ABSTRACT
This research investigated pseudobulb morphological development process of Cremastra appendiculata (D. Don) Makino by paraffin section and micro-observation combining morphological observation method, and analyzed correlation between pseudobulb morphogenesis and its part of biochemical compositions to provide clues for revealing the inhibition mechanism of germination of elder pseudobulbs by the newborn one. The results showed that pseudobulb morphogenesis of C. appendiculata can be divided into six stages: Leaf bud dormant stage, leaf bud sprouting stage, elongation growth stage of leaf bud and rhizomes, pseudobulb initial formation stage, pseudobulb swelling stage and pseudobulb full development stage. There was a close relationship between the changes of the soluble sugar and protein contents both in the newborn and elder pseudobulb development soluble sugar contents were the maximum in leaf bud dormant stage. And in annual, biennial and triennial pseudobulbs, soluble sugar contents were 23.94, 34.21 and 39.02 mg·g-1 respectively. However, soluble protein contents were the minimum in annual, biennial and triennial pseudobulbs, which were 1.30, 1.43 and 1.58 mg·g-1, respectively. With the leaf buds germinating, soluble sugar and protein contents were presenting the change trend of decline firstly, rising secondly, and then declining.
Key words: Cremastra appendiculata (D. Don) Makino, pseudobulb, morphogenesis, biochemical component.
Cremastra appendiculata (D. Don) Makino is an ornamental plant of Orchidaceae. Its dried pseudobulbs, one of the main Chinese medicinal materials, were contained in the "Chinese Pharmacopoeia" (2015 edition). C. appendiculata grows in damp ground with altitude below 2,900 m. It is distributed in the Yellow River Basin to the southwest and south and other provinces in China, such as Sichuan, Guizhou, etc. In addition, it is also distributed in Nepal, Bhutan, Sikkim, India, Vietnam, Thailand and Japan (Dong et al., 2007). The pseudobulbs of C. appendiculata has been used internally against tumors and cancers of the liver, breast, cervix and uterus (Li, 1996; Shim et al., 2004), or externally as Chinese traditional medicine for treating toxin of sores, snake bites, skin burns or scald burns by a poultice or paste (Zhang et al., 2006).
However, long-predatory overexploitation, limitation of its own reproduction mechanism and ecological environment destruction of its growing regions, C. appendiculata has become a rare and endangered species, and its wild distribution area and quantity are dropping quickly (Zhang et al., 2006). In recent years, because of unique physiological and pharmacological characteristics, domestic and overseas scholars are paying attention to C. appendiculata increasingly. So far, there are some achievements on its rapid propagation (Zhang et al., 2006; Mao and Ding, 2004; Mao et al., 2007), reproductive system (Chung and Chung, 2003.), growth and development (Zhang et al., 2010), population quantity and genetic drift (Chung et al., 2004), chemical compositions (Ikeda et al., 2005; Xue et al., 2005, 2006; Xia et al., 2006; Zhang et al., 2007; Liu et al., 2008; Liu et al., 2013; Wang et al., 2013; Liu et al., 2014), pharmacological action (Sun, 2001; Yan et al., 2002; Adriána et al., 2007; Lee et al., 2009; Ruan et al., 2009; Yang et al., 2010), symbiotic fungi (Zhu, 2009; Yagame et al., 2013), artificial cultivation (Zhang, 2008), etc. In previous study, we found that pseudobulb morphogenesis of C. appendiculata is associated with the changes of some biochemical components. So we investigated the process of C. appendiculata pseudobulb morphogenesis by paraffin section and micro-observation combining with morphology observation method, and analyzed the correlation between pseudobulb morphogenesis and its part of biochemical compositions, which will provide clues for revealing the inhibition mechanism of germination of elder pseudobulbs by the newborn one and lay a theoretical foundation for the resource conservation and the exploitation of C. appendiculata.
C. appendiculata (D. Don) Makino was collected from Gaopo Township Huaxi District in Guiyang Guizhou province of China and transplanted in facility cultivation field. According to follow-up observations of cultivated C. appendiculata, we took four parallel samples each time for different age-class pseudobulbs (annual, biennial, triennial pseudobulb in pseudobulbs string) on the 10th, the 20th and 30th of every month from March to September 2015, then the samples were frozen in liquid nitrogen and stored at -80°C refrigerator for further research.
Microstructure observation of pseudobulb
C. appendiculata pseudobulbs and growing well buds were chosen to take pictures. After that, pseudobulbs were cut into small pieces with thickness of 0.3 to 0.5 cm by a scalpel, then put into a weighing bottles containing FAA fixative (formalin-acetic acid-alcohol fixed liquid) and vacuum pumping for 15 to 30 min, and fixed for more than 5 days with FAA fixative containing 70% ethanol. Li (1987)’s method was used to make paraffin sections with thickness of 8 μm and to observe the pseudobulb microstructure under an optical microscope.
Soluble sugar content determination in pseudobulb
Soluble sugar content was determined by anthrone colorimetry (Li, 2000). The elder and newborn pseudobulbs of C. appendiculata were chopped, mixed as fresh samples and weighed. Mixture with 0.2 g samples and 15 mL distilled water was put into a large test tube, boiled in a boiling water bath for 30 min, cooled and filtered. The residue was rinsed several times by distilled water. The filtrate and irrigating fluid were collected with 50 mL volumetric flask, diluted with distilled water to scale, shaken well, until it become the crude extract, used for the determination.
Soluble protein content determination in pseudobulb
Soluble protein content was assayed by Coomassie Brilliant Blue (G-250) staining (Zhang, 2000; Shi, 2014). 0.2 g fresh pseudobulbs sample of C. appendiculata was placed in a mortar, 2 mL distilled water was added and ground into homogenate, and the homogenate was moved to 10 mL centrifuge tube. The mortar was washed three times with 6 mL distilled water, and the liquid was transferred into the centrifuge tube which was then centrifuged for 15 min with 5000 rpm. Its supernatant was transferred to 25 mL volumetric flask. Its precipitate was mixed with 5 mL distilled water, and the mixture was centrifuged for 15 min. All supernatant was merged, and diluted with distilled water to scale, shaken well, until it become the crude extract, used for the determination.
Morphogenesis process of C. appendiculata pseudobulb
Morphological changes in the generating process of pseudobulb
The results illustrate that morphogenesis process of C. appendiculata pseudobulb can be divided into six stages: Leaf buds dormant stage (Figure 1a), leaf buds sprouting stage (Figure 1b), leaf buds and rhizomes elongate growth stage (Figure 1c), pseudobulb initial formation stage (Figure 1d), pseudobulb swelling stage (Figure 1e) and pseudobulb full development stage (Figure 1f). This results is similar to the six development stages in the tissue culture of Cymbidium hybridum pseudobulb in vitro (protocorm stage, organ differentiation stage, rhizome growth period, pseudobulb initial formation stage, pseudobulb swelling stage, pseudobulb full development stage) (Zhang, 2007).
At the leaf buds dormant stage, new mature pseudobulbs of C. appendiculata have prepared for flower buds differentiation, until the reproductive grows to a certain stage, the leaf buds began to sprout. But some plants did not perform reproductive growth and directly into the leaf buds dormant stage.
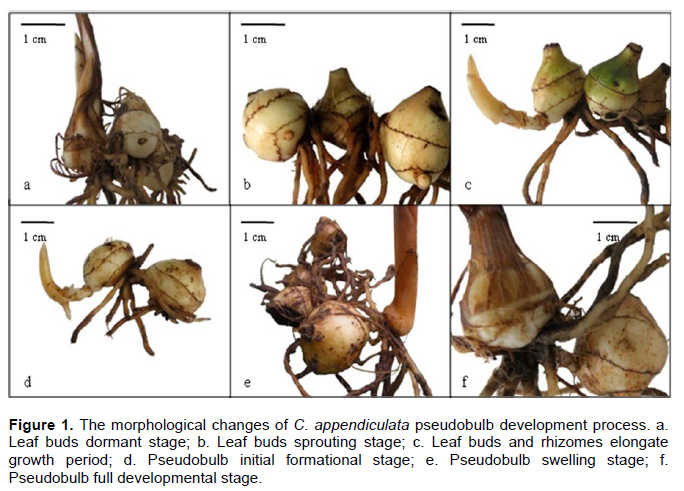
After entering the leaf buds sprouting stage, leaf buds tuber could be clearly observed on pseudobulbs. With the continued growth and differentiation of leaf buds, differentiation and growth of rhizomes also have begun, and the emergence of the rhizomes and rhizome root protrusion are observed clearly. To the pseudobulb initial formation stage, rhizomes elongation growth ended, there were a circle of roots surrounding the rhizomes at the top of rhizomes and on the base of the buds, which is a sign of newborn pseudobulb will forming on the point, and the top of rhizomes and the base of buds to begin expanding for the formation of newborn pseudobulbs, and then into the pseudobulb swelling stage. Newborn pseudobulbs developed fully and then formed mature pseudobulbs which could differentiate and develop to whole plants. From Figure 1, it is observed that there were usually two to three nodes on C. appendiculata pseudobulbs, while pseudobulb breeding buds were usually born from the first or second node of the base of the youngest pseudobulb. The whole morphogenesis process cycle of C. appendiculata pseudobulbs starts generally in the mid or at the end of March and ends in November basically each year.
Microstructure changes in pseudobulb generating process
Microstructure observations of C. appendiculata pseudobulbs had indicated that morphogenesis process of pseudobulbs can be divided into six periods: Leaf buds dormant stage (Figure 2a), leaf buds sprouting stage (Figure 2b), leaf buds and rhizomes elongate growth stage (Figure 2c to f), root differentiation stage (Figure 2g), pseudobulb initial formation stage (Figure 2h) and pseudobulb full development stage (Figure 2i). At the leaf buds dormant stage, almost the entire leaf bud was wrapped in pseudobulb by a few scaly leaves. At the leaf buds sprouting stage, the apical meristem and leaf primordium can be observed clearly, the apical meristem was ringed by polylaminate tegmentum and leaf bud obviously protruded into the epidermis of pseudobulb. The leaf buds and rhizomes elongate growth period started after the leaf bud germination. In this period, the rhizome forming between the base of leaf bud and pseudobulb, the vascular bundle was located in the center of the rhizome (Figure 2d and e), which arranged regularly in circle, the scaly leaf on the node of rhizome could be observed clearly, and the apical meristem of leaf buds and leaf primordium were also clearly visible. After entering the root differentiation stage, the vascular bundle in the rhizome was protruding to the epidermis and would break through to form real roots. The formation of roots signaled the end of the rhizomes elongation growth, then entering the pseudobulb initial formation stage. This stage was obvious different from the preceding stages, its main features were as follows: (1) The roots had been formed and the vascular bundle were scattered among the fundamental tissue of pseudobulb. (2) There were no
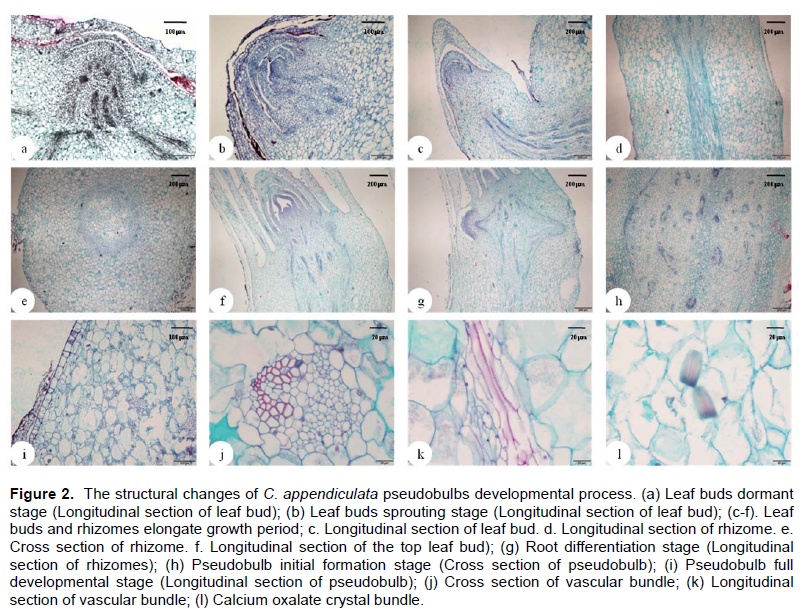
significant differences in the structure at the pseudobulb swelling stage and full developmental stage, pseudobulb mainly composed of epidermis, cortex, fundamental tissue and vascular bundle, and it was a typical structure of monocotyledon stem. (3) The part of epidermal cells and parenchyma cells of fundamental tissue contained calcium oxalate crystal bundle (Figure 2l). (4) The observed result from transverse section and longitudinal section of pseudobulb vascular bundles (Figure 2j and k) indicated that the vascular bundle was toughening type limited vascular bundle, the xylem and phloem were obvious visible, the catheters in vascular bundle was trapezoidal and V-shaped arrangement.
The change of main biochemical components in newborn and elder pseudobulb with the morphogenesis of C. appendiculata pseudobulb
The correlation between soluble sugar changes and pseudobulb morphogenesis
In the morphogenesis process of C. appendiculata pseudobulb, the changing rule of soluble sugar content in newborn and elder pseudobulbs is consistent basically, but the overall tendency was the biennial more than the triennial, and the triennial more than the annual (Figure 3). In leaf buds dormant stage, the soluble sugar content in newborn and elder pseudobulbs all showed a maximum value (annual, biennial and triennial pseudobulbs was 23.94, 34.21 and 39.02 mg•g-1, respectively.), then soluble sugar content fell badly, and tinily returned soon afterwards. The soluble sugar content in newborn and elder pseudobulbs decreased to the lowest (annual, biennial and triennial pseudobulb was 9.15, 12.79 and 7.99 mg•g-1, respectively) before the leaf buds germination, because soluble sugar was the material and energy basis of leaf bud germinating. After leaf buds sprouting, the soluble sugar content are gradually increased. However, with elongation growth of leaf buds and rhizomes, the soluble sugar content were appeared to different changing trends in different years of pseudobulbs (the soluble sugar content of annual pseudobulbs was not basically changes, it showed a downward trend in biennial pseudobulbs and got risen slowly in triennial pseudobulbs). This is because the
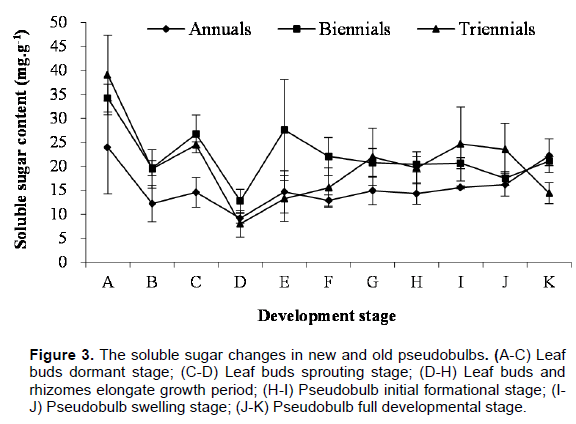
soluble sugar of annual pesudobulb was kept dynamic equilibrium between consume and produce, but biennial pesudobulb was connected to annual pseudobulbs by rhizomes and its soluble sugar content fell off due to competition from annual pesudobulb. And the production of soluble sugar was greater than the consumption for triennial pesudobulb which was away from annual pesudobulb. Entering the pseudobulb initial formation stage, the soluble sugar content in annual and triennial pesudobulb was slowly raising trend, but biennial almost had no changes. In this period, the newborn roots had been formed, and leaf buds had unearthed, plants can obtain essential substances from the soil and air, so the changes of soluble sugar content in newborn and elder pseudobulbs were not obvious. In the pseudobulb expansion period, the soluble sugar content of annual pseudobulbs was gradually rising, and it was slowing down in biennial and triennial pseudobulbs, because newborn pseudobulbs fully developed need to spend a lot of material and energy from elder pseudobulbs.
At the pseudobulb fully development stage, the soluble sugar content of annual and biennial pseudobulbs was increased gradually, because the newborn plants can carry out photosynthesis and photosynthates were transferred to the elders. On the contrary, the soluble sugar content fell sharply in triennial pseudobulbs, it might be the soluble sugar was consumed too much when the newborn pseudobulbs fully developed, and photosynthate fails to transport to triennial pseudobulbs. It is clear that the changes of soluble sugar content in pseudobulb and the development process of C. appendiculata pseudobulb had close correlation.
The correlation between soluble protein changes and pseudobulb morphogenesis
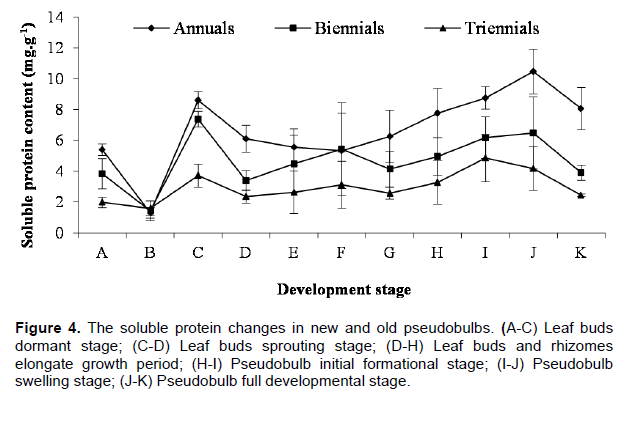
The soluble protein changes of newborn and elder pseudobulbs also showed basically consistent in the morphogenesis process of C. appendiculata pseudobulb, but its change rule was different from the soluble sugar, its change trends was the annual more than the biennial, and the biennial more than the triennial, which means the metabolism vitality of annual pseudobulbs was the strongest (Figure 4). The change rule of soluble protein and sugar was the same before leaf buds germination; merely the emergence period of their highest and lowest value was different. In the dormant stage of leaf buds, the soluble protein in elder and newborn pseudobulbs were the lowest, the contents in annual, biennial and triennial pseudobulbs was very close (1.30, 1.43 and 1.58 mg•g-1, respectively). To enter the bud germinating period, the soluble protein content increased sharply in the annual and biennial pseudobulbs, but it increased slowly in triennials, the highest value followed by 8.60, 7.38 and 3.72 mg•g-1. Since leaf buds germinating needs a mass of functional proteins (enzyme) to take part in the complex metabolic process. Then, the soluble proteins content decreased rapidly in newborn and elder pseudobulbs with leaf buds being released, especially for the biennials, it might be because the soluble proteins transform into structure proteins in the stage. Next some newborn pseudobulbs developmental stage, the soluble proteins content in newborn and elder pseudobulbs trended overall upward, it illustrated that metabolic activity of pseudobulbs was becoming stronger and stronger. In the fully developed stage, the soluble proteins content of newborn and elder pseudobulbs showed a trend of overall decline, it showed that metabolic activity of pseudobulbs wore off. In a word, the morphogenesis of pseudobulb and the soluble protein changes in pseudobulb of C. appendiculata also had close correlation.
Seed setting rate of C. appendiculata is very low in natural conditions (Chung and Chung, 2003) and its seeds hardly germinated, so pseudobulbs are almost its only reproductive organs (Chung et al., 2004) and medicinal parts. So far, about the morphogenesis of C. appendiculata pseudobulbs and its material changes are little-known. This research found the morphogenesis of C. appendiculata pseudobulbs process mainly through three kinds of morphological changes (buds → rhizomes → pseudobulbs) and six stages (leaf buds dormancy stage → leaf buds germination stage → leaf buds and rhizomes elongation stage → pseudobulbs initial formation stage → pseudobulbs swelling stage → pseudobulbs full developed stage). Zhang (2007) reported that the formation process of Cymbidium hybridum pseudobulb passed through three similar morphological changes (protocorm → rhizome → peseudobulb) in the tissue culture condition. The anatomical structure of C. appendiculata pseudobulb showed that it was comprised of epidermis, cortex, fundamental tissue and vascular bundle from outside to inside. Epidermis is one layer of parenchyma cells, the cortex is two to three layers of thick wall cells, cortex and some basic tissue cells often contain calcium oxalate crystal needle beam, toughening type limited vascular bundle are scattered in the basic organization. The typical structure of monocotyledon stems is similar to the pseudobulb anatomical structure of Cymbidium goeringii (Tang, 2013), C. sinense (Liu, 2009) and C. grandiflorum (Zhang, 2007). Under normal circumstances, monocotyledon does not have secondary structure (Liu, 1991), the buds of rhizomes grow into plants, and then its stem upper expands in different degree to form pseudobulb (Wang, 1989). By observing and analyzing the C. appendiculata pseudobulb slices, we find that its development process is basically consistent with above orchids. The macrostructure of four processes from rhizome, pseudobulb initial formation, pseudobulb intumescence, to pseudobulb sufficient development had little changes, but the position and quantity of pseudobulb vascular bundles had significant changes, from circumcresent in the centre of rhizomes to scatter in fundamental tissue of pseudobulb, from less to more. It can thus be seen that the pseudobulb intumescence of C. appendiculata was associated with the increase of vascular bundles.
In this study, we found the related morphogenesis is close to the changes of biochemical components of C. appendiculata pseudobulbs, especially the soluble sugar and soluble proteins. The cause of this phenomenon may be various; the pseudobulbs accumulated a large amount of soluble sugar in the winter. Then the sugar will be excessively consumed with the rising of temperature and the end of dormant period, cell recovery and leaf bud stirring. Followed by a small rebound might be due to the carbohydrate synthesis metabolic pathways were induced from a feedback by soluble sugar consumption. The changes of soluble proteins content seemingly implied that annual pseudobulbs metabolic activities were stronger than the elder pseudobulbs. It is well known that sugar is energy material and crucial intermediate metabolites in life activity, its levels can reflect available material and energy basic in plants (Zhan et al., 2011). The soluble proteins are the components of many important enzymes in plants and relate directly to cells energy supply and to catalyze many chemical reactions (Berry et al., 1982). Their types and levels are the result of gene expression, and the content can indirectly reflect the strength of plant metabolic activities and stress-resist ability. The relationship between the morphogenesis of pseudobulbs and other relevant biochemical components in pseudobulbs of C. appendiculata should be further explored.
The authors have not declared any conflict of interests.
This research was funded to MSZ by a grant (No. 81360613) from the Natural Science Foundation of China (NSFC) and the Project of High-level Innovative Talents in Guizhou (No. 2015-4031).
REFERENCES
|
Adriána K, Andrea V, Judit H (2007). Natural phenanthrenes and their biological activity. Phytochemistry 69:1084-1110.
|
|
|
|
Berry JA, Downton WJS (1982). Environmental regulmion of photosynthesis. In. Govindjcccd, Photosynthesis (Vol. II) . New York: Academic-Press, pp. 294-306.
|
|
|
|
|
Chinese Pharmacopoeia Commission (2015). China Pharmacopeia (Volume I). China Medical Science and Technology Press.
|
|
|
|
|
Chung MY, Chung MG (2003). The breeding systems of Cremastra appendiculata and Cymbidium goeringii: high levels of annual fruit failure in two self-compatible orchids. Ann. Bot. Fenn. 40:81-85.
|
|
|
|
|
Chung MY, Nason JD, Chung MG (2004). Implications of Clonal Structure for Effective Population Size and Genetic Drift in a Rare Terrestrial Orchid, Cremastra appendiculata. Conserv. Biol. 18:1515-1524.
Crossref
|
|
|
|
|
Dong HL, Guo SX, Wang CL, Yang JS, Xiao PG (2007). Advances in studies on chemical constituents in plants of Pseudobulbus Cremastrae seu Pleiones and their pharmacological activities. Chin. Tradit. Herbal Drugs 38:1734-1737.
|
|
|
|
|
Ikeda Y, Nonaka H, Furumai T, Igarashi Y (2005). Cremastrine, a pyrrolizidine alkaloid from Cremastra appendiculata. J. Nat. Prod. 68:572-573.
Crossref
|
|
|
|
|
Lee CL, Chang FR, Yen MH, Yu D, Liu YN, Bastow KF, Morris-Natschke SL, Wu YC, Lee KH (2009). Cytotoxic phenanthrenequinones and 9,10-dihydrophenanthrenes from Calanthe arisanensis. J. Nat. Prod. 72:210-213.
Crossref
|
|
|
|
|
Li H (1996). A report on four cases of liver carcinoma treated by topical adhesive method. J. Tradit. Chin. Med. 16:243-246.
|
|
|
|
|
Li HS (2000). Principle and technology of plant physiological and biochemical experiments. Beijing: Higher Education Press. 2000, 123124: 186191 (in Chinese).
|
|
|
|
|
Li ZL (1987). Plant Slice Technology (Second Edition). Beijing: Science Press. (in Chinese)
|
|
|
|
|
Liu J, Fu HL, Geng XJ, Liu P, Lan LQ (2009). Comparative Anatomy of tissue culture rhizome and potted pseudobulb of Cymbidium goeringii and Cymbidium sinense. J. Sichuan Univ. (Natural Science Edition) 2:485-490.
|
|
|
|
|
Liu J, Yu ZB, Ye YH, Zhou YW (2008). Chemical constituents from the tuber of Cremastra appendiculata. Yaoxue Xuebao 43:181-184.
|
|
|
|
|
Liu JP (1991). Plant Morphology and Anatomy. Beijing: Beijing Normal University Press. (in Chinese)
|
|
|
|
|
Liu L, Li J, Zeng KW, Li P, Tu PF (2013). Three new phenanthrenes from Cremastra appendiculata (D. Don) Makino. Chin. Chem. Lett. 24:737-739.
Crossref
|
|
|
|
|
Liu L, Ye J, Li P, Tu PF (2014). Chemical constituents from tubers of Cremastra appendiculata. China J. Chin. Mater. Med. 39:250-53.
|
|
|
|
|
Mao TF, Ding Y (2004). Tissue cultuer and canltet regeneartion of Cremastra appendiculata. Plant Physiol. Commun. 40:716.
|
|
|
|
|
Mao TF, Liu T, Liu ZY, Zhu GS, Huang YH (2007). Rapid Propagation of Cremastra appendiculata in Vitro. J. Chin. Med. Mater. 30:1057-1059.
|
|
|
|
|
Ruan XL, Shi DW (2009). Anti-tumor and antibacterial effects of Pseudobulbus Cremastrae seu Pleiones. J. Chin. Med. Mater. 32:1886-1888.
|
|
|
|
|
Shi TX, Gu LL, Chen ZL, Chen QF (2014). Content analysis of flavonoids, soluble protein, soluble sugar in F.cymosum Leafs. Jiangsu Agric. Sci. 42:252-255.
|
|
|
|
|
Shim JS, Kim JH, Lee J, Kim SN, Kwon HJ (2004). Anti-angiogenic activity of a homoisoflavanone from Cremastra appendiculata. Planta Med. 70:171-173.
Crossref
|
|
|
|
|
Sun HX (2001). Study of some Chinese medicine and its volatile constituents anti-fungal activities. China J. Chin. Mater. Med. 26:99-102.
|
|
|
|
|
Tang FP (2013). Anatomical Studies on the Development of Cymbidium goeringii Pseudobulb in Darkness. Adv. Ornam. Hortic. China 4:274-277.
|
|
|
|
|
Wang GX (1989). Preliminay Study on Stems Cymbidium plants. Acta Hortic. Sin. 4:314-315.
|
|
|
|
|
Wang Y, Guan SH, Meng YH, Zhang YB, Cheng CR, Shi YY, Feng RH, Zeng F, Wu ZY, Zhang JX, Yang M, Liu X, Li Q, Chen XH, Bi KS, Guo DA (2013). Phenanthrenes, 9,10-dihydrophenanthrenes, bibenzyls with their derivatives, and malate or tartrate benzyl ester glucosides from tubers of Cremastra appendiculata. Phytochemistry 94:268-276.
Crossref
|
|
|
|
|
Xia WB, Xue Z, Li S, Wang SJ, Yang YC, He DX, Ran GL, Kong LZ, Shi JG (2006). Chemical constituents from tuber of Cremastra appendiculata. China J. Chin. Mater. Med. 30:1827-1830.
|
|
|
|
|
Xue Z, Li S, Wang S, Wang Y, Yang Y, Shi J, He L (2006). Mono-, Bi-, and triphenanthrenes from the tubers of Cremastra appendiculata. J. Nat. Prod. 69:907-913.
Crossref
|
|
|
|
|
Xue Z, Li S, Wang Sj, Yang YC, He DX, Ran GL, Kong LZ, Shi JG (2005). Studies on chemical constituents from the corm of Cremastra appendiculata. China J. Chin. Mater. Med. 30:511-513.
|
|
|
|
|
Yagame T, Funabiki E, Nagasawa E, Fukiharu T, Iwase K (2013). Identification and symbiotic ability of Psathyrellaceae fungi isolated from a photosynthetic orchid, Cremastra appendiculata (Orchidaceae). Am. J. Bot. 100:1823-1830.
Crossref
|
|
|
|
|
Yan J, Li CS, Chen SL, Zhang JR, Zhao TE (2002). The Effects of Twenty-one Traditional Chinese Medicines on Tyrosinase. J. Chin. Med. Mater. 25:724-726.
|
|
|
|
|
Yang MH, Cai L, Tai ZG, Zeng XH, Ding ZT (2010). Four new phenanthrenes from Monomeria barbata Lindl. Fitoterapia 81:992-997.
Crossref
|
|
|
|
|
Zhan YF, Yang Y, Dang XM, Cao ZM, Zhang XM (2011). Research on Changes of Soluble Sugar and Soluble Protein Contents during Development of Long Cowpea Pod. J. Changjiang Vegetables 18:49-51.
|
|
|
|
|
Zhang C (2007). Growth Regulation of Plantlets and Formation and Development Characters of Pseudobulbs of Cymbidium hybridium. Master' Degree Thesis, Jiangsu: Nanjing Agricultural University.
|
|
|
|
|
Zhang JC, Shen Y, Zhu GY, Yang MS (2007). Studies on Chemical Constituents from Cremastra appendiculata. J. Hebei Univ. (Natural Science Edition) 27:262-303.
|
|
|
|
|
Zhang LX (2008). Physiological Characteristics and Ecological Adaptability of Cremastra appendiculata (D.Don) Makino. (Master' Degree Thesis) Guizhou: Guizhou University.
|
|
|
|
|
Zhang MS, Peng SW, Wang W (2010). Macro research on growth and development of Cremastra appendiculata (D.Don.) Makino (Orchidaceae). J. Med. Plants Res. 4:1837-1842.
|
|
|
|
|
Zhang MS, Wu SJ, Jie XJ, Zhang LX, Jiang XH, Du JC, Qi JL, Liu Z, Yang YH (2006). Effect of endophyte extract on micropropagation of Cremastra appendiculata (D. Don.) Makino (Orchidaceae). Propagation of Ornamental Plants, 6:83-89.
|
|
|
|
|
Zhang ZL (2000). Plant Physiology Experimental Instruction. Beijing: Higher Education Press. (in Chinese)
|
|
|
|
|
Zhu GS (2009). Establishment of a Rice Enhancer Trap Mutant Library by T-DNA Insertion. Doctoral Dissertation, Hubei: Huazhong Agricultural University.
|
|