol. 18:91-98.
|
|
|
|
|
Staehelin J, Hoigne J (1982). Decomposition of ozone in water: rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 16(10):676-681.
Crossref
|
|
|
|
|
Staehelin J, Hoigne J (1985). Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ. Sci. Technol. 19(12):1206-1213.
Crossref
|
|
|
|
|
Stiborová M, Martínek V, Semanská M, Hodek P, DraÄínský M, CvaÄka J, Schmeiser H, Frei E (2009). Oxidation of the carcinogenic non-aminoazo dye 1-phenylazo-2-hydroxy-naphthalene (Sudan I) by cytochromes P450 and peroxidases: a comparative study. Interdiscip. Toxicol. 2(3):195-200.
Crossref
|
|
|
|
|
Sweeney M (1981). Comparative studies in the ozonolysis of lignin and coal. Thermochim. Acta 48(3):263-275.
Crossref
|
|



