ABSTRACT
This study described some aspects of the reproductive biology of Blackchin tilapia (Sarotherodon melanotheron, Cichlidae) from Brimsu reservoir. A total of 457 specimens were sampled using a monofilament gill net of mesh size 25 mm from September, 2009 to September, 2010. Standard length for the species ranged between 7.5 and 17.6 cm, with both males and females having a unimodal modal length of 10.0 to 10.9 cm. The length at sexual maturity (L50) was 11.26 and 11.34 cm for males and females, respectively. The pattern of fluctuation in gonadosomatic index indicates that the species had an extended spawning season starting from February to August.
Key words: Sarotherodon melanotheron, reproductive biology, sexual maturity.
Inland fishery contributes to a significant portion of the total fish catch in Ghana. The fisheries sector is one of the key sectors in Ghana, employing more than 2 million people directly or indirectly (Fisheries Commission, 2011). Abban et al. (2004) noted that in Africa, tilapias are among the most commercially and thus socio-economically important inland fish.
Although cichlids constitute a significant percentage of catch in reservoirs and lakes (Kwarfo-Apegya and Ofori-Danson, 2010; Quarcoopome and Amevenku, 2010) and most coastal waters in Ghana (Blay and Asabere-Ameyaw, 1993; Abban et al., 2004), their habitats are threatened by pollution, siltation of lagoons, over-fishing, destructive fishing methods, habitat degradation and destruction (Abban et al., 2000; Ofori-Danson, 2000). In some cases, there has been decline or disappearance of some species in reservoirs as a result of the change from an originally lotic system to a lacustrine condition (Quarcoopome and Amevenku, 2010).
Sarotherodon melanotheron is a cichlid which inhabits fresh to brackish water environments. This species is native to West Africa and it is found from Senegal to Zaire. The abundance of S. melanotheron in coastal lagoons in West Africa has earned it the name “West African lagoon tilapia” (Eyeson, 1979). Falk et al. (2000) identified three subspecies by analysing allozyme data viz (1) S.m. heudelotii, ranging from Senegal to Sierra Leone; (2) S.m. melanotheron, ranging from Côte d'Ivoire tosouthern Cameroon; and (3) S.m. nigripinnis, ranging from Equatorial Guinea to the mouth of the Congo River.
Although the species is primarily brackish, it has been found to invade freshwater bodies (Dial and Wainright
1983; Orhihabor and Adisa-Bolanta, 2009; Kuton and Kusemiju, 2010).
The blackchin tilapia comprises more than 59% of fish caught in brackish environments in Ghana (Welcome, 1972; Blay and Asabere, 1993) thus making the species important in the fisheries of brackish water systems in Ghana (Ekau and Blay, 2000; Koranteng et al., 2000; Abban et al., 2004). The species is primarily planktivorous (Pauly, 1976; Ndimele et al., 2010), feeding mainly on phytoplankton and zooplankton. It exhibits elaborate parental care with the male undertaking brooding. However, Eyeson (1992) reported residual maternal brooding in brackish environments in Cape Coast and Elmina. Panfili et al. (2004) noted that S. melanotheron is able to withstand saltier environments by limiting its growth, reducing the size-at-maturity, and changing its fecundity.
Studies on some biological aspects, including reproductive biology (Eyeson, 1983, 1992), growth and mortality parameters (Blay and Asabere-Ameyaw, 1993; Blay, 1998; Ekau and Blay, 2000), genetics (Abban et al., 2000) and feeding ecology (Ofori-Danson and Kumi, 2006) of the species have been carried out in different habitats. However, most of these researches were conducted in brackish water. The result is a dearth of information on biology of the species found in coastal freshwater habits in Ghana. Nonetheless, given the ability of this species to adapt to different environments, continuous studies of various aspects of the biology, especially its reproductive biology is needed, since this has a bearing on the recruitment pattern.
The Brimsu reservoir, an important source of livelihood for fishers and people living within the catchment area, has been reported to experience high levels of siltation (Akayuli et al., 2007). In spite of the immense importance of this reservoir, there is inadequate information on its fauna and flora. However, given the current increase in human population which has its attendant repercussions on food security and livelihood of fishers, the need to study inland fisheries has gained relevance. Studies on the biology of fish in reservoirs to ascertain their potential for enhancing inland capture fisheries, as well as culture based fisheries will provide information on the state of the fisheries in the reservoir and provide baseline data for management of the reservoir. Reproductive parameters such as age and length at maturity are critical for stock assessment.
The aim of this research was to describe the reproductive pattern of the blackchin tilapia in the Brimsu reservoir.
Study area
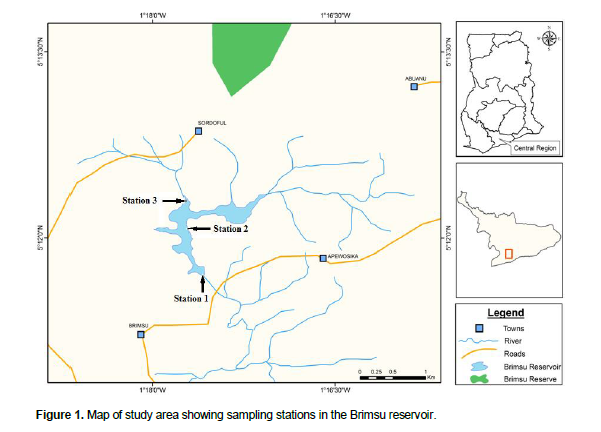
The Brimsu reservoir is located about 15 km northeast of Cape Coast in the Central Region of Ghana. The reservoir was formed by the construction of a dam across the Kakum River in 1928 and is located on 5° 11’N and 1° 16’W (Figure 1). The reservoir was created primarily to supply domestic water to Cape Coast and the surrounding communities. It also provides livelihood opportunities to fishermen in the nearby communities. The reservoir is fed primarily by the Kakum River and it has a catchment area of 867 km2 and a surface area of about 278 ha (Bosque-Hamilton et al., 2004). At full capacity, the reservoir has a storage volume of 2.3 × 106 m3 (Gordon, 2006) and maximum depth of approximately 7 m.
Fish were collected monthly (from September, 2009 to September, 2010) in three stations (Figure 1) using a gill net of mesh size 25 mm, length of 50 m and a depth of 1.5 m. The nets were set overnight (11:00 pm) and fish sampled in the morning of the following day (6:00 am). After collection, they were kept under ice to minimize post mortem decomposition and later taken to the laboratory for further analysis. Physico-chemical parameters of the water including pH, dissolved oxygen, temperature and conductivity were also measured by means of YSI Probe (Model 63). The total length (TL) of each fish was determined as the length measured from the tip of the snout to the end of the caudal fin while standard length (SL) was the length measured from the tip of the snout to the base of the caudal fin to the nearest 0.1 cm, using a fish measuring board (Bagenal and Braum, 1978). The body weight (BW) was ascertained by weighing each specimen to the nearest 0.01 g using an electronic balance. Fish were dissected to determine their sex, and their gonads weighed. The specimens were sorted by sex to determine the changes in monthly sex ratio by means of Chi square test (χ2). The ovaries were then staged (I to V) using a modification of the classification described by Witte and Van Densen (1995).
Estimation of absolute fecundity
Absolute fecundity was determined as the potential number of oocytes capable of being released at the next spawning. This was estimated by manual counting of the number of ripe oocytes ready to be spawned. Ripe ovaries (Stage IV) from gravid females were stored in 10% formalin for at least one week to ensure hardening of the ova. The ovaries were then washed and rinsed with water prior to counting the eggs after teasing out the ovarian tissues. The whole count method was employed for individuals with low fecundity while the sub-sampling technique was used for individuals with high fecundity (Bagenal and Braum, 1978). The gonadosomatic index (GSI) of the fish was computed according to the equation of Marcus and Kusemiju (1984) as follows:
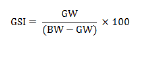
Where GW is the gonad weight and BW is the body weight. Visceral fat from each specimen was examined macroscopically and staged using a 1 to 5 point scale (adapted from Kwei, 1970). The least point (1 point) was scored for specimens with the least amount of visceral fat whereas 5 points were scored for specimen with the most amount of visceral fat. The mean visceral fat for each month was then determined as:
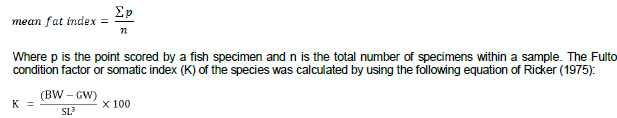
Where SL is the standard length in centimeters, BW is the body weight and GW is gonad weight in grams. In order to determine the spawning pattern of the species, the frequency distribution of ova diameter of gravid females was undertaken. The ovaries were preserved in 10% formalin for at least a week to enable it to harden and also facilitate the separation of the eggs from the ovarian tissues. The ova were placed on a microscope glass slide and the diameter of each measured to the nearest 0.1 mm with a stage micrometer under a dissecting microscope. Data from the measurements were grouped into diameter classes of 0.2 mm intervals after which a frequency distribution was plotted for each species to ascertain the spawning pattern of the cichlids. The length at which 50% of the individuals were mature was estimated by fitting frequency data of mature individuals by length class using a cumulative frequency method. This was used to estimate the length of the onset of sexual maturity (Pitt, 1970).
A total of 457 specimens of S. melanotheron were obtained from the Brimsu reservoir during the study. Of the total sample, 227 were males and 230 were females (Figure 2). The Chi square test (χ2) showed no significant changes in the sex ratio throughout the period of study (p > 0.05).
Length-frequency distribution
The length of S. melanotheron ranged from 7.5 to 17.6 cm (Figure 3) showing a unimodal distribution, with the 10.0 to 10.9 cm size group as the modal class. The length-frequency distributions for males and females were also unimodal with modal class of 10.0 to 10.9 cm for both sexes. The smallest fish obtained for both sexes fell within the 7.0 to 7.9 cm size group. Although both sexes had the biggest individual in the 17.0 to 17.9 cm size group, there were more males that were bigger (> 14.0 cm) than females. The overall distribution of the species was also unimodal with modal class of 10.0 to 10.9 cm.
Monthly variation in physico-chemical parameters
Temperature, transparency and conductivity fluctuated in a similar trend within the period with a maximum value between January and February, 2010 (Figure 4). Higher pH values were recorded after April, whereas the dissolved oxygen content attained peaks in November, 2009 and June, 2010.
Seasonal changes in condition factor
The condition factor for males ranged between 3.95 and 4.94; while that of the females ranged between 4.08 and 4.99 (Figure 5). Two distinct periods of improved condition were observed during the study; one in December (dry season) and the other which extended from May to August. Condition of the two sexes reduced from 4.94 in January, 2010 to a minimum of 4.03 in April, 2010. Afterwards, the condition of the two sexes increased until May, 2010 when that of the males began to decline to 4.49 ± 0.15 in September, 2010. However,there was improvement in the condition of the females after May, 2010, with July and August, 2010 recording significant differences in the condition of females than males as indicated by the standard error (s.e) bars.
Changes in the fat index
There was reduction in the mean visceral fat index of the blackchin tilapia from 2.92 ± 0.07 in September, 2009 to 2.21 ± 0.14 in October, 2009 (Figure 6). This was followed by an increase in the accumulation of fat in the species to a peak of 3.79 ± 0.05 in December, 2010. Subsequently, there was a gradual reduction in the accumulation of fat from April to June, 2010. Thereafter, there was a sharp increase in fat storage in July, 2010 which declined in August, 2010.
Monthly variation in GSI
The GSI of the males showed three peaks in October and November, 2009, April, 2010 and June, 2010 (Figure 7). April, 2010 recorded the highest GSI (0.17 ± 0.01), while the lowest GSI of 0.03 ± 0.001 was obtained in September, 2009. There was a steady increase in the GSI of female from 0.23 ± 0.12 in September, 2009 to a maximum of 4.189 ± 0.53 in February, 2010, followed bya decline to 0.19 in August, 2010 and a slight increase in September, 2010 to 0.78 ± 0.15. Generally, the females had higher GSI values than the males.
Seasonal fluctuation in ripe gonads
The proportion of males with advanced stage of gonad maturity reduced from 23.53% in September, 2009 to 0.00% in November, 2009 (Figure 8). This was followed by a steady increase to 100% in April, 2010 after which the proportion of ripe males reduced to 0.00% in August, 2010. No ripe females were recorded in September and October, 2009. The percentage of ripe females began to rise gradually from October, 2009 to a peak of 60 % in February, 2010, followed by a decline to 0.00% in May, 2010. The subsequent months had no females with ripe ovaries. The fecundity of the species ranged from 187 to 732 with a mean of 434.4 ± 24.5 for specimens ranging between 10.5 and 14.0 cm standard length. The relationship between the standard length (SL) and fecundity (Fec) of the cichlid was linear with a stronger correlation for the relationship, r = 0.77 (Figure 9).
Size frequency distribution of ova diameter
The frequency distributions of oocyte diameter of three ripe (hydrated) ovaries of S. melanotheron are shown in Figure 10. The ovum diameter ranged from 0.3 and 4.1 mm for fish measuring 12.8 and 14.8 cm SL. Two distinct peaks which were completely separated from each other were observed in each of the ovaries.The first peak at the 0.7 to 0.8 mm class and the other at the 2.7 to 2.8 mm class for fish measuring 13.2 cm; 0.7 to 0.8 mm and 2.5 to 2.6 mm for fish measuring 12.8 cm; and 0.9 to 1.0 mm and 3.5 to 3.6 mm for fish measuring 14.8 cm.
Length at maturity
The length at first sexual maturity of the blackchin tilapia, S. melanotheron was 11.26 and 11.34 cm SL for male and female, respectively (Figure 11). The least matured male and female in the population were 9.30 and 9.50 cm SL, respectively.
Spawning is an important aspect in the study of the reproductive biology of fish (Hislop et al., 1978; Malison and Held, 1996; Coward and Bromage, 1999; Rideout et al., 2005; Ellis et al., 2012). Primary factors that influence the events leading to spawning includes, nutritional state of the female, physiological/endogenous factors and ecological factors. Photoperiod and temperature are important ecological factors which influence reproduction of fish (Scott, 1990). In tropical warmwater fish, ovulation and oviposition are linked. This is a result of the short lifespan of the ovulated eggs. Oviposition and mating have been known to be triggered by such factors as flooding (Lake, 1967) and rainfall (Schwassmann, 1978). These conditions provide maximum food to enhance the survival of the fry.
Majority of teleost are seasonal breeders while a few breed continuously (Sundararaj, 1981). Tilapias are known to breed asynchronously year-round in the tropics, with relatively small number of eggs been produced per spawn (Srisakultiew and Wee, 1988). In the current study, it was observed that the blackchin tilapia is a batch (serial) spawner with an extended breeding season beginning in February and ending in August. This agrees with work done by Guèye et al. (2012), which indicated that S. melanotheron has an extended spawning season.
The presence of two distinct classes of oocytes occurring simultaneously in the ripe ovaries of the species gives an indication that the species is a discontinuous spawner. The species exhibits group-synchronous ovarian development with determinate fecundity (Plaza et al., 2011). The greater number of females with ripe ovaries within the month of February indicates a higher proportion of hydrated oocytes in the species. This also corresponded with the highest GSI of females within the study period. This could probably mark the commencement of spawning of the species in the reservoir. Hence the fecundity of this cichlid is determined at the start of the period of spawning. Thespecies compensated for the low fecundity with large-sized eggs.
There was one major peak in the fluctuation in the GSI of female S. melanotheron. This ranged from November, 2009 to August, 2010 with a peak in February, 2010. The increase in GSI of S. melanotheron from November, 2009 to a peak in February, 2010 corresponded with an increase in the proportion of fish with ripe ovaries. The period was also characterized by an increase in fat storage and condition factor. This improvement in the condition and increased storage of fat might have probably aided in the production of reproductive materials for the spawning period which begun in February till August, 2010. Thereafter, the proportion of females with riped ovaries reduced to a minimum, Indicating an arrested stage where reproductive activities is reduced to enable the recruitment and development of reproductive materials for the next spawning season. The prolonged breeding of fish may be attributed to the breeding of various size groups in succession during the spawning season (Karamchandani et al., 1967; Desai, 1973).
Panfili et al. (2004) observed that the species had a short spawning season from May to July in an estuary. However, Blay (1998) noted that the species had two major recruitment seasons in the Benya Lagoon and three seasons in the Kakum River Estuary. These short periods of breeding have been attributed to greater reproductive capacity of the species (Iles, 1970; Blay, 1998). The current study of the species in a reservoir indicates that in freshwater habitats the species displays one spawning season which extends over longer periods. This may be due to the low fishing pressure and also suitable physico-chemical parameters of the reservoir. The species therefore changes its reproductive pattern in varying environments.
The species was generally in good condition during the period. However there were two major periods when the condition peaked viz December and also between May and July. Improved condition of fish can be attributed to enhanced feeding intensity in fish as well as development of body tissue in preparation of spawning activities. There was no clear relationship between the gonadosomatic index (GSI) and the condition factor. However, the period of spawning coincided with reduced condition of the species. The reduction in condition could be attributed to cessation of feeding due to fully developed ovary which invariably leaves limited space for food intake (Desai, 1973).
There was a general absence of female with mature ovaries after the spawning season. This agrees with the work of Hyder (1970), who observed that in cichlids, after the spawning season, testes and ovaries are in resting phase from July to September. Also after the period of spawning, the females tended to recover slightly faster than the males. The period of reduction in condition was related to the period of reduction in visceral fat. This could be due to the channeling of stored fat for reproduction. This indicates that although the species is predominantly found in brackish water systems, it performs well in purely freshwater systems.
Temperature has been identified as a factor affecting sexual maturation and spawning in tilapias (Lowe-McConnell 1979; Eyeson 1983). The optimum temperature for reproduction of tilapia ranges from 25 to 30°C (Popma and Lovshin, 1996). The temperature range for the current research falls within the optimum range for reproduction of the species. Increase in photoperiod, rainfall and water temperature, together with a decrease in water pH has been identified as cues for gonadal maturation (Brummett, 1995; Cornish and Smit, 1995; El-Naggar et al., 2000). The increase in temperature from September to February corresponded with an increase in transparency. Thereafter there was a decrease in the transparency of the reservoir due to inflow of runoffs from the catchment area. This could have served as a cue to trigger spawning of the blackchin tilapia.
Size at maturity of cichlids has been shown to bear a positive correlation with the surface area of the water body (Lowe-McConnell, 1958, 1982; De Silva, 1986; Legendre and Écoutin, 1989, 1996; Duponchelle and Panfili, 1998) as well as the environmental condition, fishing pressure and pollution. Eyeson (1983) reported that in a confined environment S. Melanotheron can be sexually active at 4 to 6 months old and at a size as small as 4 to 4.5 cm (SL). In natural environments, however, Blay (1998) reported that the species matured at 3 months in the Kakum River estuary and 5 months in the Benya lagoon at 4.6 cm SL and 5.5 cm SL, respectively. The results of the current studies indicated that the length at maturity was 11.26 and 11.34 cm for male and females, respectively in the Brimsu reservoir. The length at maturity for the cichlids showed that the males matured at a smaller size than females. The length at first sexual maturity for females recorded in this study was larger compared with 4.6 cm reported for the adjacent Kakum River Estuary. This may be an indication of improved environmental conditions as well as low fishing activity within the reservoir.
S. melanotheron in the Brimsu reservoir has an extended spawning period, starting from February to August. The eggs of this species are also released in batches or serially. The species matures at a much bigger size in the reservoir than its adjoining estuary.
The authors have not declared any conflict of interests.
REFERENCES
|
Abban EK., Asante KA, Falk TM (2000). Environment of the Black-chinned tilapia, Sarotherodon melanotheron, and their potential effects on the genetic structure and stocks in Ghana. In: Abban EK, Casal CMV, Falk TM, Pullin RSV (Eds.) Biodiversity and sustainable use of fish in the coastal zone ICLARM Conference Proceedings. pp. 14-16.
|
|
|
|
Abban EK, Agyakwah S, Falk TM (2004). Socio-economic importance of Tilapiine fishes. In Abban EK, Casal CMV, Falk TM, Pullin RSV (Eds.), Biodiversity, Management and Utilization of West African Fishes World Fish Center Conference Proceedings. pp. 1-3.
|
|
|
|
|
Akayuli CF, Asenso-Gyambibi D, Owusu J, Owusu-Adade K (2007). Geotechnical survey, dregability of soil sediment and siltatation volume of the Brimsu water reservoir. J. Appl. Sci. Technol. 12(1&2):71-77.
|
|
|
|
|
Bagenal TB, Braum E (1978). Eggs and early life history. In Bagenal T (Ed.) Methods for the assessment of fish production in freshwater (3rd ed.), Oxford: Blackwell Scientific Publications Ltd. pp. 165-201.
|
|
|
|
|
Blay J, Asabere-Ameyaw A (1993). Assessment of the fishery of a stunted population of the cichlid, Sarotherodon melanotheron (Rüppel), in a "closed" lagoon in Ghana. J. Appl. Ichthyol. 9:1-11.
Crossref
|
|
|
|
|
Blay J (1998). Growth and mortality parameters of Sarotherodon melanotheronmelanotheron (Teleostei: Cichlidae) in two brackish water systems in Ghana. Ghana J. Sci. 38:47-55.
|
|
|
|
|
Bosque-Hamilton EK, Nana-Amankwah E, Karikari AY (2004). A preliminary comparative limnological assessment of three coastal water supply reservoirs in Ghana. J. Ghana Sci. Assoc. 6(1):128-138.
|
|
|
|
|
Brummett RE (1995). Environmental Regulation of Sexual Maturation and Reproduction in Tilapia. Rev. Fisher. Sci. 3(3):231-248.
Crossref
|
|
|
|
|
Cornish DA, Smit GL (1995). The Correlation Between the Environmental Factors and the Reproduction of Oreochromis mossambicus. J. Water. S.A. 21:259-264.
|
|
|
|
|
Coward K, Bromage NR (1999). Spawning periodicity, fecundity and egg size in laboratory-held stocks of a substrate-spawning tilapiine, Tilapia zillii (Gervais). Aquaculture 171:251-267.
Crossref
|
|
|
|
|
De Silva SS (1986). Reproductive biology of Oreochromis mossambicus populations of a man-made lake in Sri Lanka: A comparative study. Aquac. Fisher. Manag. 17:31-48.
Crossref
|
|
|
|
|
Desai VR (1973). Studies on the fisheries and biology of Tor tor (Hamilton) from the River Narmada. Proc. Indian Natl. Sci. Acad. B Biol. Sci. 39(2):228-248.
|
|
|
|
|
Dial RS, Wainright SC (1983). New distributional records for non-native fishes in Florida. Florida Scientist 46:8-15.
|
|
|
|
|
Duponchelle F, Panfili J (1998). Variations in age and size at maturity of female Nile tilapia, Oreochromis niloticus, populations from man-made lakes of Côte d'Ivoire. Environ. Biol. Fishes 52:453-465.
Crossref
|
|
|
|
|
Ellis JR, Milligan SP, Readdy L, Taylor N, Brown MJ (2012). Spawning and nursery grounds of selected fish species in UK waters. Science Series Technical Report 147, Cefas Lowestoft, P 56.
|
|
|
|
|
Ekau W, Blay J (2000). Validation of daily increment deposition and early development in the otoliths of Sarotherodon melanotheron. J. Fish Biol. 57:1539-1549.
Crossref
|
|
|
|
|
El-Naggar GO, El-Nady MA, Kamar MG, Al-Kobaby AI (2000).Effect of photoperiod, dietary protein and temperature on reproduction in Nile tilapia (Oreochromis niloticus). In The 21st Century Proceedings from the Fifth International Symposium on Tilapia Aquaculture. Hotel Sofitel Rio Palace Convention Center Rio Die Janeiro-RJ, Brazil September 3-7:352-358.
|
|
|
|
|
Eyeson KN (1979). Studies on egg production, spawning and fry development in Tilapia melanotheron. Ghana J. Sci. 17(1):25-34.
|
|
|
|
|
Eyeson KN (1983). Stunting and reproduction in pond-reared Sarotherodon melanotheron. Aquaculture 31:257-267.
Crossref
|
|
|
|
|
Eyeson KN (1992). Residual bi-parenting oral-brooding in the blackchin tilapia, Sarotherodon melanotheron (Rüppell), J. Fish Biol. 41:145-146.
Crossref
|
|
|
|
|
Falk TM, Teugels GG, Abban EK (2000). Genetic characterization of the West African populations of Sarotherodon melanotheron (Teleostei, Cichlidae), pp. 8-11. In Abban EK, Casal CMV, Falk TM, Pullin RSV (eds.) Biodiversity and sustainable use of fish in the coastal zone. ICLARM Con. Proc. 63:71.
|
|
|
|
|
Fisheries Commission (2011). Annual report. Ministry of Food and Agriculture, Ghana.
|
|
|
|
|
Gordon C (2006). Background Paper for The Multi-stakeholder consultation process for dams development in Ghana. Volta Basin Research Project, University of Ghana. View
|
|
|
|
|
Guèye M, Tine M, Kantoussan J, Ndiaye P, Thiaw OT, Albaret JJ (2012). Comparative analysis of reproductive traits in black-chinned tilapia females from various coastal marine, estuarine and freshwater ecosystems. PLoS ONE 7(1):e29464.
Crossref
|
|
|
|
|
Hislop JRG, Robb AP, Gauld JA (1978). Observations on effects of feeding level on growth and reproduction in haddock, Melanogrammus aeglefinus (L.) in captivity. J. Fish Biol. 13:85-98.
Crossref
|
|
|
|
|
Hyder M (1970). Gonadal and reproductive patterns in Tilapia leucosticta (Teleostei: Cichlidae) in an equatorial lake. Lake Naivasha, Kenya. J. Zool. Lond. 162:179-195.
Crossref
|
|
|
|
|
Iles TD (1970). Dwarfing or stunting in the genusTilapia (Cichlidae), a possibly unique recruitment mechanism. Rapports et procès-verbaux
|
|
|
|
|
Karamchandani SJ, Desai VR, Pisolkar MD, Bhatnagar GK (1967). Biological investigations on the fish and fisheries of Narmada river (1958-66). Bull. Cent. Inl. Fish. Res. Inst. Barrackpore, 10:40. (Mimeo)
|
|
|
|
|
Koranteng KA, Ofori-Danson PK, Entsua-Mensah M (2000). Fish and fisheries of the Muni lagoon in Ghana, West Africa. Biodivers. Conserv. 9:487-499.
Crossref
|
|
|
|
|
Kuton MP, Kusemiju K (2010). Species diversity and richness of cichlids in three Southwest lagoons in Ghana. J. Sci. Res. Dev. 12:22:33.
|
|
|
|
|
Kwarfo-Apegyah K, Ofori-Danson PK (2010). Spawning and recruitment patterns of major fish species in Bontanga reservoir, Ghana, West Africa. Lakes and Reservoir: Res. Manag. 15:3-14.
|
|
|
|
|
Kwei EA (1970). The migration and biology of the Spanish mackerel, Scomber japonicus (Houttyn). Ghana J. Sci. 11(2):75-85.
|
|
|
|
|
Lake JS (1967). Rearing experiments with five species of Australian freshwater fishes. Inducement to spawning. Aust. J. Mar. Freshw. Res. 18:137-153.
Crossref
|
|
|
|
|
Legendre M, Ecoutin JM (1989). Suitability of brackish water tilapia species from Ivory Coast for lagoon aquaculture. Aquat. Living Resour. 2(2):71-79.
Crossref
|
|
|
|
|
Legendre M, Ecoutin JM (1996). Aspects of the reproductive strategy of Sarotherodon melanotheron: A comparison between a natural population (Ebrié lagoon, La Cote d'Ivoire) and different culture populations. In: Pulin RSV, Lazard J, Legendre M, Kothias JBA, Pauly D (Eds.). The Third International Symposium of Tilapia in Aquaculture, (pp. 326-338). ICLARM, Conference Proceedings 41.
|
|
|
|
|
Lowe-McConnell RH (1958). Observations on the biology of Tilapia nilotica Linné in East African waters. Revue de Zoologieet de Botanique. Africaines. 57(1-2):129-170.
|
|
|
|
|
Lowe-McConnell RH (1979). Ecological aspects of seasonality in fishes of tropical waters. In Miller PJ (ed.), Fish phenology: Anabolic adaptiveness in teleosts. Symposia of the Zoological Society of London, No. 44, Academic Press, London, pp. 219-241.
|
|
|
|
|
Lowe-McConnell RH (1982). Tilapias in fish communities. In: Pulin RSV, Lowe-McConnell RH (Eds.), The biology and culture of tilapias, (pp. 83-113). ICLARM Conference Proceedings 7.
|
|
|
|
|
Malison JA, Held JA (1996). Reproductive biology and spawning. pp. 11-18 In: Summerfelt RC (Ed). Walleye culture manual. NCRAC Culture Series 101. North Central Regional Aquaculture Center Publications Office, Iowa State University, Ames.
|
|
|
|
|
Marcus O, Kusemiju K (1984). Some aspects of the biology of the clupeid Ilisha africana (Bloch) off the Lagos coast, Nigeria. J. Fish Biol. 25:679-689.
Crossref
|
|
|
|
|
Ndimele PE, Kumolu-Johnson CA, Aladetohun NF, Ayorinde OA (2010). Length-weight relationship, condition factor and dietary composition of Sarotherodon melanotheron, Rüppell, 1852 (Pisces: Cichlidae) in Ologe Lagoon, Lagos, Nigeria. Agric. Biol. J. North Am. 1(4):584-590.
|
|
|
|
|
Ofori-Danson PK (2000). Status of fish stocks in the Yejisegment of Lake Volta. In: Abban EK, Casal CMV, Falk TM, Pullin RSV (Eds.). Biodiversity and sustainable use of fish in the coastal zone (pp. 34-35). World Fish Conference Proceeding 63.
|
|
|
|
|
Ofori-Danson PK, Kumi GN (2006). Food and feeding habits of Sarotherodon melanotheron Rüppell, 1852, (Pisces: Cichlidae) in Sakumo lagoon, Ghana. West Afr. J. Appl. Ecol. 10:21-34.
|
|
|
|
|
Orhihabor BJ, Adisa-Bolanta AS (2009). Aspects of the biology Sarotherodon melanotheron and Tilapia guineensis (Perciformes: Cichlidae) in Buguma Creek, River State, Nigeria. Niger. J. Agric. Food Environ. 5(2-4):5-9.
|
|
|
|
|
Panfili J, Mbow A, Durand JD, Diop D, Diouf K, Thior D, Ndiaye P, Laë R (2004). Influence on salinity on the life-history traits of the West African black-chinned tilapia (Sarotherodon melanotheron): Comparison between the Gambian and Saloum estuaries. Aquat. Living Resour. 17:5-74.
Crossref
|
|
|
|
|
Plaza G, Espejo V, Almanza V, Claramunt G (2011). Female reproductive biology of the silverside Odontesthes regia. Fish. Res. 111:31-39.
Crossref
|
|
|
|
|
Popma TJ, Lovshin LL (1996). Worldwide Prospects for Commercial Production of Tilapia. Research and Development Series 41, International Center for Aquaculture and Aquatic Environments, Department of Fisheries and Allied Aquacultures, Auburn University, Alabama 36849, USA.
|
|
|
|
|
Pitt TK (1970). Distribution, abundance and spawning of yellowtail flounder, Limanda ferruginea, in the New Foundland area of the North West Atlantic. J. Fish. Res. Board Canada 27(12):2261-2271.
Crossref
|
|
|
|
|
Quarcoopome T, Amevenku FYK (2010). Fish community structure of Weija Reservoir after 28 years of impoundment. J. Appl. Sci. Technol. 15(1):126-131.
Crossref
|
|
|
|
|
Pauly D (1976). The biology, fishery and potential for aquaculture for Tilapia melanotheron in a small West African lagoon. Aquaculture 7:33-49.
Crossref
|
|
|
|
|
Ricker WE (1975). Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Canada 191:1-382.
|
|
|
|
|
Rideout RM, Rose GA, Burton MP (2005). Skipped spawning in female iteroparous fishes. Fish Fisher. 6:50-72.
Crossref
|
|
|
|
|
Sundararaj BI (1981). Reproductive Physiology of Teleost Fishes: A review of present knowledge and needs for future research. Aquaculture Development And Coordination Programme ADCP/REP/81/16. FAO, Rome.
|
|
|
|
|
Schwassmann HO (1978). Times of annual spawning and reproductive strategies in Amazonian fishes. In Rhythmic activity of fishes, edited by Thorpe JE. London, Academic Press, pp. 187-200.
|
|
|
|
|
Scott AP (1990). Salmonids.In: Munro AD, Scott AP, Lam TJ Reproductive seasonality in Teleosts: Environmental Influences pp. 33-51.
|
|
|
|
|
Srisakultiew P, Wee KL (1988). Synchronous spawning of Nile tilapia through hypophysation and temperature manipulation. The second International Symposium on Tilapia in Aquaculture pp. 275-284. In: Pllin RSV, Bhukaswan T, Tonguthai K, Maclean JL (eds.). The Second Symposium of Tilapia in Aquaculture. ICLARM Conference Proceedings 15:623.
|
|
|
|
|
Welcome RL (1972). An evaluation of the acadja method of fishing as practised in the Coasta Lagoons of Dahomey, (West Africa). J. Fish Biol. 4:39-55.
Crossref
|
|
|
|
|
Witte F, Van Densen WLT (1995). Fish Stocks and Fisheries of Lake Victoria. A Handbook for Field Observations. Cardigan, Britain: Samara Publishing.
|
|