ABSTRACT
The ultimate objective of this study was, to explore community structure and biomass of zooplankton in relation to key environmental drivers to bear out the productivity of Lake Tinishu Abaya. Zooplankton samples collection was carried out for a year from January to December 2016 from two sampling sites (open and shore) using 30 µm mesh size net within the euphotic depth. Biomass of zooplankton was anticipated using length-weight regressions adjusted for the representative microcrustacean and biovolume of Rotifera from linear dimensions applied to simple geometric formula appropriate to body shape. In parallel, in-situ and laboratory measurements of the various physicochemical factors were performed using the standardized method. The results of the present study are given strongly generalized that Rotifera had the highest number of species (11 species) followed by Cladocera (5 species) and Copepods (2 species). Diversity parameter, the Shannon diversity index was higher for Rotifera (2.45) than cladocerans (2.395) and copepods (2.384). The mean total biomass of copepods, cladocerans, and Rotifera were 174.4, 46.76 and 13.67 µg/L at open site and 101.8, 51.35 and 10.9 µg/L at shore site, respectively. Physicochemical factors responsible for the observed variations in the biological features of the lake generalized that the lake water was fresh, well oxygenated, slightly warm, alkaline, very turbid, and with relatively high inorganic nutrients. The presence of fairly high diversity, abundance, and biomass of zooplankton in the study shows that the ecosystem of Lake Tinishu Abaya is chemically, physically and biologically productive which supports most of the aquatic life.
Key words: Biomass, community structure, Lake Tinishu Abaya, productive, zooplankton
Zooplankton community is made up of a mixture of species belonging to many taxonomic groups whose morphology, reproductive strategies and feeding habits are very diverse, resulting in spatial and temporal variations in productivity profiles. In freshwater environments, zooplankton plays a relevant role in energy transfer and nutrient transport and regeneration, owing to their position in the food web as the main direct energy transfer and nutrient transport and regeneration, owing to their position in the food web as the main direct consumers of phytoplankton. The magnitude of energy fluxes is determined by the herbivory of the various components of the zooplankton community, which depends on the community structure, and is a result of the process of colonization and establishment of species (Armengol, 1980; Armengol and Miracle, 1999). Among the dominant zooplankton in freshwater ecosystems are rotifers and microcrustaceans made up of cladocerans and copepods (Wetzel, 1983). Zooplankton occupies an open position in the trophic link between primary producers and higher trophic levels; they are also good bioindicators of the physical and chemical conditions of aquatic environments which cause changes in the qualitative and quantitative composition of zooplankton and influence their densities (Karabin, 1985). Zooplankton abundance, expressed as number per area or volume units does not necessarily provide accurate information about community biomass because zooplankton consists of a great variety of groups or animal species of a large size range (Matsumura-Tundisi et al., 1990). Moreover, the biomass of the zooplankton species is an important and necessary parameter to calculate the secondary production of this community (Melão and Rocha, 2004). Biomass reflects the instantaneous quantity of live organic matter in a given area and also provides a means of analyzing an ecosystem’s productivity, irrespective of taxonomic composition. An increase in zooplankton biomass has often been associated with a rise in the trophic status of the environment. Studies that consider biomass values are important because they allow comparison of different environments, providing a common unit to evaluate zooplankton groups.
Generally, eutrophic environments support higher biomass of smaller individuals than more oligotrophic ones (Esteves and Sendacz, 1988). The establishment of length-weight regressions are fundamental when determining the biomass of aquatic communities and also in most studies of food web how aquatic ecosystems function. For microcrustaceans (cladocerans and copepods), weight estimates from interactions and secondary productivity (Ghidini and Santos-Silva, 2009; Ghidini and dos Santos-Silva, 2011), which contribute to our knowledge of length-weight regressions have been the most frequently used technique employed to approximate the biomass of these organisms. Alternatively, the determination of zooplankton size is an important tool for building the size–weight regressions, which are useful when only size data is available. Due to these reasons, estimation of zooplankton size and dry weight constitutes an important contribution for the study of trophic-web structure in aquatic ecosystems, considering its relationship with the trophic status of the water bodies (Pinto-Coelho et al., 2005a; Pinto-Coelho et al., 2005b). Furthermore, an accurate measurement of biomass is necessary to understand the structure and dynamics of biological communities (Bird and Praire, 1985). Basic knowledge on the biomass (length-weight) of different zooplankton species provides the necessary data for the calculation of secondary production, as well as information about the competitive strategies responsible for their success in a given environment. Measurement of zooplankton biomass is essential in studies of production ecology of zooplankton. Therefore, the aim of the present work was intended to quantify biomass, abundance, and community structure of the zooplankton species to bear out the ecological productivity of Lake Tinishu Abaya. The study was used to establish length-weight regressions for the representative microcrustacean (cladoceran and copepod) species in a tropical Rift Valley Tinishu Abaya, Ethiopia.
Study area
Lake Tinshu Abaya, or interchangeably called, Small Abaya, is a small freshwater lake located in the Rift Valley nearly 160 km southwest of Addis Ababa, which is the capital city of Ethiopia. It located at 7°29´03.65´´N, 38°03`17.79´´E, and 1835 m above sea level. The lake is situated in a remote area 15 km from a small village in the township of Silttie. It is a shallow lake, having a surface area of 1253 ha (Kassahun et al., 2011), with a maximum and a mean depth of 3.7 m and 2.9 m, respectively. During this study, two major perennial rivers (Rivers Dacha and Boboda) and a single outlet (River Badober) were always active. The former two rivers are relatively big. The lake has some commercially important fish species including the native Tilapia zilli and Barbus species, while Nile tilapia (Oreochromis niloticus), was stocked from the nearby Lake Ziway in 1997 (Kassahun et al., 2011). The major food items found in the gut of O. niloticus in the study area are phytoplankton, the macrophytes, detritus, and zooplankton (Yirga and Brook, 2018). The lake has nearly an oval shape (Figure 1). For this study two study sites (open water/center and shore/offshore site) was selected purposely. An open-water site located in the center; 2.5 km far from the shoreline and the shore site is so close (nearly 50 m) to the edge of the lake. This site was considered as a direct recipient of wastes from agricultural land as well as domestic materials; thus the site was taking into consideration as impaired by human activities compared to the open-water site.
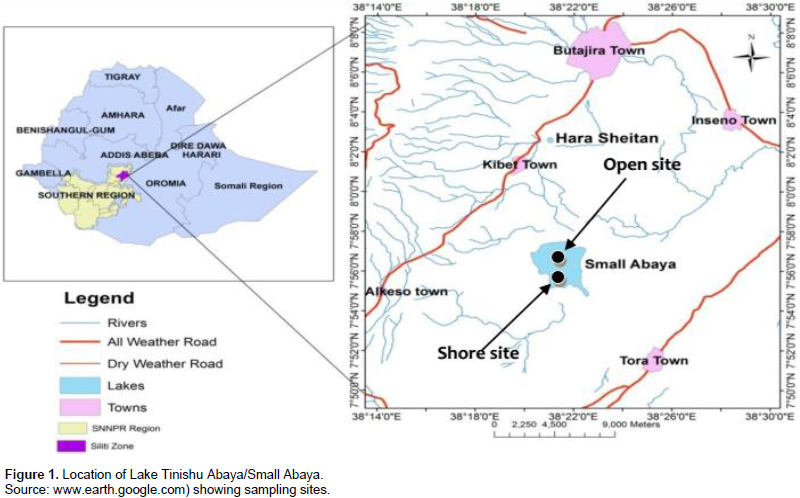
Water sampling and measurements of physico-chemical parameters
For all physical and chemical analysis, sample collection procedures were carried out on a monthly basis for a year between January 2016 and December 2016 from two sampling stations (open and shore). Water samples were taken with bottle sampling from the surface. The collected water samples were transported to Limnology Laboratory of Addis Ababa University for further physicochemical analysis. In-situ measurements of the parameters temperature, dissolved oxygen, conductivity, and pH was measured using a portable multimeter (Model HQ 40d Multi Hach Lange). Water transparency was measured using a standard Secchi disc 30 cm in diameter. The euphotic depth (Zeu), the depth at which 1% of the surface photosynthetic active radiation is detected, for the study area, was calculated from the relation Zeu = 4.6/Kd (Kalff, 2002). Kd (mean vertical extinction coefficient of downwelling irradiance, in m-2) was computed from the relation as Kd=1.44/ZSD (ZSD-Secchi depth, in m) Holmes (1970). Turbidity was measured using portable digital turbidimeter (Model OAKTON: T-100). In the Laboratory, total suspended solids (TSS) were determined through the standardized gravimetric method for examination of TSS in water analysis by Howard 1933. Total Dissolved Solids (TDS), the portion that passes through a filter, in a sample correlates to electrical conductivity (Glenn, 2005) (TDS=0.65 × Electrical conductivity). The major inorganic nutrients analyzed in the present study were nitrogen (nitrite-NO2, nitrate-NO3-N, ammonium-NH4-N), phosphorus (soluble reactive phosphorus-SRP and total phosphorus-TP), and dissolved silicate (SiO2) and all were determined using the standard method of APHA (1995) at Limnology laboratory of Addis Ababa University, Ethiopia. Trophic state of the lake was determined using Carlson's (1977) trophic state index (TSI) determination method.
Zooplankton sampling, identification and estimation of abundance and biomass
Zooplankton sample collection was carried out from two sampling sites (open and shore) from January to December 2016, using 30 µm net sampler with vertical halls within the euphotic depth (1.5 – 2 m below the surface). The sample was immediately preserved with 4% formalin. For the identification and enumeration of abundance, 10 ml of sub-sample was taken from the preserved volume of zooplankton sample. Zooplankton identification was held under a stereoscope microscope. The major zooplankton groups were counted using a counting grid as separate groups. In the counting grid/chambers, individuals/liter were counted randomly up to a minimum of 400 individuals under stereoscope microscope (magnification 40x) with a fixed camera, and the final estimation of zooplankton abundance (individual/L) of lake water, was estimated for each site and months using the formula of Edmondson and Winberg (1971). Biomass of the crustacean (copepods and cladocerans) zooplankton was determined using length-weight relationships (Dumont et al., 1975), or gravimetric method. The dry weight of each zooplankton was measured on a PerkinElmer AD6 Autobalance after drying (60°C in an oven for 30 ± 6 h. (Adameneh, 2010). The body length (total body length, excluding caudal setae for copepoda and cladoceran) (Mengistou and Fernando, 1991b; Dumont et al., 1975) of zooplankton was measured using measuring bored with is fixed with the microscope. Length-Weight regression equations were derived for the different species of copepods (230-adults, 148-copepodites, and 166-naupli) and cladocerans (340 adults) to Lake Tinishu Abaya and used this equation throughout the study period. Biomass of rotifer taxa/species was calculated from length measurements and bio-volume approximations whereas, bio-volume of rotifers was computed from linear dimensions applied to simple geometric formulae appropriate to body shape (Ruttner-Kolisko, 1977). A wet weight/dry weight conversion factor of 0.1 (Doohan, 1973) is used for all genera except Asplanchna, for which a factor of 0.039 (Dumont et al., 1975).
Statistical analysis
The relationships between the dominant zooplankton species and significant environmental variables were analyzed using a constrained Redundancy Analyses (RDA, CANOCO for Windows 4) using PAST software (Leps and Smilauer 2003). Pearson correlation 'r' was used to check the affinities of various physico-chemical parameters and its correlation with zooplankton abundance and biomass. Shannon and Evenness indices were applied to show the diversity of zooplankton. One way analysis of variance (ANOVA) was used to analyze the temporal distribution pattern of physico-chemical parameters and zooplankton abundance and biomass. Since only two study site had, a t-test was used to saw check the spatial variation of the various environmental parameters and zooplankton communities. SPSS software package version 20 was used for ANOVA and t-test statistical analysis.
Physico-chemical features
The different physico-chemical features which were responsible for the diversity, abundance, and biomass of zooplankton in Lake Tinishu Abya were measured (Table 1 and Figure 2). As expected from a small-sized and shallow lake, and occurrence of complete mixing, most of the physicochemical parameters was more significantly varied seasonally (p<0.05) than spatially (p>0.05). The value of surface temperature ranged from 18.5 to 27°C at the open station and 18.5 to 29.2°C at shore station, while surface dissolved oxygen (DO) ranged from 5.85 to 15.1 mg/L and 6.1 to 11.62 mg/L at open and offshore stations, respectively. Surface temperature and dissolved oxygen were correlated negatively and strongly (r= -0.46). The minimum and the maximum value of temperature observed in December (dry period) and June (rainy period), respectively at both shore stations. The maximum value of DO was recorded in January (dry season). The pH of the study lake was ranged from a minimum of 8.11 to a maximum of 9.27 at the open station and 8.15 to 9.22 at a shore station. Total alkalinity was varied from 1.44 - 8.26 and 0.96 - 6.94 meq/L at open and shore stations, respectively. There was a seasonal effect on the distribution of pH (p=0.000) and alkalinity (p=0.004). High pH and alkalinity values were seen during the dry season (January to May and October to December). pH and alkalinity were correlated positively and significantly (r= 0.98). The electrical conductivity (EC) of surface water for the study lake varied from 181.1 to 1006 µScm-1 at open station and 147.7 to 1006 µScm-1 at shore station. There was a significant variation of electrical conductivity between months (p<0.05).
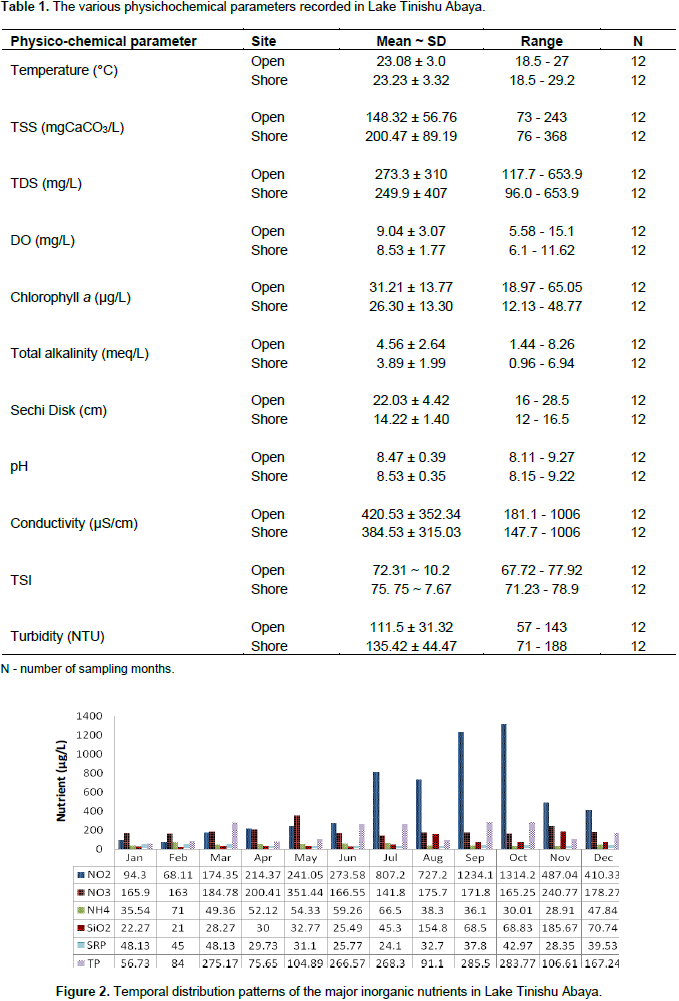
The high values of conductivity were recorded during the dry period (January to March). In this study, both sites have shown a fairly high amount of total dissolved solids (TDS). It was varied from a low value of 117.7 mg/L to a high value of 653.9 mg/L at the open station and 96 to 653.9 mg/L at a shore station. There was a seasonal effect (p<0.05) on the distribution of TDS with high values were observed in the dry period (January to March), and sharply decreased from April to September. EC and TDS were correlated perfectly and positively (r= 1). In the study lake, TSS was estimated and it was ranged from 73.24 - 243 mgCaCO3/L and 76 - 368 mgCaCO3/L at open and shore stations, respectively. TSS was significantly varied seasonally (P=.000) by means of high values were reported in the rainy period (June to August) and low values were observed from November to January (Dry period). The other most important parameters measured in the present study was water turbidity. Lake Tinishu Abaya was highly turbid throughout the year and it was varied from 57 to 143 NTU at the open station and 71 to 188 NTU at a shore station. Turbidity was significantly varied seasonally (P= 0.008) with high values were reported during the main rainy season (June and July). Comparatively, low turbidity was reported in the dry season (January to April). Water transparency (Secchi disk) of the lake was also detected during the study. Secchi disk of Lake Tinishu Abaya varied from a low value of 16 cm to a high value of 28.5 cm at the open station and 12 to 16.5 cm at a shore station. There were significant seasonal variations of Secchi disk between sites (0.042) and months (p=0.025). Secchi disk was higher at the open station than a shore station and during the time of the rainy season (June - September). The euphotic depth (Zeu), the depth at which 1% of the surface photosynthetic active radiation (PAR) is detected, for the study area, was calculated at the open station, and it was ranged from a low value of 0.51 m to a high value of 0.91 m.
The concentration of chlorophyll-a that was recorded in the study lake was varied from 18.97 to 65.05 µg/L at the open station and 12.13 to 48.77 µg/L at a shore station. The annual mean chlorophyll values were 31.21 and 26.3 µg/L at open and shore stations, respectively (Table 1). There was a seasonal effect (p=0.029) in the distribution of chlorophyll a and it was high during the dry season (January to May and October to December). Pearson correlation coefficient analysis indicated that chlorophyll a was correlated positively and strongly with pH (r=0.543), conductivity (r=0.485), Secchi disk (r=0.467), and alkalinity (r=0.576) while it was correlated negatively and strongly with NO2 (r= -0.550), TP (r= -0.411) and TSS (r= -0.658). The major inorganic nutrients analyzed in the present study were nitrogen (nitrite-NO2, nitrate-NO3-N, ammonium-NH4-N), phosphorus (soluble reactive phosphorus-SRP and total phosphorus-TP), and dissolved silicate (SiO2) (Figure 2). The annual mean concentration of NO2, NO3, NH4, SRP, TP, and SiO2 were, 503.82, 192.14, 47.44, 36.11, 172.13, and 62.80 µg/L at the open station and 626.32, 42.26, 51.34, 42.26, 184.41 and 62.48 µg/L at the offshore station, respectively. Nitrite and ammonium nutrients were significantly varied seasonally (p<0.05) with high concentrations as reported during the rainy season (June to September). Conversely, nitrate was high in the dry season (January to May and October to December). Phosphorus nutrients showed a significant seasonal variation (p<0.05). A clear oscillation was observed in the concentrations of TP. It was maximum from May to July and from September to October. The peak value of SRP was seen from January to March (dry period). Reasonably, low dissolved silica was reported in the study lake throughout the study period. Carlson's trophic state index (CTSI) was varied from 67.72 to 77.92 and 71.23 to 78.9 with a mean value of 72.31 ~ 10.2 and 75.75 ~ 7.67 at the open and shore station, respectively (Table 1). Based on TSI values, the overall trophic state of Lake Tinishu Abaya was a hypereutrophic system. The trophic state index in terms of chlorophyll-a was lower than the trophic state indices of Secchi disk and total phosphorus which indicated that the hypereutrophic state of Lake Tinishu Abaya is as a result of the presence of high concentration of phosphorus and low water transparency rather than algal bloom/algal turbidity.
Zooplankton diversity and abundance
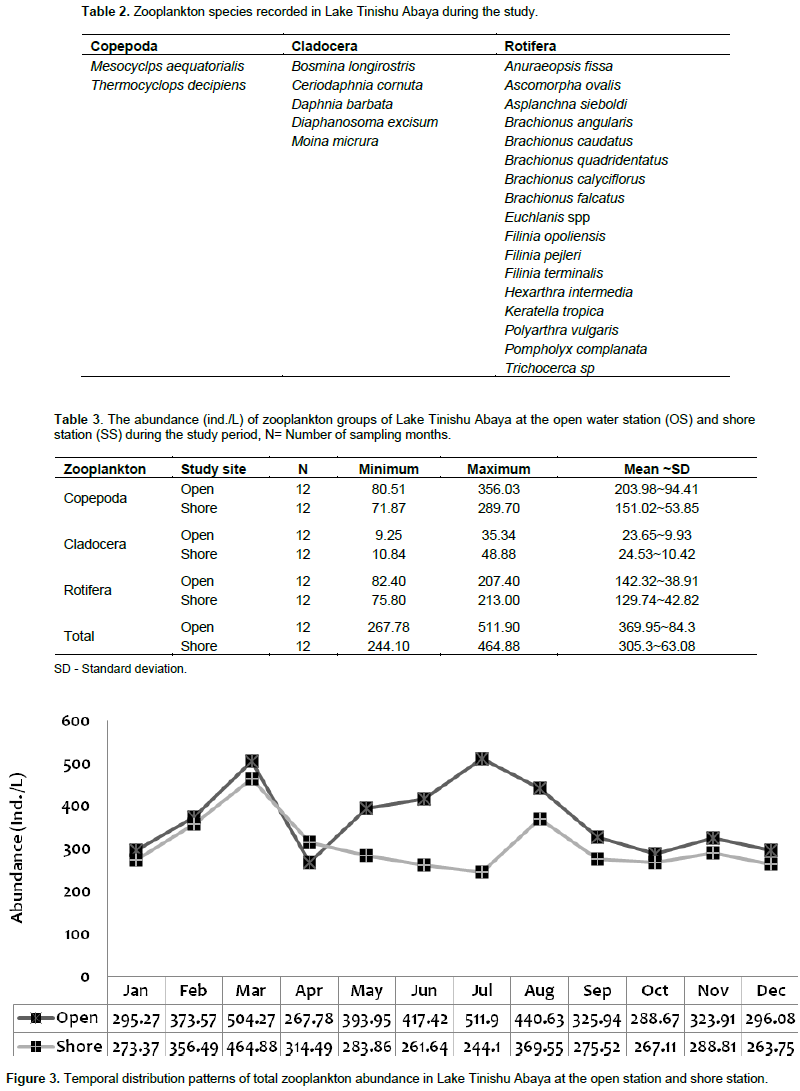
During the study, a total of 24 species of zooplankton that comprise Rotifera, Copepoda, and Cladocera were identified in Lake Tinishu Abaya for the first time (Table 2). Rotifera had the highest number of species (11 species) followed by Cladocera (5 species), and Copepoda (2 species) (Table 2). The Shannon diversity index was higher for Rotifera (2.45) than cladocerans and (2.395) copepods (2.384). This showed the small-sized Rotifera were more diverse than the larger crustacean in the study area. Evenness index was lower for copepods (0.9043) and cladocerans (0.9138) than Rotifera (0.9657) which indicated the two crustacea were distributed randomly in the lake. The annual mean total zooplankton abundance was 369.95 ~ 84.3 at the open site and 305.3 ~ 63.08 ind./L at the shore site. It varied from a low value of 267.8 to 511.9 ind./L and 244.1 to 464.88 ind./L at the open site and shore station, respectively (Table 3). The total abundance of zooplankton was dominated by copepods (54%) followed by Rotifera (40%) and cladocerans (6%). There was significant variation in the total zooplankton abundance between months (p=0.045) by means of high and low values which was reported during the rainy season (June-September) and the dry season (January-May), respectively (Figure 3). The temporal variations of the larger crustaceans (copepods and cladocerans) and small-sized rotifers are shown in Figure 4. There was a seasonal effect (p=0.01) on the distribution of copepods abundance with high values (60 - 65%) which occurred during the rainy season (June-August). The adult copepods T. decipiens widely dominated (25 - 45%) the abundance of zooplankton in most of the sampling months. The abundance of T. decipiens was high (>50%) in all the rainy season (June - September) and low (<15%) during the dry season (January - April and November - December (Figure 5). M. aequatorialis, on the other hand, contributed relatively low percentage (5%) for the total zooplankton abundance. The high numerical abundance of M. aequatorialis was observed in the dry season (January-April) and from the pre-rainy season (May) towards the rainy season (June-September), low abundance of M. aequatorialis was shown (Figure 5).
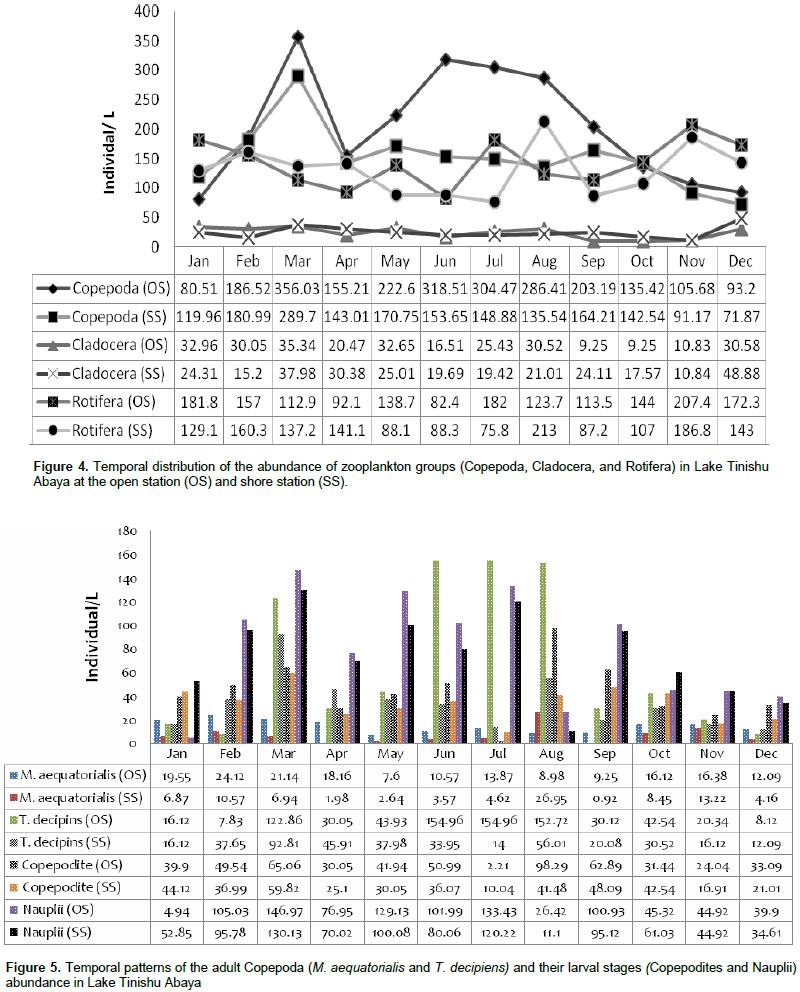
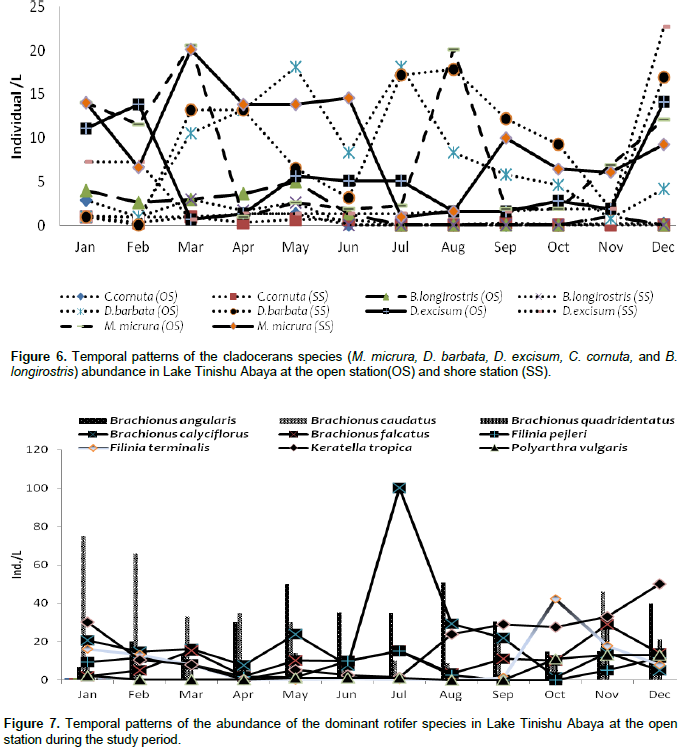
The pre-adult stages, copepodite, and nauplii contributed a significant amount (35%) of the total zooplankton abundance. The former contributed 23% of the total zooplankton abundance. The high numerical abundance of the pre-adult stages of copepods was seen mostly during the rainy season (June, August, and September) (Figure 5). Unlike the copepod crectaceans, cladocerans consisted of low abundance (<10%) throughout the study period. The peak (25%) abundance of cladocerans was reported in January and December. M. micrura, D. barbata, and D. excisum significantly (90%) dominated the abundance of cladocerans. The remaining 10% of the cladoceran abundance was comprised of C. cornuta and B. longirostris. The abundance of D. barbata was relatively high during the rainy season (June to September) (Figure 6). On the other hand, the abundance of most of the cladoceran species (M. micrura, D. excisum, C. cornuta, and B. longirostris) was high mostly during the dry period (January to March and November to December) (Figure 6). Rotifera was the second most important group of zooplankton in terms of abundance and there was a notable significant temporal variation (p=0.019) in the abundance of Rotifera. In contrast to the copepods, high abundance (50 - 55%) of Rotifera was observed during the dry season (January, November, and December). B. angularis considerably (55 - 70%) (January to March), B. caudatus dominated (>45%) the abundance of Rotifera. Most of the Rotifera species (K. dominated the Rotifera abundance in most of the sampling months (March to August) (Figure 7). . At the beginning of the study period (January to March), B. caudatus was dominated (>45%) the abundance of Rotifera. Most of the Rotifera species (K. tropica, B. falcatus, F. pejleri, A. ovalis, P. complanata, P. vulgaris, Trichocera sp, Euchlanis sp, A. sieboldi) were high during the dry seasons.
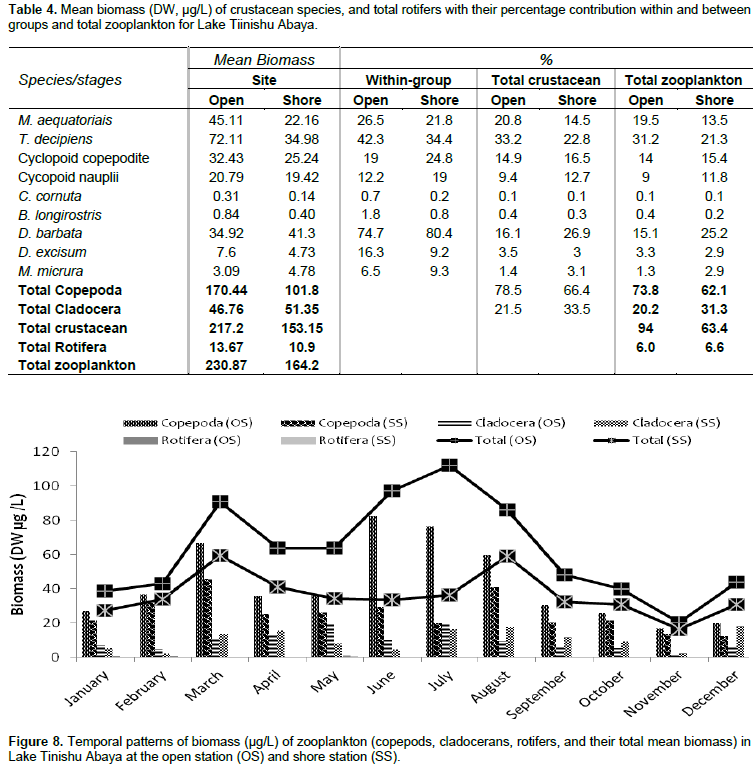
Zooplankton biomass
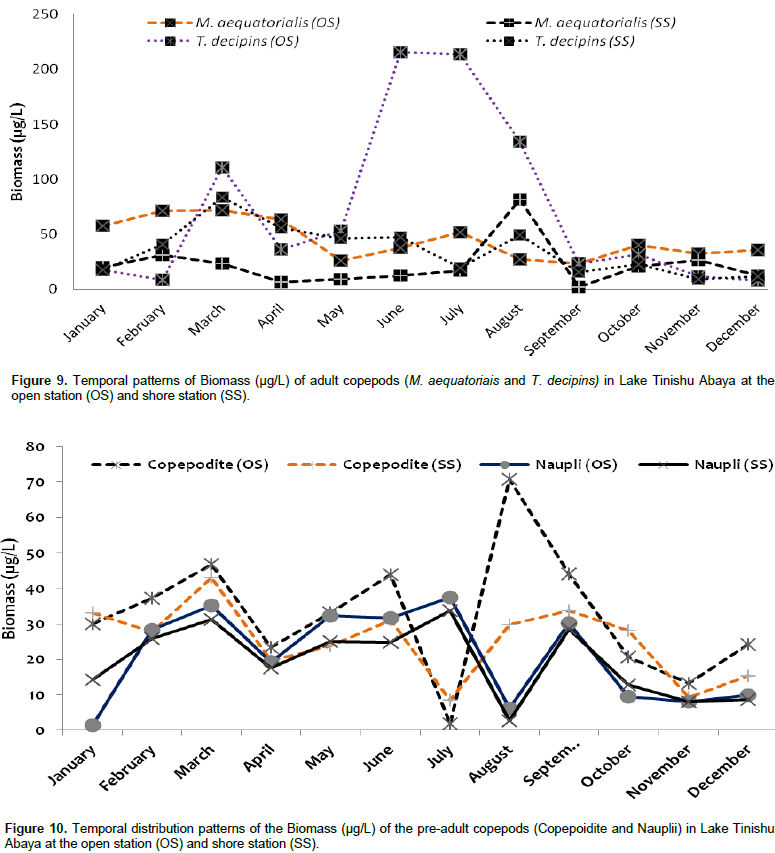
The overall mean total zooplankton biomass of Lake Tinshu Abaya was 230.87 and 164.2 µg/L at open and shore stations, respectively. The mean total biomass of copepods, cladocerans, and Rotifera were 174.4, 46.76 and 13.67 µg/L at open sites and 101.8, 51.35 and 10.9 µg/L at shore sites, respectively (Table 4). The biomass of zooplankton was widely dominated by copepods (74%), and subsequently cladocerans (20%) and Rotifera (6%) (Table 4). The high biomass of zooplankton in the study area was contributed by T. decipiens (31%), M. aequatoriais (19.5%), D. barbata (15%), copepodite (14%), and nauplii (9%) at the open water site and D. barbata (25%), T. decipiens (21%), copepodite (15%), M. aequatoriais (13.5%), and nauplii (12%) at a shore site (Table 4). There was significant temporal variation (p=0.037) in the biomass total of zooplankton with high values observed mostly during the main rainy season (June-August) (Figure 8) coincides with high ambient inorganic nutrients and water turbidity. There was significant variation in the mean biomass of copepods between sites (p=0.025) and months (p=0.0015). The biomass of copepods was high at open sites and during the rainy period (June to August) and in the post-rainy month (December) (Figure 8). The two adult copepods, T. decipiens, and M. aequatoriais contributed the highest (35 - 51%) biomass of zooplankton. The former comprised 31.2% and the later comprised 19.5% of the total zooplankton biomass (Table 4). The larval stages of copepods, copepodites, and nauplii, together comprised relatively high percentage (about 25%) of the total zooplankton biomass (Table 4). The high biomass of the two larval stages (copepodites and nauplii) (Figure 9) and the adult T. decipiens (Figure 10) were observed during the rainy season (June to September). The high biomass of M. aequatoriais, on the other hand, was seen during the dry period (January to April) (Figure 10).
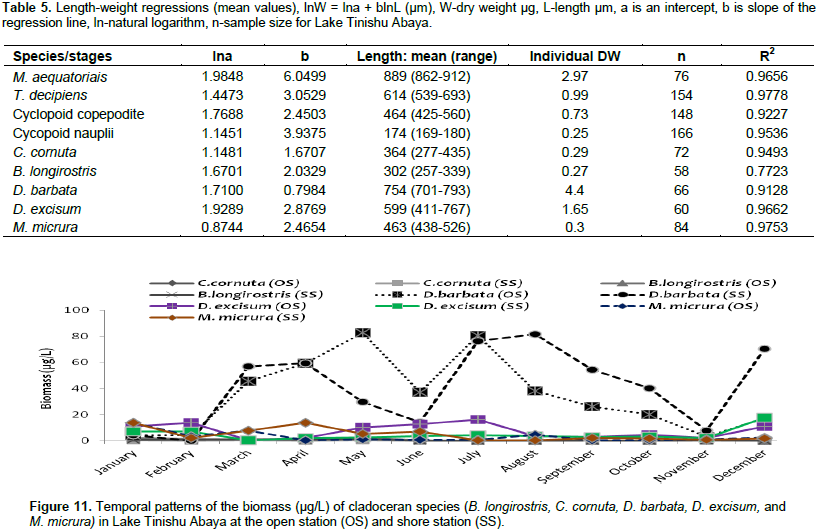
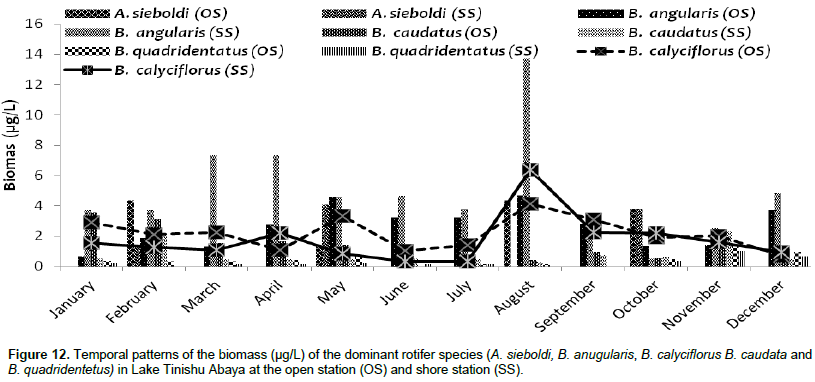
The large-sized cladocerans consisted of relatively high biomass next to the copepods (Table 4). The biomass of cladocerans was significantly varied seasonally (p=0.02) with high values observed during the main rainy season (June, July, and August) concurrently with high water turbidity. D. barbata had large body-size (754 µm mean length) and high body dry weight (4.4 µg mean dry weight) relative to the other crustacea species (Table 5) and the species consisted of the highest (75%) biomass of cladoceran. This species also comprised the highest (25%) biomass of the total zooplankton at a shore station. D. excisum contributed relatively high (16%) biomass for cladoceran, next to D. barbata. Though the maximum abundance of cladocerans was contributed by M. micrura, it contributed low (only 9%) biomass of cladocerans. C. cornuta and B. longirostris contributed an insignificant amount (<5%) for the biomass of cladoceran. The biomass of D. barbata and D. excisum was relatively high during the rainy time (June-September). The reverse was true for M. micrura, C. cornuta and B. longirostris having high biomass during the dry period (Figure 11). The biomass of Rotifera was approximately 15 times lower (6% ) than the crustacea. The total biomass of Rotifera was ranged from 5.54 - 18.69 µg/L and 5.17 - 20.7 µg/L at open and shore sites, respectively. There was a seasonal effect (0.03) in the biomass of Rotifera by means of high values which was estimated during the dry season (January, February, May, and December) (Figure 6). The highest (75%) biomass of Rotifera was contributed by four Brachionus species (B. angularis, B. calyciflorus, B. caudatus and B. quadridentatus) (Figure 12). The maximum percentage (45%) contribution for the total rotifer biomass was contributed by B. angularis followed by B. calyciflorus (17%), B. caudatus (12%), and B. quadridentatus (10%). The individual dry weight of A. sieboldi was much higher (4.1 µg/L) compared to all the other species of Rotifera. However, its percentage contribution for the total biomass of Rotifera was relatively low (10%) in relation to its body size. There was a seasonal effect in the distribution of the different species of Rotifera biomass. At the beginning of the study period (January to March), Filinia sp. contributed the highest (35%) percentage whereas, in May, August and October A. sieboldi contributed the maximum percentage (41%) of the biomass of Rotifera.
Redundancy analysis (RDA)
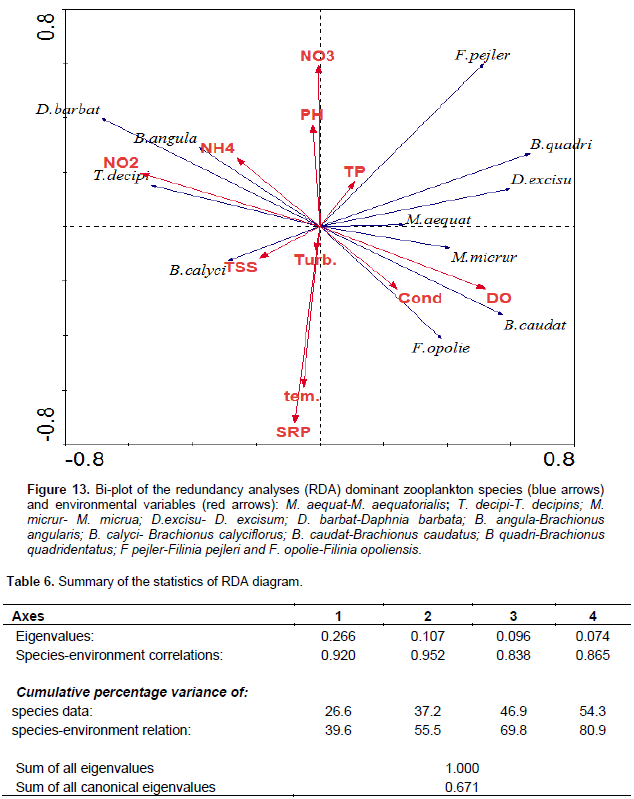
The correlation of environmental parameters and dominant zooplankton species was analyzed using a constrained Redundancy Analyses (RDA, CANOCO ) graph (Figure 13). The first and second axes explain 55.5% of the cumulative percentage variance of species-environment relation (Table 6). The first axis was correlated positively with conductivity, DO and TP and it was negatively correlated with turbidity, temperature, pH, TSS, NO2, NH4, and SRP. The second axis correlated positively with pH and most of the nutrients and it was correlated negatively with TSS, turbidity, temperature, conductivity and DO (Figure 13). The occurrence of T. decipiens and B. angularis correlated positively and strongly with NO2 and NH4. Whereas B. calyciflorus occurrence correlated positively and strongly with TSS and turbidity. F. opoliensis, B. caudatus, and M. micrura coincided positively with conductivity and DO. D. barbata occurrence correlated negatively but strongly with DO and conductivity. The occurrence of B. quadridentatus, D. excisum, and M. aequatorialis correlated negatively and strongly with TSS, turbidity and temperature and nutrients of NH4 and NO2 while they correlated positively but weakly with TP (Figure 13).
Zooplankton diversity and abundance
Diversity indices have been used as an important tool by ecologists to understand community structure in terms of richness, evenness or a total number of existing individuals (Allan, 1975). In the present study, the diversity of zooplankton communities of Lake Tinishu Abaya is fairly high, which indicated that the lake water is plausibly productive. The diversity of zooplankton in the study area, reflects typical tropical aspects, with Rotifera being by far the dominant taxa richness (Mengistou and Fernando, 1991b; Adamneh, 2010; Matheos, 2011). Rotifer diversity dominating over the crustacea in the present study is in agreement with reports from other Ethiopian Lakes (Mengistou and Fernando, 1991a; Fetahi et al., 2011; Fetahi et al., 2014). In his studies on zooplankton communities from East African lakes, Green (1993) found that Rotifera has more species in the plankton than copepods and cladocerans. Green and Seyoum (1991) also found that the mean momentary Rotifera species number from Ethiopian water samples was higher than the mean for the rest of the world. Size relationships of plankton and fish have been documented in many different environments. Lakes with resident Salmonoid fish have crustacea zooplankton always smaller than 0.75 mm and substantial rotifer populations, while lakes without fish contained much longer crustacea and few Rotifera (1962). In Lake Tinishu Abaya, Oreochromis niloticus fish were introduced into the lake in 1997 (Kasshun et al., 2011). This may ravenously prey the large-sized cladocerans and there may be a top-down effect. The lower percentage of Cladocera, in turn, could have resulted in a high diversity of Rotifera. The smaller zooplankters are known to be less impacted by blue-green algae (Bouvy et al., 2000) could have persuaded their dominance over the large-sized-crustacea. Rotifera benefited from the high turbidity of the lake as they are less affected than larger filter feeders (Kirk, 1991).
This explanation may seem to spot on for the diversity of Rotifera over the crustacean in the study area ever since the lake water is very turbid throughout the study period. In the study area, the abundance of zooplankton was significantly dominated by the cyclopoid copepods. This is in accordance with several other authors, who reported the dominance of copepod abundance in tropical and subtropical lakes (Burgis, 1974; Lewis, 1979; Mengistou and Fernando, 1991a; Amarasinghe et al., 1997; Irvine and Waya, 1999; Eshete et al., 2004; Adamneh, 2010; Fetahi et al., 2011). The dominance of the abundance of copepods and Rotifera over the cladocerans in Lake Tinishu Abaya could have been the result of their competitive advantage. Feeding experiment did show that Rotifera and smaller zooplankton were dominant in the presence of blue-green filament (Fulton and Paer, 1988). The low percentage of cladocerans abundance compared to copepods and rotifers in the study area could have been due to the high pressure of predation by juvenile tilapia. Cladocera seems to be affected by the high turbidity of the lake and the food quality. It has been indicated in several studies that the formation of large colonies or filaments limits their exploitation by zooplankton through a physical constraint on ingestion, nutritional inadequacy, and toxicity (HrbáÄek, 1962; DeMott and Moxter, 1991; Haney et al., 1994, Gilbert, 1990). As expected from small-sized and the occurrence of frequent water mixing lake, spatial variations of zooplankton abundance is less expected. The polymictic nature of the lake and relatively equivalent amount of major inorganic nutrients (nitrate, phosphate, and silicate) could have resulted in the absence of spatial variations of zooplankton abundance in the study area. The abundance of zooplankton, on the other hand, showed a significant variation between a month and this variation probably due to proximal causes such as changes in resource availability, weather, and predator pressure. Since zooplankton is an important diet of planktivorous fish (Fernando and Ponyi, 1981), the effect of predation on zooplankton temporal variation cannot be ruled out in Lake Tinishu Abaya. The breeding season of tilapia (O. niloticus) in Lake Tinishu Abaya occurred between December and May peaking in January, February and March where a minor peak was also observed in June, July and August (Kassahun et al. 2011), may play a role in the seasonal structuring of the zooplankton community.
Copepoda and Rotifera that dominated the abundance of zooplankton in the study lake correlated negatively with chlorophyll a (food) and indicated that there is a grazing pressure on phytoplankton, which is a well-established fact for other Ethiopian lakes (Adamneh et al. , 2008). In this study, large seasonal changes in water turbidity and Secchi disk were obviously observed. The turbidity and Secchi disk was positively and strongly correlated the total zooplankton abundance which seems that the two physicochemical variables are also important factors for the seasonal variations of zooplankton communities. Compared to the other factors, copepods abundance was correlated positively and strongly with Secchi disk (r= 0.504) and pH (r =0.545) which indicated that the two physiochemical factors had a profound effect on the seasonality of the copepod abundance. Several studies revealed that temporal variations of tropical zooplankton were associated with turbidity, water level, temperature, and stratification (Irvine and Waya, 1999; Eshete , 2003; Eshete et al., 2004; Melao and Rocha, 2004; Isumbisho et al., 2006). The relative total abundance of zooplankton was high during the rainy season which coincided with the complete mixing period, high ambient inorganic nutrients, high water turbidity, and low water transparency. Seasonal increase in zooplankton abundance during the rainy periods was also reported in Lake Naivasha, Kenya and Lake George, Uganda (Mavuti and Literick, 1981), and in Ethiopian Lakes Hawassa (Mengistu and Fernando, 1991b), Tana (Ayalew, 2006), Ziway (Adamneh et al., 2008; Mathios, 2011) and Hayq (Fetahi et al., 2011). The allochthonous dissolved nutrients washed into the lake via rivers Dacha and Bobodo, during the rainy time persuade high phytoplankton biomass (chlorophyll a) that might in turn, support the occurrence of high zooplankton abundance. T. decipiens significantly dominated the total zooplankton abundances throughout the sampling period. The RDA analysis (Figure 11) indicated that the abundance of T. decipins is strongly correlated with nutrient availability (NO2, NO3, NH4), and other various physicochemical variables (pH, turbidity, and TSS). The dominance of T. decipiens abundance over the other species of zooplankton could be the result of its omnivorous feeding habits; this species is reported to feed on Microcystis and nauplii in Lake George Uganda (Burgis, 1969).
It may have gained an advantage from the dominance of blue-green algae and the fact that it is less attractive for a predator due to its small body size. The average total length of this species is 614 µm in Lake Tinishu Abaya (Table 5), which is considered as small in size, may benefit from predator and could lead to its extensive dominance in the study lake. Size variation in the distribution and abundance of copepod species was evident in the present study, and larger adult M. aequatorialis were very low compared to the small-sized T. decipiens. A possible reason for the absence of a high abundance of M. equatorialis could be the presence of high juvenile (<10 cm in total length) O. niloticus (Brook and Yirga, 2018), which may pose a significant predation on the larger adult copepods, M. aequatorialis. The presence of high juvenile Tilapia may avoid the macrophytes due to chemical cues from the fish. Larger copepods like M. aequatorialis, preferred macrophytes to avoid their predation. However, the distribution of macrophytes in the study lake was low (personal observation). In shallow lakes where the water transparency is generally low (a feature of Lake Tinishu Abaya), diel vertical migration may hardly be observed. Rather, in such shallow lakes, large-bodied zooplankton migrates horizontally from the open water to the littoral into the macrophytes seeking a refuge against predators (Burks et al., 2002), though the compositions of macrophytes in the study lake is very low. This may result in the occurrence of low abundance of large copepods, M. eaquatorialis compared to the small-sized T. decipiens. However, it is difficult to explain such fluctuations in species composition due to a lack of extensive studies on macrophytes as habitat effect on large copepods in this study lake. Several studies indicated Chaoborus as a major component of the limnetic food web in tropical lakes (e.g. Lake Chad; Saint-Jean, 1983; Lake Malawi; Irvine, 1997; or in shallow reservoirs (Aka et al., 2000; Pagano et al., 2003). Chaoborus can eliminate 2 - 90% of the population of its prey per day (Pastorok, 1980) or 20 - 29 prey items per individual per day (Pagano et al., 2003). An experimental study on Chaoborus predation on zooplankton also indicated that large zooplankton like adult Mesocyclops species is significantly affected.
Consistent high selection of Chaoborus for M. aequatorialis was reported from Lake Malawi (Irvine, 1997) and an increase in vulnerability of Mesocyclops with an increase in size from Lake Valencia (Lewis, 1979) and Lake Tana (de Graaf, 2003). In the study lake, we confirmed the presence of these predators may have as outcome the lower abundance of M. aequatorialis. Moina micrura and D. barbata, and D. excisum contributed the maximum abundance of cladoceran while C. cornuta and B. longirostris contributed an insignificant amount. Except the D. barbata, the abundance of most of the cladoceran species (M. micrua, D. excisum, C. cornuta, and B. longirostris) was relatively high during the dry season. The presence of a high abundance of most cladoceran in most of the dry season may be due to the occurrence of high phytoplankton biomass (chlorophyll-a) and Rotifera. The RDA graph also indicated that the M. micrura and D. excisum was strongly correlated with surface temperature and dissolved oxygen, which showed they regulate the distribution of the two species. The high abundance of D. excisum reported during dry seasons. The high abundance of D. barbata was observed during the rainy season and this could have been due to the high ambient inorganic nutrients and water turbidity. D. barbata was correlated strongly and positively with phosphorous nutrients than others (RDA graph/Figure 13). In the study lake, the abundance of Rotifera was dominated by Brachionus species more than half of the sampling periods. The four species of Brachionus (B. angularis, B. caudatus, B. calcyflorus, and B. quadridentatus) contributed the highest abundance of Rotifera. B. angularis dominated Rotifera abundance from January to March (Dry period). The other Rotifera which contributed a significant amount to the total abundance of Rotifera were H. intermedia, K. tropica, and F. terminalis. Most of the Rotifera species abundance was high during the dry season probably as a result of high phytoplankton biomass (chlorophyll a). Generally, the abundance of Rotifera was high during the rainy season concurrently with high temperature, high water transparency, high dissolved oxygen, and high phytoplankton biomass (chlorophyll-a) whereas they are low in time of the rainy season coincides with high water turbidity.
Zooplankton biomass
Biomass reflects the instantaneous quantity of live organic matter in a given area and also provides a means of analyzing an ecosystem’s productivity, irrespective of taxonomic composition (Pace, 1986). The total mean standing biomass of the relatively larger crustaceans (copepods and cladocerans) and small-sized-Rotifera in the study lake was fairly high which shows the lake water is considered moderately productive which supports most of the aquatic lives including fish production. The biomass of the Lake Tinishu Abya is comparable with other nearby rift valley lakes of Ethiopia such as L. Ziway (Adamneh, 2010) (90.87 mg DW m-3 for total zooplankton biomass, 78.3 mg DW m-3 for copeoda, 9.4 mg DW m-3, for cladocera, and 3.1 mg DW m-3 for rotifera), L. Hawassa (Mengistou and Fernando, 1991a (44.9 mg DW m-3 for total zooplankton biomass, 36.93 mg DW m-3 for copepoda biomass) and L. Hayq (Fetahi et al., 2011) (236.4 mg DW m-3 for total zooplankton biomass, 161.46 mg DW m-3 for copepoda biomass, 75 mg DW m-3 for cladocera biomass). The occurrence of the reasonably high biomass of zooplankton in the present study could have been due to the presence of high inorganic nutrients which discharge from the watershed through runoff, high water turbidity through the year, and comparatively high algal biomass (chlorophyll a), well oxygenated, and good water temperature. The occurrence of large body-sized cladoceran species, particularly D. barbata, may help in the presence of high zooplankton biomass in the study area. An increase in zooplankton biomass has often been associated with a rise in the trophic status of the environment. Studies that consider biomass values are important because they allow comparison of different environments, providing a common unit to evaluate zooplankton groups.
Generally, eutrophic environments support higher biomass of zooplankton than more oligotrophic ones (Esteves and Sendacz, 1988). The trophic status of Lake Tinishu Abaya is eutrophic, which results in the sort of high biomass of zooplankton in the study lake. The two crustaceans (copepods and cladocerans) have contributed the highest biomass of zooplankton in the study area. The presence of high relative abundance and having large body size for the crustacean may have favored the occurrence of higher biomass than the small-sized Rotifera. In addition to the extensive dominance of their abundance, the higher contribution of copepods for the total zooplankton biomass may also be due to grazing presser on phytoplankton. Biomass of copepod correlated positively and strongly with the abundance of Bacilorophyceae (p=0.002) and Cyanophyceae (p=0.084), indicated the occurrence of high grazing between the two groups. There was a seasonal variation in the distribution of biomass by which the biomass of crustacea was high mostly during the rainy season when most of the inorganic nutrient and the turbidity of the lake water became high. Biomass of the small-sized Rotifera, on the other hand, was high during the dry season when surface temperature, dissolved oxygen, pH, and alkalinity increased. In the post-rainy season (December) and the dry season (March), high mean biomass of zooplankton was shown. High phytoplankton biomass (chlorophyll-a ) were observed in March which may, in turn, result to occurrence of high zooplankton biomass in March. In addition, the occurrence of unusual rainfall in March (personal observation) may augment the entering of nutrients from the watershed to the lake owing to the escalating biomass of zooplankton in this month.
The zooplankton communities found in Lake Tinishu Abaya, comprised of the two Crustacea, cyclopoid copepod and cladoceran, and the small-sized Rotifera. Rotifera accounted for the highest number of species while copepods had the highest abundance of zooplankton. There was a temporal pattern in the distribution of zooplankton with Rotifera abundance being high during the dry period coincides with high chlorophyll a (phytoplankton biomass), whereas, crustacea abundance was prominent during the rainy period concurrently with high ambient inorganic nutrients and water turbidity. The standing biomass of zooplankton in the study lake was comparatively high and was dominated by copepods followed by cladocerans then Rotifera. Due to the high biomass, the lake can be said to be productive. The various physico-chemical factors responsible for the observed temporal variations in the physical, chemical and biological features of the lake are discussed and generalized that the lake water was well oxygenated, slightly warm, alkaline, contained more TSS, TDS, and EC, very turbid, low transparency and with relatively high inorganic nutrients which support most of the aquatic lives. Generally, based on the results of the diversity, abundance, and biomass of zooplankton communities, and the various physichochemical drivers, the authors concluded that Lake Tinishu Abaya is reasonably a productive inland freshwater ecosystems in the rift valley of Ethiopia.
The authors have not declared any conflict of interests.
The authors thank the Department of Zoological Sciences of Addis Ababa University for assisting with laboratory facilities. Bureau of Cultural and Tourism for Selttie Woreda (Ethiopia) is also appreciated for allowing the study to be conducted in the area.
REFERENCES
|
Adamneh D (2010). Zooplankton community structure, population dynamics and production and its relation to abiotic and biotic factors in Lake Ziway, Ethiopia, Ph.D.D Thesis, Wien University.
|
|
|
|
Adamneh D, Herzig A, Jersabek C, Tadesse Z (2008). Abundance, species composition and spatial distribution of planktonic rotifers and crustaceans in Lake Ziway (Rift Valley, Ethiopia). International Review of Hydrobiology, 93:210-226.
Crossref
|
|
|
|
Aka M, Pagano M, Saint-Jean L, Arfi R, Bouvy M, Cecchi P, Corbin D, Thomas S (2000). Zooplankton Variability in 49 Shallow Tropical Reservoirs of Ivory Coast (West Africa). International Review of Hydrobiology, 85:491-504.
Crossref
|
|
|
|
Allan JD (1975). The distributional ecology and diversity of benthic insects in cement creek, Colorado. Ecology, 56:1040-1053.
Crossref
|
|
|
|
Amarasinghe PB, Vijverberg J, Boersma M (1997). Production biology of copepods and cladocerans in three south-east Sri Lankan low-land reservoirs and its comparison to other tropical freshwater bodies. Hydrobiologia, 350:145-162.
Crossref
|
|
|
|
American Public Health Association (APHA) (1995). Standard Methods for the Examination of Water and Wastewater, 19 ed. Washington, DC.
|
|
|
|
Armengol J (1980). Colonización de Los embalses espa-oles por crustáceos planctónicos y evolución de la structure de suscomunidades. Oecologia aquatica 4:45-70.
|
|
|
|
Armengol X, Miracle MR (1999). Zooplankton communities in doline lakes and pools, in relation to some bathymetric parameters and physical and chemical variables, Journal of Plankton Research, 21(12):2245-2261.
Crossref
|
|
|
|
Ayalew W (2006). Dynamics of the major phytoplankton and zooplankton communities in Lake Tana, Ethiopia, a Ph.D. thesis, Addis Ababa University.
|
|
|
|
Bouvy M, Falcao D, Marinho M, Pagano M, Moura A (2000). The occurrence of Cylindrospermopsis (Cyanobacteria) in 33 Brazilian tropical reservoirs during the 1998 drought. Aquatic Microbial Ecology, 23:13-27.
Crossref
|
|
|
|
Burgis MJ (1969). A preliminary study of the ecology of zooplankton in Lake George, Uganda. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 17: 297-302.
|
|
|
|
Burgis MJ (1974). Revised estimates of the biomass and production of zooplankton in Lake George, Uganda. Freshwater Biology 4:535-541.
Crossref
|
|
|
|
Carlson RE (1977). A trophic state index for lakes. Limnology and Oceanography. 22(2):361-369.
Crossref
|
|
|
|
de Graaf M (2003). Lake Tana's Piscivorous Barbus (Cyprinidae, Ethiopia). Ecology, Evolution, and Exploitation. Ph.D. thesis, Wageningen University, Wageningen, The Netherlands, 255 p.
|
|
|
|
DeMott WR, Moxter F (1991). Foraging on cyanobacteria by copepods: response to chemical defenses and resource abundance. Ecology, 72:1820-1834.
Crossref
|
|
|
|
Doohan M (1973). An energy budget for adult Brachionus piicatilis Muller (Rotatoria). Oecologia, 13:351-362.
Crossref
|
|
|
|
Dumont HJ, Van De Velde I, Dumont S (1975). The dry weight estimate of biomass in a selection of Cladocera, Copepoda, and Rotifera from the plankton, periphyton, and benthos of continental waters. Oecologia, 19:75-97.
Crossref
|
|
|
|
Edmondson WT, Winberg GG (1971). A Manual on Methods or the Assessment of Secondary Productivity in Fresh Waters. IBP Handbook no. 17. Oxford: Blackwell.
|
|
|
|
Eshete D (2003). Ecology and Potential for Fishery of the Small Barbs (Cyprinidae, Teleostei) of Lake Tana (Ethiopia). Ph.D. thesis, Wageningen University, Wageningen, The Netherlands, 190 p.
|
|
|
|
Eshete D, Vijverberg J, Nagelkerke-Leo AJ, Ferdinand AS (2004). Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (Lake Tana, Ethiopia). Hydrobiologia, 513:39-49.
Crossref
|
|
|
|
Esteves KE, Sendacz S (1988). Relações entre a biomass do zooplâncton e o Estado trófico de reservatórios do Estado de São Paulo. Acta Limnologica Brasiliensia, 11:587-604
|
|
|
|
Fábio de A, Juliana DD, Louizi de S, Magalhães B, Cláudia CB (2012). Length-weight regressions of the microcrustacean species from a tropical floodplain. Acta Limnologica Brasiliensia, 24(1):1-11
Crossref
|
|
|
|
Fernando CH, Ponyi JE (1981). The free-living freshwater cyclopoid Copepoda (Crustacea) of Malaysia and Singapore. Hydrobiologia, 78:113-123.
Crossref
|
|
|
|
Fetahi T, Schagerl M, Mengistou S (2014). Key drivers for phytoplankton composition and biomass in an Ethiopian highland lake Hayq. Limnologica, 46:77-83.
Crossref
|
|
|
|
Fetahi T, Seyoum M, Michael S (2011). Zooplankton community structure and ecology of the tropical-highland Lake Hayq, Ethiopia. Limnologica, 41:389-397.
Crossref
|
|
|
|
Fulton RS, Paer HW (1988). Effects of the blue-green alga Microcystis aeruginosa on zooplankton competitive relations. Oecologia, 76(3):383-389.
Crossref
|
|
|
|
Ghidini AR, dos Santos-Silva EN (2011). Composition, species richness and patterns of nycthemeral vertical distribution of planktonic cladocereans in a black water Amazonian lake. Nauplius, 19(2):109-122.
Crossref
|
|
|
|
Ghidini AR, Santos-Silva EN (2009). Biomassa de quatro espécies de Cladocera (Crustacea: Branchiopoda) e sua variação nictemeral no Lago Tupé, Amazonas, Brasil.
|
|
|
|
Gilbert JJ (1990). Differential effects of Anabaena affinis on cladocerans and rotifers: Mechanisms and implications. Ecology, 71:1720-1740.
Crossref
|
|
|
|
Glenn (2005). Total Dissolved Solids from conductivity. Technical Support, In-Situ Inc. Technical Note 14.
|
|
|
|
Green J, Seyoum M (1991). Specific diversity and community structure of Rotifera in a salinity series of Ethiopian inland waters. Hydrobiologia, 209:95-106.
Crossref
|
|
|
|
Green L (1993). Zooplankton Associations in East African Lakes Spanning a Wide Salinity Range. Hydrobiologia, 267:249-256.
Crossref
|
|
|
|
Haney JF, Forsyth DJ, James MR (1994). Inhibition of zooplankton filtering rates by dissolved inhibitors produced by naturally occurring cyanobacteria. Archiv fur Hydrobiologie. Stuttgart, 132:1-13.
|
|
|
|
Holmes RW (1970). The Secchi disc in turbid coastal waters. Limnology and oceanography, 15:688-694.
Crossref
|
|
|
|
Howard CS (1933). Determination of Total Dissolved Solids In Water Analysis. Industrial & Engineering Chemistry Analytical Edition, 5(1):4-6.
Crossref
|
|
|
|
HrbáÄek J (1962). Species composition and amount of the zooplankton in relation to fish stock. Rozpr. Cesk. Akad. Ved., Rada. Mat. Prir. Ved. 10:1-116.
|
|
|
|
Irvine K (1997). Food selectivity and diel vertical distribution of Chaoborus edulis (Diptera, Chaoboridae) in Lake Malawi. Freshwater Biology 37:605-620.
Crossref
|
|
|
|
Irvine K, Waya R (1999). Spatial and temporal patterns of zooplankton standing biomass and production in Lake Malawi. Hydrobiologia, 407:191-205.
Crossref
|
|
|
|
Isumbisho M, Sarmento H, Kaningini B, Micha JC, Descy JP (2006). Zooplankton of Lake Kivu, East Africa, half a century after the Tanganyika sardine introduction. Journal of Plankton Research, 28:971-989.
Crossref
|
|
|
|
Kalff J (2002). Limnology: Inland Water Ecosystems. Prentice-Hall, Inc, NJ. 592 p
|
|
|
|
Karabin A (1985). Pelagic zooplankton (Rotatoria + Crustacea) variation in the process of lake eutrophication. 1. Structural and quantitative features. Polish Journal of Ecology, 33:567-616.
|
|
|
|
Kassahun A, Fekadu T, Zenebe T (2011). Adaptability, growth and reproductive success of the Nile tilapia, Oreochromis niloticus (Pisces: Cichlidae) stocked in Lake Tinshu Abaya, south Ethiopia). Ethiopian Journal of Biological Sciences 10(2):153-166.
|
|
|
|
Kirk K (1991). Suspended clay reduces Daphnia feeding rate: behavioral mechanisms. Freshwater Biology 25:357-365.
Crossref
|
|
|
|
Lampert W (1987). Laboratory studies on zooplankton-cyanobacteria interactions. New Zealand Journal of Marine and Freshwater Research, 21:483-490.
Crossref
|
|
|
|
Leps J, Smilauer P (2003). Multivariate Analysis of Ecological Data using CANOCO, 1st edition. Cambridge University Press, United Kingdom, 283 p.
Crossref
|
|
|
|
Lewis WM (1979). Zooplankton Community Analysis. Studies on a Tropical System. Springer-Verlag. New York, P 163
Crossref
|
|
|
|
Matheos H (2011). Ecosystem structure, trophic link, and functioning of a shallow rift valley lake: the case of Lake Ziway (Ethiopia). MSc. Thesis, Addis Ababa University.
|
|
|
|
Matsumura-Tundisi T, Arnold C, Evaldo LG, Jose G, Tundisi-Odette R (1990). Predation on Ceriodaphnia cornuta and Brachionus calyciflorus by two Mesocyclops species coexisting in Barra Bonita reservoir (SP, Brazil). Hydrobiologia, 198(1):141-151
Crossref
|
|
|
|
Matsumura-Tundisi T, Rietzler A, Tundisi JG (1989). Biomass (dry weight and carbon content) of plankton crustacea from Broa reservoir (São Carlos, SP.–Brazil) and its fluctuation across one year. Hydrobiologia, 179(3):229-236.
Crossref
|
|
|
|
Mavuti KM, Literick MR (1981). Species composition and distribution of zooplankton in a tropical lake, Lake Naivasha, Kenya. Archiv Fur Hydrobiologie. Stuttgart 93:52-58.
|
|
|
|
Melão G, Rocha O (2004). Life history, biomass, and production of two planktonic cyclopoid copepods in a shallow subtropical reservoir. Journal of Plankton Research, 26(8):909-923.
Crossref
|
|
|
|
Mengistou S, Fernando C (1991a). Seasonality and abundance of some dominant crustacean zooplankton in Lake Awassa, a tropical Rift Valley lake in Ethiopia. Hydrobiologia 226: 137-152.
Crossref
|
|
|
|
Mengistou S, Fernando C (1991b). Biomass and production of the major dominant crustacean zooplankton in a tropical Rift Valley lake, Awassa, Ethiopia. Journal of Plankton Research, 13: 831-851.
Crossref
|
|
|
|
Pace ML (1986). An empirical analysis of zooplankton community size structure across lake trophic gradients, Limnolology and Oceanography 31: 45-55.
Crossref
|
|
|
|
Pagano M, Koffi MA, Cecchi P, Corbin D, Champalbert G, Saint-Jean L (2003). An experimental study of the effects of nutrient supply and Chaoborus predation on zooplankton communities of a shallow tropical reservoir (Lake Brobo, Cête ÄIvoire). Freshwater Biology, 48:1379-1395.
Crossref
|
|
|
|
Pastorok RA (1980). Selection of prey by Chaoborus larvae: a review and new evidence for behavioral flexibility. In: Evolution and Ecology of Zooplankton Communities, ed. W.C. Kerfoot, pp. 538-553. University Press of New England, Hanover.
|
|
|
|
Pinto-Coelho R, Pinel-Alloul B, Méthot G, Havens KE (2005a). Crustacean zooplankton in lakes and reservoirs of temperate and tropical regions: variation with trophic status. Canadian Journal of Fisheries and Aquatic Science, 62:348-361
Crossref
|
|
|
|
Pinto-Coelho RM, Bezerra-Neto JF, Morais-JR CA (2005b). Effects of eutrophication on size and biomass of crustacean zooplankton in a tropical reservoir. Braz. J. Biol. 65(2):325-338.
Crossref
|
|
|
|
Ruttner-Kolisko A (1977). Suggestions for biomass calculations of plankton rotifers. Arch. Hydrobiol Beih. Ergebn. Limnology, 8:71-76.
|
|
|
|
Saint-Jean L (1983). The zooplankton in Lake Chad. Monogr. Biol. 53:199-232.
|
|
|
|
Wetzel RG (1983). Limnology, 2nd ed. Saunders College Publishing, Philadelphia.
|
|
|
|
Yirga E, Brook L (2018). Seasonality in the diet composition and ontogenetic dietary shifts of (Oreochromis Niloticus L.) (Pisces: Cichlidae) In Lake Tinishu Abaya, Ethiopia. Int. J. Fish. Aquatic. Res. 3(1):49-59.
|