ABSTRACT
The study was carried out to determine effect of brewery effluent on the anatomical and morphological characters of Talinum triangulare. Seeds from matured T. triangulare were germinated and transplanted into plastic containers. The seeds were irrigated with 10, 20, 30 and 40% effluent concentrations respectively. A control experiment irrigated with normal water was also set aside. Data were collected on weekly basis until after the thirteenth week when the experiment was terminated. The plant showed significant reduction in the leaf area, seed number, shoot height, this morphological response is also associated with a reduction in various anatomical structure that were studied. The significant reduction was more obvious on plants irrigated with the 30 and 40% effluent concentrations. Stem tissue such as epidermis, cortical cell distance and vessel diameter showed significant reduction. The reduction in the morphological and anatomical character might be an adaptive mechanisms employed by the plant in order to cope with the heavy concentration of the effluent. This study showed that brewery effluents have toxic effect on the T. triangulare and the effects were more pronounced on those irrigated with 30 and 40% effluent concentrations. The physical and morphological characters observed in plants are the repercussion of the various endogenous characters among which are the anatomical characters.
Key words: Brewery effluent, vegetables, irrigation, plant cells.
Brewery effluent is waste generated by breweries industries in the process of production of a particular product. Some breweries effluents are known to contain high concentration of lead, mercury which has been the source of pollution into the plant and the society. The use of industrial effluents process for irrigation of food crops especially leafy and fruit vegetable has increase in the recent year in urban areas due to shortage of clean water (Arora et al., 2008). The use has also been promoted by urban farmers due to the belief that such effluents contain high nutrients that can promote rapid vegetative growth of their crops which reduce or eliminate the cost of the fertilization either in organic or inorganic form. Fatoba et al. (2011) reported that vegetable are produced throughout the year due to the availability of industrial effluent to irrigate them. However, there should be caution in the use of effluent for irrigation of plants that are tender and herbaceous like vegetables. Uaboi-Egbenni et al. (2009) reported that in Nigeria, most of the urban farmers divert effluents to farm land to irrigate their vegetable farm to meet up the risen demand of vegetable in our society. Ramasubramanian (1993) observed the germination percentage and development of Phaseolus mungo growth rate in sand culture decrease with an increase in the concentration of some effluents obtained from some industries. The release of toxic materials contaminates the earth environment and interferes with human health. This also affects the quality of life or the natural functioning of the ecosystem including in both living organisms and their non-living environment. The release of such an effluent into the plant body is also a source of pollution into the plant (Uaboi-Egbenni et al., 2009). Although, industrial processes are desirable, at the same time, the serious and irreversible damage done to the environment through their apparently innocuous discharges of effluents are unquantifiable. Until now, effluents are discharged into rivers, estuaries, lagoons (Ajmal, 1984). Industrial effluents have a great inhibitory effect on the germination and development of many plants species, the percentage of surviving plant decrease with an increase in treatment concentration (Rajni and Chauchan, 1996).
Talinum triangulare is commonly known as water leaf which belongs to the family Portulacaceae, it grows from a short taproot and can be up to 2.5 m in height. T. triangulare is an herbaceous annual and perennial plant with a broad, worldwide distribution. The demand for waterleaf is high in Nigeria especially in western part of this country, and it is therefore a major source of income for farmers. Its high demand is attributed to its nutritional value and importance as a softener when cooking the common fibrous leafy vegetables such as Afang (Gnetum africana), Atama (Heinsia crinata), and Editan (Lasienthera bulchozianum). It is also cooked with green Amaranthus (Amaranthus curentus) and fluted pumpkin (Telfairia occidentalis).
Due to the high demand for this vegetable in Nigeria, Urban farming of T. triangulare through effluent irrigation has grown tremendously. Several researchers have reported the effect of such effluent irrigation on the nutrient and heavy metal content of vegetables (Arora et al., 2008; Fatoba et al., 2011). This research work was designed to assess the morphological and anatomical structure of T. triangulare in response to the irrigation of brewery effluent.
The seeds from matured T. triangulare plants were collected from the forest behind the Department of Botany Obafemi Awolowo University, Ile-Ife (7° 28´ 0´´ N, 4º 34´ 0´´ E). Identification of this plant was done in the Herbarium (IFE) of the Department of Botany Obafemi Awolowo University; Ile-Ife and voucher specimens were deposited. The effluent used was collected from the International Breweries Ilesa, Osun State (Nigeria) (7° 37´ 0´´N, 4° 44´ 0´´E). The soil was collected behind the Department of Botany, Obafemi Awolowo University, Ile-Ife. This experiment was conducted in the screen house to protect the plants from direct rainfall contaminations, trampling and to avoid being destroyed by rodents. Seeds of T. triangulare were planted in two big bowls in front of Botany Department, Obafemi Awolowo University, Ile-Ife. After germination, three T. triangulare plants were transplanted into each of the plastic container provided which had been filled with soil and arranged in a complete randomized design to avoid competition. The effluent was serially diluted to give representative concentration of 10, 20, 30 and 40%. Three replicates of each of the treatment were used and the fifteen plastic containers were labeled based on their means of irrigation such as control, 10, 20, 30 and 40% effluent concentration. A central experiment was also set aside in the plastic container which was irrigated with normal water continue from the seventh week after planting until thirteenth week when the experiment was terminated. Quantitative characters recorded on weekly basis are: plants shoot height, leaf length, leaf breadth, leaf area, number of branches and number of fruits. The mean values of these quantitative characters were recorded. The leaf area was calculated according to the formula of Hoyt and Bradfied (1962) shown as:
LA=LL × LB × 0.75
Where, LA is the leaf area; LL is the leaf length, LB is the leaf breadth, and 0.75 is the correction factor for the shape of the leaf.
For the purpose of studying the epidermal structures, sizeable portion of mature leaves for each of the concentration were cut from standard median portion. Epidermal peels were made by free hand method, the abaxial and adaxial epidermis were preserve in 50% ethyl alcohol until when require, the epidermal surface were then stained in 1% safranin ‘O’ for about 5 min and mounted on clean slide in 25% glycerol for microscopic examination (Dutta, 2003). Ten slides from each of the upper and the lower epidermis were prepared per concentration and studied. Out of the slides prepared, ten were selected per concentration for size measurements. Also, frequencies counting per field of view were also made from the slides. Finally, different counts were made from different portion of each slide.
Stomata index was calculated according to Dilcher (1974):
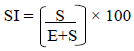
Where S.I is the stomata size, S is the stomata per unit area and E is the number of epidermal plus subsidiary cells in the same unit area. The transverse section of the stem were made by using microtome and stained with aniline blue for 5 min and mounted in glycerine on clean glass slide with the edge cover slip for microscope observation. Epidermis, cortical distance, vessel distribution and the layer of both collenchyma and parenchyma cell were studied and their sizes measured with ocular micrometer. Photomicrographs of the section were taken. Transverse sections of the root were cut using a rotring sledge microtome. Permanent slides were prepared by staining sections in aniline blue for about 5 min and later counter stain with 1% aqueous solution of safranin “O” for 4 to 5 min. This was then mounted in 25% glycerine on clean glass slides with the edges of the cover slip for microscopic studies. Arrangements of cortical cell, epidermis, vascular bundles distribution were observed. Photomicrographs of slides were taken with Amscope MT microscope camera version 3.001 attached to a light microscope. Illustrations of the foliar epidermal features and transverse sections of both stem and root were done by Camera Lucida under × 25 objective power of Leitz DIALUX research microscope. Mean of all the parameter were subjected to analysis of variance to calculate the least significant difference (LSD) with the use of statistical package for Social science (SPSS) software IBM SPSS Version 21 and the level of testing significance was set at 0.05%. Duncan’s multiple range tests were used to analyze the data obtained.
There is no significant difference in the number of leaf of T. triangulare when the various concentrations of the effluent were compared with the control (Table 1). There is a significant difference between the control and the various effluent concentrations in the length and width of leaf of T. triangulare. The number of branches, shoot height and seed number similarly decreased significantly with an increase the effluent concentrations (Table 1).
The number of stomata on the abaxial surface of the leaf of T. triangulare decreases significantly at 10% effluent concentration (Figures 1 and 2), there was also a significant increase at 20% effluent concentration, then a decrease at 30% effluent concentration and a sporadic increase at 40% effluent concentration (Table 2). On the adaxial surface, there was a significant increase at 20% effluent concentration in the stomata number and then a significant decrease in the stomata number from 30 to 40% effluent concentrations (Figures 1 and 2). The size of guard cell significantly increased at 40% effluent concentration. The length of epidermal cell at both abaxial and adaxial surfaces significantly decreased at 40% effluent concentration (Table 2).
The vessel diameter reduced significantly at 10% effluent concentration, however at both 20 and 30% effluent concentrations, there was no significant difference except at 40% effluent concentration where there was a reduction in the vessel diameter (Table 3 and Figure 3). There was a significant increase in the number of vascular bundle at both 30 and 40% effluent concentrations (Table 3). The epidermal thickness of the stem decreased significantly as the effluent concentration increases. The thickness of both parenchyma and collenchyma cells similarly decreased significantly as the effluent concentration increases (Table 3). In the root of T. triangulare studied, the epidermal layer, cortical layer and vessel diameter decreased significantly as the effluent concentration increases (Table 4 and Figure 4).
The brewery effluent reduces the leaf area of the plant significantly. Stevovic et al. (2010) reported that leaves from plant growing in polluted area were significantly reduced than leaves from an unpolluted area. Similarly, Rafia et al. (2009) reported that the reduction found in the leaf area of Phaseolus mungo and Lens culinaris may be considered as an adaptive advantage that enables leaves to develop and function in habitats marked by strong variations of heavy metals such as lead toxicity with solar radiation, air temperature and humidity. The increase in the leaf area reported under 40% effluent concentrations could be as result of the adaptive measures employed by some characters in the plant in order to withstand the effluent concentrations. Morphological features and physiological responses are linked to adaptive characteristics of plants in stressed environments (Dzomeku, 2012).
The continuous and consistent reduction in the seed number of T. triangulare is an indication that the effluent are in no way favourable to their growth. This might subsequently affects the availability of this vegetable in the market for consumers. Also, significant reduction has been reported to occur in all morphological parameters such as leaves length as the concentration of the effluent increases (Iqbal, 1985; Jahan and Zafar, 1992). Various morphological attributes of plant have been reported to be important features used by plant to withstand unfavorable condition.
Many diverse changes in anatomical characters of different plant have been previously reported by a number of authors (Khudsar and Igbal, 2001; Papadakis et al., 2004; Akinlabi et al., 2014; Ekpemerechi et al., 2014). There was a remarkable reduction in the number of stomata from the control to 10% effluent concentration. A significant reduction in stomata was reported in Cenchrus ciliaris and Cynodon dactylon in response to Cadmium stress. It has been reported that transpiration may decrease because of lower stomata index and size per unit of leaf area (Molas, 1997). The decrease in stomata size may be an avoidance mechanism against the inhibitory effect of a pollutant on physiological activities such as photosynthesis (Verma et al., 2006). These modifications are important response to the environmental stress which affected plant used in controlling the absorption of pollutants (Gostin, 2009). Decrease in stomata size on both the adaxial and the abaxial surfaces in all the treatment aside the control is an indication of T. triangulare survival strategy in the presence of pollutants from breweries effluent. Reduced stomata help in increasing the rate of photosynthesis without excessive transpiration. This type stomata size modification is an indication of the presence of heavy metal toxicity which could prove that brewery effluents may have contained toxic heavy metals. Due to the important of the adaxial surface in photosynthesis and gas exchange, the impact of the toxicity of the effluent was most felt on the leaf surface.
The length of the epidermal cell decreases as the concentration of the effluent increases, a significant increase was recorded at 40% effluent concentration. Epidermal cell number increase significantly as the effluent concentration increases. This could be an adaptive measure to neutralize the adverse effect of the effluent and the growth of T. triangulare. The result presented here is supported by earlier work of Gostin (2009) that showed a significant increase in epidermis in the leaves of plant growing in the polluted environment as compared to the leaves collected from non-polluted area. Also, Gomes et al. (2011) reported increased epidermal thickness in Brachiaria decumbens due to Cadmium stress.
There is a significant decrease in the vessel diameter as the effluent concentration increases, this constantly reduces the flow of assimilates to the plant parts. This is one of the reasons for the stunted growth in T. triangulare as the effluent concentration increases. The result of this work is supported by Kasim (2005) who reported that xylem vessel of Vicia faba showed significant reduction in their diameter in response to higher effluent concentration relative to their corresponding control. This result is also in conformity with the observation of Ghouse and Yunus (1972) and Khudsar et al. (2000), who reported that soil pollution, can cause a decrease in vessel abundance in several herbs and shrub. The increase in the concentration of effluents affects the internal organs of T. triangulare negatively. Among these internal organs are the vascular bundles which collapsed and ruptured as a result of increase in effluent concentration. Flow of assimilates, mineral nutrients and water will subsequently be reduced. Reduction in number of conducting elements has been reported in literature as being an adaptive measure to secure water flow. Proliferation of parenchyma cells in the stem of T. triangulare was promoted at very low concentrations of the brewery effluent. The effluents toxic effect resulted in drastic reduction of the number of parenchyma and collenchyma cells at 40% effluent concentration. This showed that at low concentration, T. triangulare was able to maintain the productions of parenchyma which constitute adaptive mechanism to regulate ion concentrations entering the stem. But, at high concentrations which seem to be toxic, the number of parenchyma and collenchyma cells was reduced in order to prevent excess ions from the effluents entering the xylem and reduce toxicity from effluent conduction to the aerial parts. The increase in concentration of effluent causes increase in the activity of phellogen to produce numerous intercellular spaces leading to the formation of lenticels.
Root as first organ of plant in different stages of development utilizes different mechanisms to cope with the presence of any pollutant in the soil. However, in this study, cortical layer suffer significant reduction in the highest percentage effluent concentration when compared to the control. Literatures have reported a remarkable decrease in cell size which might be the result of a decrease in elasticity of cell walls of the root (Sieghardt, 1984; Barcelo et al., 1986). This was supported by the work of Kasim (2005) who find out significant reduction in the cortical cell of root of Sorghum bicolor induced by some heavy metal which are also likely to be present in the effluent used for this study.
The physical and morphological characters observed in plants are the repercussion of the various endogenous characters among which are the anatomical characters. The response of anatomical characters to the various effluent concentrations in T. triangulare depicts the observed exomorphological features. The vegetable should not be grown in the area where breweries are sited and also, in an environment where there is shortage of water. About 10% concentration of this effluent can be used to irrigate this vegetable. However, it must be noted that brewery effluents have a great adverse effect on both the anatomical and morphological structure of the T. triangulare studied. The effect of effluent is more pronounced on T. triangulare irrigated with 30 and 40% effluent concentrations.
The authors have not declared any conflict of interests.
REFERENCES
|
Ajmal M (1984). Effect of Industrial Dairy processing effluent on soil and crop plants. Environmental Pollution 33(2):97-106.
Crossref
|
|
|
|
Akinlabi AA, Jimoh MA, Saheed SA (2014). Effect of altitudinal gradients on morphoanatomical characters of Chromolaena odorata (L.) King and Robinson. FUTA Journal of Research in Sciences 2:150-156.
|
|
|
|
|
Arora M, Kiran B, Rani S, Rani A, Kaur B, Mitta LS (2008). Heavy metal accumulation in vegetable irrigated with water from difference sources. Food Chemistry 111:811-815.
Crossref
|
|
|
|
|
Barcelo J, Poschenrieder CH, Andreu I, Gunse B (1986). Cadmium induced decrease of water stress resistance in bush beans plants (Phaseolus vulgaris L. cv. Contender). In Effects of Cd on water potential, relative water content, and cell wall elasticity. Journal of Plant Biology 125:17-25.
|
|
|
|
|
Dilcher DL (1974). Approaches to the identification of angiosperm leaf remains. The Botanical Review. 40:1-157.
Crossref
|
|
|
|
|
Dutta AC (2003). Botany for Degree Student Resived, 6th Edition, Oxford University Press, New Delhi, India. P.240.
|
|
|
|
|
Dzomeku BM (2012). Leaf anatomy variation in relation to stress tolerance among some woody species on the Accra plains of Ghana. Journal of Plant Development 19:13-22.
|
|
|
|
|
Ekpemerechi SE, Lala MA, Jimoda LA, Odiwe AI, Saheed SA (2014). Effect of air pollution on the foliar morphology of some species in the family Euphorbiaceae in Southwestern Nigeria. Journal of Science and Technology 34:21-29.
|
|
|
|
|
Fatoba PO, Olorunmaiye KS, Adepoju AO (2011). Effect of soaps and detergents wastes on seed germination, flowering and fruiting of Tomato (Lycopersicon esculentum) and Okra (Abelmoschus esculentus) plants. Ecology, Environment and Conservation 17(1):7-11.
|
|
|
|
|
Ghouse AKM, Yunus, M (1972). Preparation of Epidermal peels from leaves of gymnosperms by treatment with hot 60% HNO3. Stain Technology 47(6):322-324.
|
|
|
|
|
Gomes MP, Marques TC, Nogueira MOG, Castro, EM, Soares AM (2011). Ecophysiological and anatomical changes due to uptake and accumulation of heavy metal in Brachiaria decumbens. Scientia Agricola 68:566-573.
Crossref
|
|
|
|
|
Gostin NI (2009). Air pollution Effects on the Leaf structure of some Fabaceae species. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 37(2):57-63.
|
|
|
|
|
Hoyt P, Bradfield R (1962). Effect of varying leaf area by defoliation and density on dry matter production of Corn. Agronomy Journal 54:523-525.
Crossref
|
|
|
|
|
Iqbal MZ (1985). Cuticular and anatomical studies of white clover leaves from clean and air polluted areas. Pollution Research 4:59-61.
|
|
|
|
|
Jahan S, Zafar I (1992). Morphological and anatomical studies of leaves of different plants affected by motor vehicles exhaust. Medical Journal of Islamic World Academy of Sciences 5:21-23.
|
|
|
|
|
Kasim WA (2005). The correlation between physiology and structural alterations induced by Copper and Cadmium stress in broad beans (Vicia faba L.). Egyptian Journal of Biology 7:20-32.
|
|
|
|
|
Khudsar T, Igbal M (2001). Cadmium-induced changes in leaf epidermis, photosynthesis rate and pigment concentrations in Cajanus cajan. Journal of Plant Biology 44:149-157.
Crossref
|
|
|
|
|
Khudsar T, Wound YS, Igbal M (2000). Morphological and anatomical variations of Cajanus cajan (Linn.) Hirth raised in Cadmium rich soil. Journal of Plant Biology 4:59-64.
|
|
|
|
|
Molas J (1997). Changes in morphological and anatomical structure of cabbage (Brassica oleracea L.) outer leaves and in ultrastructure of their chloroplasts caused by an in vitro excess of Nickel. Photosynthetica 34:513-522.
Crossref
|
|
|
|
|
Papadakis IE, Dimassi KN, Bosabalidis AM, Theorios IN, Patakas A, Giananakoula A (2004). Effects of B excess on some physiological and anatomical parameters of 'Navalia' orange plants grafted on two rootstocks. Environmental and Experimental Botany 51:247-257.
Crossref
|
|
|
|
|
Rafia A, Saba H, Hajra N, Farha A, Marina R (2009). A viable alternative mechanism in adapting the plants to heavy metal environment. Pakistan Journal of Botany 41(6):2729-2738.
|
|
|
|
|
Rajni A, Chauchan SVS (1996). Effect of Tannery effluent on seed germination and total biomass in some varieties of Hordeum vulgare L. Acta Oecologica 18:112-115.
|
|
|
|
|
Ramasubramanian S (1993). Analysis of Industrial effluent and their impact on the growth and metabolism of Phaseola mungo L. Communications in Soil Science and Plant Analysis 24(17-18):2241-2249.
Crossref
|
|
|
|
|
Sieghardt H (1984). Eine anatomisch-histo- chemische Studie zur Bleiverteilung in Pri- m/irwurzeln yon Pisum sativum L. Mikroskopie 41:25-133.
|
|
|
|
|
Stevovic S, Vesna SM, Dusica C (2010). Environmental impact on morphological and anatomical structure of Tansy. African Journal of Biotechnology 16(9):2413-2421.
|
|
|
|
|
Uaboi-Egbenni PO, Okolie PN, Adeyuyitan OE, Sobande AO, Akinyemi O (2009). Effect of industrial effluent on the growth and anatomic structure of Abelmoschus esculentus. African Journal of Biotechnology 8(14):3251-3260.
|
|
|
|
|
Verma RB, Siddigi TO, Igbal M (2006). Foliar response of Ipomoea pestigridis L. to coal-smoke pollution. Turkish Journal of Botany 30(5):413-417.
|
|