ABSTRACT
Botanical piscicides over the years have been used singly in fishing efforts. This research investigated the joint action of binary mixtures of Carica papaya + Nicotiana tabacum, Anacardium occidentale + Senna occidentalis, and A. occidentale + Luffa cylindrica. Relative toxic units (RTU) estimations and synergistic ratio (SR) models were used in the joint action evaluation. The 96 h LC50 (95% confidence limit [CL]) results revealed that the mixture of C. papaya and N. tabacum showed antagonism (reduction in toxicity) in both predetermined mixture ratio 1:1 and equitoxic mixture ratio 1:2. The RTU value for mixture ratio 1:1 was 0.298, while SR was 0.221. The mixture ratio 1:2 had RTU of 0.421 and SR of 0.384. The mixture of A. occidentale and S. occidentalis also followed the antagonistic model with mixture ratio 1:1, having RTU of 0.927 and SR of 0.577, while the mixture ratio 1:2 had RTU of 0.489 and SR of 0.360. The mixture ratio 1:1 of A. occidentale and L. cylindrica was synergistic with RTU of 2.145 and SR of 1.442. Most of the binary mixtures of the botanical piscicides were antagonistic.
Key words: Joint action, binary mixtures, botanical piscicides, Clarias gariepinus.
Botanical piscicides are substances of plant origin used by fishermen and fish farmers. They are applied during fishing or eradication of unwanted, predatory, and exotic fish species (Cagauan et al, 2004). For sustainable aquatic environment, there is a fast growing preference for piscicides of plant origin for catching fish and clearing ponds. Botanical ichthyotoxins are less expensive, biodegradable, readily available, easy to handle and safe for mankind and the environment (Singh et al., 1996). Piscicidal plants used in fishing actually stupefy the fishes; they do not kill the whole fish stock like chemicals (Kamalkishor and Kulkani, 2009). Because piscicidal plants are biodegradables, hence fast recovery in fishes exposed to them. Plant extracts used as piscicides in fisheries are considered advantageous when viewed against the backdrop of using persistent chemicals (Gabriel et al., 2009). Different species of plants employed as piscicides have different effects, depending on the species of fish targeted (Van Andel, 2002). The use of toxic plants for catching fish is a common practice worldwide. The ichtyotoxic characteristics of some of these plants make them potent tools for catching or stupefying fish all over the world. Forty years ago, some local fishermen in Nigeria have been reported to have used specific biocides derived from plants for fishing (Sambasivam et al., 2003). Derris elliptica (Family Papilionaceae) are widely available in the tropics and their twigs and roots have been used as natural piscicides in artisanal fisheries and aquaculture ponds in Nigeria. The use of plant piscicides, such as Tephrosia candida, Tephrosia purpurea, Mundulea sericea, Acacia pennata (Weiss, 1973), Adenia cissampeliodes (Morah, 1985), Tetrapleura tetraptera, Parkia filicoides, and Tephrosia vogelii is common among fish farmers in controlling pests and predators. Many plants contain chemicals, which have traditionally been used in fish culture in almost all parts of the world (Jenness, 1967). The best-known plants are Derris species, which produce rotenone and Tephrosia species, which contain tephrosin, a substance similar to rotenone (natural biocide).
The phytochemical screenings of some botanical piscicides were performed in petroleum ether, chloroform, and methanolic extracts. The chloroform and methanolic extracts of flowers, barks, leaves, and seed were found to contain flavonoids, alkaloids, steroids, glycosides, and anthraquinones (Kathirvel and Sujatha, 2012). The two main groups of phytochemicals that occur in most plants used for the stunning fish are rotenones and saponins. This groups represent nearly all varieties of fish poisons, although plants with sufficient levels of ichthyoethereol, triterpene, and other ichthyotoxins are also used (Béarez, 1998). Many researchers have worked on botanical piscicides, but much work has not been done on their joint action or combined effects of their binary mixtures. Because of its ruggedness and ability to survive acclimatization, Clarias gariepinus is an ideal candidate species for this kind of experiments.
Therefore, this study is a deliberate effort to evaluate the joint action effects of the binary mixtures of some botanical piscicides on post juvenile C. gariepinus.
Post juveniles C. gariepinus (average length, 7.75±2 cm, average weight 37.55±2 g) were obtained from Daddy-T fish farm in Surulere, Lagos State, Nigeria. They were transported in a stress free manner to the Zoological Garden, University of Lagos, Nigeria.
The fishes were acclimatized for 7 days in a rectangular glass holding tank measuring 47 × 35 × 28 cm containing 20 L of tap-water de-chlorinated by keeping it for 24 h. The main physicochemical parameters were evaluated by means of specific instruments. More specifically, the DO of the sample was determined using a portable Exstik II DO 600 meter. The pH, salinity, temperature, total dissolved solids (TDS) and electrical conductivity were estimated by means of Mettler – Tornado meter.
Collection of botanical samples and preparation of aqueous stock solutions
All the plant samples were collected in the morning between 7.00 and 11.00 am, because it is better to collect them before the starting of the photochemical reactions which takes place in the afternoon. After collection, the samples were placed in clean sac bags to retain their natural state (Ogunkule and Tonia, 2006). Luffa cylindrica L M. Roem (Sponge) pods were collected from the bush behind Erastus Akingbola Postgraduate Hall, University of Lagos Nigeria (06°31’ 03.20’’N, 003°23’ 87.80’’E). Anacardium occidentale L (Cashew) barks were collected from Works and Physical Planning, University of Lagos Nigeria (06° 31’10.10’’N, 003° 23’ 44.10’’E). Carica papaya L (Pawpaw) leaves were collected from Okota Lagos Nigeria (06° 31’ 58.16’’N, 003°21’ 00.79’’E). Senna occidentalis (L.) Link (Yellow Flower) barks were collected from the front of Amina Hall, University of Lagos Nigeria (06° 30’ 56.03’’N, 003° 23’ 08.89’’E). Nicotiana tabacum L (Tobacco) leaves were purchased from Mushin market Lagos (06° 31’ 58.16’’N, 003° 21’00.79E). The botanicals were properly identified in the Department of Botany of the University of Lagos Nigeria (Table 1a).
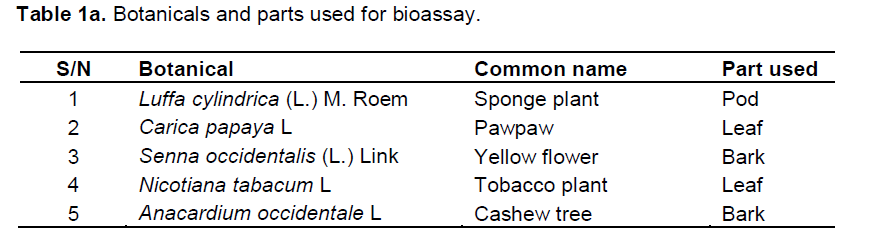
Preparation of botanicals
Each of the botanicals collected was air dried at room temperature for 21 days and oven dried at 32°C for 30 min to make it more bristle before pounding into powder with clean mortar. The powder of each botanical was sieved with 100 micron sieve to obtain fine powder which was stored separately in dry airtight bottle containers.
Preparation of aqueous extracts/stock solutions of the botanicals
The stock solution of each botanical was prepared by dissolving 50 g of powder of each botanical in 1 L of water and put into a transparent jerry-can of 5 L volume and left to ferment for 3 days, finally filtered to obtain their aqueous extracts (Fafioye, 2005).
Bioassay
Pilot tests were carried out to establish concentrations of the extracts to be used in the actual experiments. The results of the pilot tests led to the preparation of five concentrations for each botanical in 96 h acute toxicity tests. Twenty fishes were randomly assigned to each concentration in four replicates of 5 fishes in each glass test tanks measuring 46×36×30 in a static bioassay without renewal test solutions.
Assessment of quantal response (Mortality)
Death was taken to be no movement of any part of the fish body on gentle prodding with a glass rod after 2 min of observation.
Joint action toxicity of botanical mixtures
The joint action toxicity of the botanical mixtures was determined by two models: (1) the concentration-addition model by Anderson and Weber (1975), modified by Otitoloju (2003) with Relative Toxic Units (RTU) estimations; (2) synergistic ratios (SR) model (Hewlett and Plackett, 1969; Chukwu et al., 2009).
The concentration-addition model assumes that, when toxic substances with similar act are mixed in any proportion, they will add together to give the observed response. In evaluating the joint action, a predicted response value (s) e.g. LC50 is derived by summing up the LC50 values of individual toxicants according to the proportion of their contribution in the mixture. The predicted LC50 value (s) is then compared to the observed LC50 value of the mixture so as to classify the type of interaction among the components of the mixture as follows: (1) Additive if the observed LC50 value of the mixture is equal to the predicted LC50 value, (2) Synergistic if the observed value of the mixture is less than the predicted LC50 value, and (3) Antagonistic if the observed LC50 value of the mixture is greater than the predicted LC50 value.
Statistics used for data analysis
The dose-response data for both toxicity tests (single and joint action) were analyzed by probit analysis using Statistical Package for Social Sciences (SPSS) 17 version, to get the 96 h LC50 values.
The main physicochemical parameters calculated during the experiment were summarized in Table 1b.
Single action toxicity tests of the botanicals against post juvenile C. gariepinus
The dose response tables for both toxicity tests (single and joint action) revealed that the mortality of the exposed fishes were both concentration and duration dependent (Tables 2 to 12).
On the basis of 96 h LC50 values, aqueous extract of C. papaya was found to be the most toxic botanical tested against post juvenile C. gariepinus followed by A. occidentale, N. tabacum, L. cylindrical, and S. occidentalis (Table 13). Toxicity factor showed that C. papaya was 1.04, 1.69, 2.06, and 2.31 times more toxic than A. occidentale, N. tabacum, L. cylindrical, and S. occidentalis, respectively.
Joint action toxicity tests of the plant extracts against post juvenile C. gariepinus
It is important to note that the botanicals can be mixed (combined) in any order to get the 96 h LC50 values of the mixture, provided you know the 96 h LC50 values of individual botanical. The 96 h LC50 values of the bioassays test with the binary mixtures in the ratio of 1:1 and equitoxic ratio of 1:2 for C. papaya and N. tabacum against post juvenile C. gariepinus was 53.280 and 30.781 ml/L, respectively. A. occidentale and S. occidentalis was 21.378 and 34.215 ml/L, while A. occidentale and L. cylindrica recorded 8.55 and 17.795 ml/L for mixture ratio of 1:1 and mixture ratio of 1:2, respectively. The result also revealed that the 96 h LC50 value for mixture ratio of 1:2 was about 2 times more toxic than 1:1 for C. papaya and N. tabacum against post Juvenile C. gariepinus. For A. occidentale and S. occidentalis, mixture ratio 1:1 was 1.60 times more toxic than mixture ratio 1:2, while for A. occidentale and L. cylindrica, mixture ratio 1:1 was 2.08 times more toxic than mixture ratio 1:2 (Table 14).
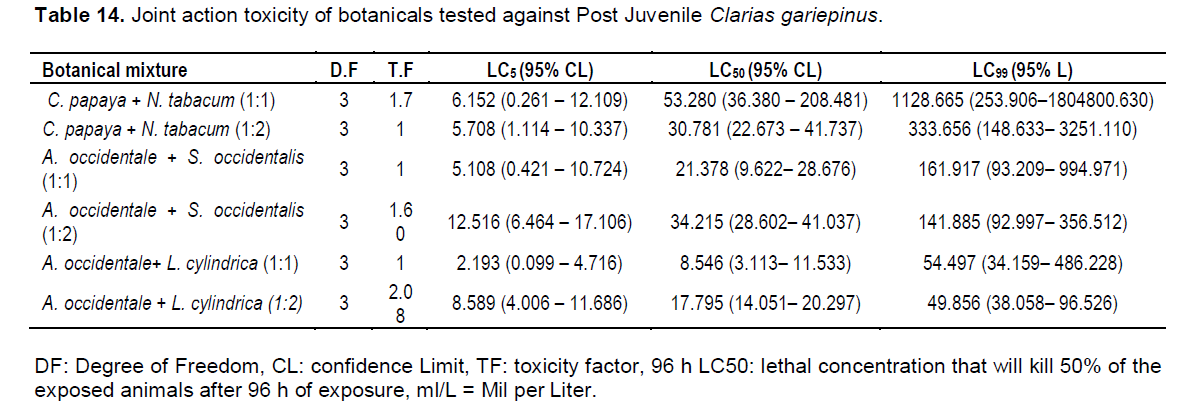
Analysis based on concentration addition model and synergistic ratio model
The 96 h LC50 (95% CL) results revealed that the mixture
of the extracts of C. papaya and N. tabacum showed antagonistic action for both test concentrations. For mixture ratio 1:1, RTU = 0.298 and SR= 0.221, while for mixture ratio 1:2, RTU = 0.421, SR = 0.384. Also, the mixture of A. occidentale and S. occidentalis followed antagonistic trend with values are RTU = 0.927 and SR = 0.577 for mixture ratio 1:1, for mixture ratio 1:2, RTU = 0.489, SR = 0.360 (Table 15a, b and c). Only for the mixture of A. occidentale and L. cylindrica was observed a synergistic action between the extracts. In fact, the mixture ratio 1:1, RTU = 2.145 and SR = 1.442 (Table 15c).
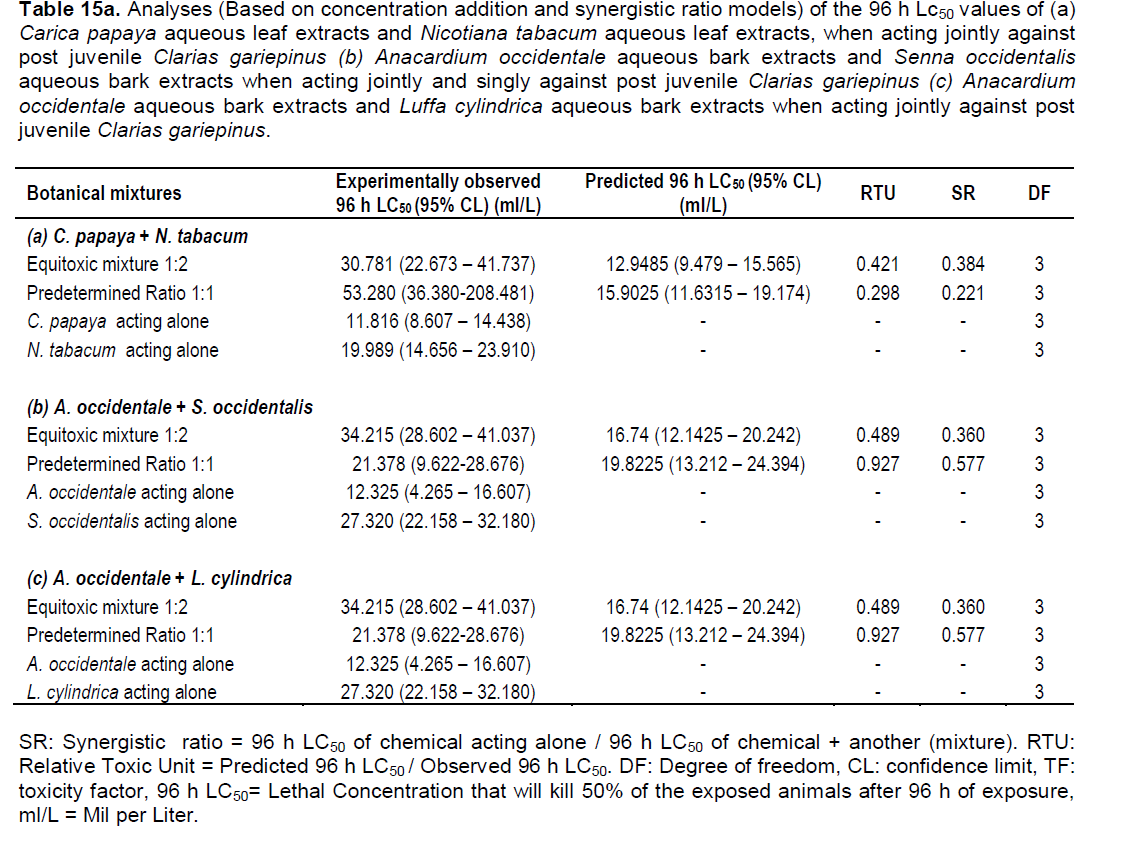
The data show that they are more effective when used singly than jointly. This result was similar to that of Otitoloju (2003) when he looked at the relevance of joint action toxicity evaluation in setting realistic environmental safety limits of heavy metals, as most of the combined effects of the heavy metals showed antagonism. Also, Chukwu et al. (2009) observed antagonism when they studied the combined effects of binary mixtures of commonly used agrochemicals; pattern of toxicity in fish. Otitoloju and Don-Pedro (2006) observed antagonism when they determined the types of interactions exhibited by binary mixtures of heavy metals tested against hermit crab. This study on the joint action of botanical piscicides, using RTU and SR models, has clearly demonstrated that the effects of the binary mixtures of some piscicidal plant species on post juvenile C. gariepinus are antagonist. Therefore, the botanical piscicides are better used singly than jointly as their binary mixtures are not effective.
The use of poisonous botanicals in hunting and fishing is not particularly a new development, but it is almost as old as our ancestors (Finlayson et al., 2002). Early studies on the effects of botanicals on fishes include Campbell (1991) who examined the use of fish poison by the natives of California for their survival. Chuck (2003) described some piscicidal plants used by the primitive people in South America, North America, Australia, Asia, and Africa. Fafioye (2005) evaluated the toxicity of Raphia vinifera fruit extracts on biochemical composition of Nile Tilapia. Olufayo (2009) studied the haematological characteristic of C. gariepinus juvenile exposed to powdered root of D. elliptica Obomanu et al. (2009) worked on the haematology, plasma enzymes, and organ indices of C. gariepinus after intra muscular injection with aqueous leaves extracts of Lepidagathis alopecuroides. Usman et al. (2005) examined the toxicity of methanol extract of Euphorbia lateriflora to the juvenile C. gariepinus. Adeyemo (2005) studied the haematological and histopathological effects of cassava mill effluent on C. gariepinus, Abalaka and Auta (2010) studied the toxic effects of aqueous and ethanol extracts of Parkia biglobosa pods on C. gariepinus adults. Kamalkishor and Kulkani (2009) worked on the fish stupefying plants used by the Gond tribal of Mendha village of Central India. Muhammad and Tufail (2010) examined the possibility of replacing rotenone by locally grown herbal extract. However, much work has not been done on the joint action or combined effects of the binary mixtures of the botanical piscicides.
The authors have not declared any conflict of interests.
REFERENCES
|
Abalaka SE, Auta J (2010).Toxic Effects of Aqueous and Ethanol Extracts of Parkia on gariepinus Adults. World J. Biol. Res. 3(1):9-17.
|
|
|
|
Adeyemo OK (2005). Haematological and histopathological effects of cassava mill effluent in Clarias gariepinus. Afr. J. Biomed. Resour. 8(3):179-183.
|
|
|
|
|
Anderson PD, Weber LJ (1975). The toxicity of aquatic population of mixtures containing certain heavy metals, Proceedings of the international Conference on Heavy Metals in the Environment, Institute of Environmental Studies, University of Toronto, pp. 933-953.
|
|
|
|
|
Béarez P (1998). Focus: First archaeological indication of fishing by poison in a sea environment by the Engoroy population at Salango (Manabi, Ecuador). J. Archaeol. Sci. 25:943-948.
Crossref
|
|
|
|
|
Cagauan AG, Galaites MC, Fajardo LJ (2004). Evaluation of botanical piscicides on Nile Tilapia (Oreochromis niloticus L.) and mosquito fish (Gambusiaaffinis Baird and Girard). Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines. Sept. 12 – 16. pp. 179-187
|
|
|
|
|
Campbell P (1991). Fish poison. Survival skill of native California, Gibbs Smith Publishing, Layton Utah, pp. 433-434.
|
|
|
|
|
Chuck K (2003). Fishing with poison. The bulletin of primitive Technol. 25:277-301.
|
|
|
|
|
Chukwu LO, Samuel OB, Olaogun MO (2009). Combined effects of binary mixtures of commonly used Agrochemical: Pattern of toxicity in Fish, J. Agric. Biol. Sci. 5(6):883-891.
|
|
|
|
|
Fafioye OO (2005). Plants with piscicidal activities in southwestern Nigeria. Turk. J. Fish. Aquatic Sci. 5:91-97.
|
|
|
|
|
Finlayson BJ, Schnick RA, Cailteux RL, DeMong L, Horton WD, McClay W, Thompson CW (2002). Assessment of antimycin as use in fisheries and its potential for re-registration. Fisheries 27(6):10-18.
Crossref
|
|
|
|
|
Gabriel UU, Obomanu FG, Edori OS (2009). Haematology, plasma enzymes and organ indices of Clarias gariepinus after intramuscular injection with aqueous leaves extracts of Lepidagathis alopecuroides. Afr. J. Biochem. Res. 3(9):312-316.
|
|
|
|
|
Hewlett PS, Plackett RL (1969). A unified theory for quantal responses to mixtures of drugs, non-interactive action. Biometrics 15:591-610.
Crossref
|
|
|
|
|
Jenness J (1967). The use of plants as fish poison within the kainji basin. In: Feed W (ed.). Fish and Fisheries of Northern Nigeria. Ministry of Agriculture of Northern Nigeria. 226 pp.
|
|
|
|
|
Kamalkishor HN, Kulkani KM (2009). Fish Stupefying Plants Used By the Gond Tribal of Mendha Village of Central India. Indian J. Trad. Knowl. 8(4):531-534.
|
|
|
|
|
Kathirvel A, Sujatha V (2012). Phytochemical Studies of Cassia occidentalis Linn. Flowers and Seeds in Various Solvent Extracts. Int. J. Pharmacogn. Phytochem. Res. 3(4):95-101.
|
|
|
|
|
Morah FNI (1985). Constituents of the stem of Adenia cassia. J. Sci. Educ. 1:177-122.
|
|
|
|
|
Muhammad A, Tufail S (2010). Replacement rotenone by locally grown Herbal extracts. Int. J. Agric. Biol. 12(1):77-80.
|
|
|
|
|
Obomanu FG, Gabriel UU, Edori OS (2009). Haematology, plasma enzymes and organ indices of Clarias gariepinu safter intramuscular injection with aqueous leaves extracts of Lepidagathis alopecuroides. Afr. J. Biochem. Res. 3(9):312-316.
|
|
|
|
|
Ogunkule ATJ, Tonia AL (2006). Ethnobotanical and phytochemical studies on some species of Senna in Nigeria. Afr. J. Biotechnol. 5(21):2020 -2023.
|
|
|
|
|
Olufayo MO (2009). Hematological characteristics of Clarias gariepinus Juveniles exposed to Derris elliptica root powder. Afr. J. Food Agric. Nutr. Dev. 9(3):920-933.
|
|
|
|
|
Otitoloju AA (2003). Relevance of joint action toxicity evaluations in setting realistic environmental safe limits of heavy metals. J. Environ. Manag. 67(2):121-128.
Crossref
|
|
|
|
|
Otitoloju AA, Don-Pedro KN (2006). Determination of types of interactions exhibited by binary mixtures of heavy metals tested against hermit crab, Toxicol. Environ. Chem. 88(2):331-343.
Crossref
|
|
|
|
|
Sambasivam S, Chandran R, Karpagam G, Khan SA (2003). Toxicity of leaf extracts of oleander, Thevetia neriifolia on tilapia. J. Environ. Biol. 24(2):201-204.
|
|
|
|
|
Singh A, Singh DK, Mishra TN, Agarwal RA (1996). Molluscicides of plant origin. Biol. Agric. Hortic. 13:205-252.
Crossref
|
|
|
|
|
Usman JI, Auta J, Adamu AK and Abubakar MS (2005). Toxicity of Methanol extract of Euphorbia lateriflora to the juvenile of Clarias gareipinus, Chem. Class J. 2:59-61.
|
|
|
|
|
Van Andel T (2002). The diverse uses of fish-poison plants in Northwest Guyana. Econ. Bot. 54:865-875.
|
|
|
|
|
Weiss EA (1973). Some indigenous tree and shrubs used by local fishermen on the East Africa Coast. Econ. Bot. 27(2):174-192.
Crossref
|
|