ABSTRACT
This study examined the potential of catfish pituitary cells to proliferate in culture media and that of hormones produced in their primary culture to induce spawning in African catfish. The trypsinized pituitary cells were cultured in three culture media RPMI 1640, McCoy 5a and M2 media with addition of 10% fetal bovine serum. Female catfish (800±200 g) were induced using the culture medium that gave the shortest population doubling time and highest cell count (RPMI 1640). The eggs were fertilized with sperm cells in vitro. The fertilization and hatchability rates were determined. The blood samples of the induced spawners were collected at O (before injection), 3, 6, 9 and 12 h, respectively after injection. The collected samples of blood plasma were analyzed using enzyme-linked immunosorbent assay for quantitative determinations of Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH). A significant increase in the cell counts over the initial seeding density of 4.1 × 106/ml after 12 h of culture was established in each of the three culture media used. The induced spawning bio-assay which served as a biochemical marker for gonadotrophin specific function showed that 97.5 g/kg of mature oocytes were spawned from cultured pituitary cells (CPC), 127.5 g/kg from induction with fresh pituitary gland (FPG) and 157 g/Kg from gonadotrophin releasing hormone analogue (Ovaprim) induced catfishes (800±200 g). The fertilization rate of cultured pituitary cells (CPC) differed significantly (P<0.05) without significant differences in hatchability (P>0.05). The results of the plasma gonadotrophin revealed that the highest levels of plasma LH and FSH were reached between 9 and 12 h of ovulation in all the inducers used. The two pituitary based hormones (FSH and LH) played complementary roles during ovulation and spawning of catfish eggs. This study provides an insight to the possibility of using hormones from the primary culture of pituitary cells to induce spawning in African catfish.
Key words: Pituitary cells, primary culture, induced spawning, ovulation.
The African catfish, Clarias gariepinus Burchell 1822, is the favourite fish for aquaculture in West Africa (Adewumi and Olaleye, 2011). This species of fish dwells in a variety of freshwater environments including still water (lakes, ponds and pools); and flowing water (rivers, rapids and dams). C. gariepinus has been vastly cultured and subjected to intensive research in aquaculture due to its ease of culturing, fast growth rate, high resistance to disease, tolerance of a wide range of temperature, low dissolved oxygen as well as high salinity levels and most importantly high commercial value (Teugels, 1986a). The major limitation in the aquaculture of C. gariepinus is that the species does not breed freely in captivity (Adebayo and Fagbenro, 2004). This is as a result of stress induced ovarian atresia (Lubzens et al., 2010). Hormonal induction of reproduction is necessary for the catfish to overcome atresia. This is done either by injection of pituitary gland from the donor fish of equivalent weight to the female spawner (Fagbenro et al., 1998; Salami et al., 2006) or by injection of synthetic gonadotrophin releasing hormone analogues (GNRH-a). The synthetic equivalents are quite expensive for many local farmers and hatcheries in Africa while the use of raw pituitary gland could be cumbersome. Pituitary gland is the main source of the major hormones responsible for reproduction in animals. The pituitary cells have been reported to undergo continuous mitotic process (Yeung et al., 2006). The pituitary gland can be cultured and proliferated in vitro in order to use their secretions for induction of spawning in catfish (Lugo et al., 2008, Chen et al., 2010). Cells growth, division and multiplication can be achieved by addition of fetal bovine serum and culture medium to the pituitary cells in a culture plate at 30°C and 5% CO2 (Gupta et al., 2005; Gupta and Saxena, 1999; Huhtaniemi, 2000; Lubzens et al., 2010). This study evaluated the efficacy of the hormones produced in the primary culture of pituitary cells to inducing spawning and their effects on the plasma gonadotrophin levels after induction.
Site of the study
This study was carried out at the Animal Reproduction, Animal Biotechnology and Wet Laboratories of the Department of Animal Sciences, Faculty of Agriculture, Obafemi Awolowo University, Ile-Ife, Nigeria.
Isolation of pituitary gland
The brain case of the head of catfish was removed using the sharp pointed edge of a surgical knife and carefully lifted up to expose the pituitary gland. It was gently removed with the aid of a forceps, dropped in a petri dish containing physiological normal saline solution.
Homogenization and trypsinization of the pituitary tissues:
Ten donor catfish weighing between 500 and 700 g were anaesthetized for the culture experiment. Then, 0.5 ml of trypsin (SIGMA) was added to the squashed pituitary tissues to aid dissociation of pituitary cells (Lugo et al., 2008; Makkar et al., 2011) and warmed in water bath with shaker (GZ Series, Germany) for one hour at 30°C. The initial pituitary cell counts per millimeter were determined using hemocytometer (Marienfeld, Germany) following Oyeleye and Omitogun (2007) procedure.
Culture media preparation
The trypsinized pituitary cells were divided into 3 parts and cultured in three culture media: M2, McCoy 5a and RPMI 1640. 225 µl of the culture medium containing 10% (25 µl /250 µl) of Fetal Bovine Serum was measured into the culture microplate. 20 µl of the trypinised pituitary cells was gently added to the medium and mixed thoroughly with micro-pipette (Gupta and Saxena, 1999).
Culture in CO2 incubator
The cells were cultured for 12 h at 30°C and 5% CO2 in CO2 incubator (Labline, USA). The cultured cells were harvested at different time interval of 3, 6, 9 and 12 h of incubation and the cell counts were determined using haemocytometer (Marienfeld, Germany). The growth curve for each culture medium was drawn from the cells counted. Proliferation rates and population doubling time of the cells were determined at each time interval.
Induced spawning bio-assay
Induction of spawning was carried out under optimal laboratory conditions required for catfish oocyte ovulation and maturation. Three inducers were used; fresh pituitary cells, cultured pituitary cells and gonadotrophin releasing hormone analogue (Ovaprim; Syndel, Canada) which served as control. An aliquot of 1 ml each of both fresh and cultured pituitary gland cells as well as 0.4 ml/kg of Ovaprim (Fagbenro et al., 1998; Olaleye, 2005) were injected intramuscularly into quadruplicate female catfish per inducer. The mature and ovulated eggs were later fertilized in vitro with milt from the male catfish. Incubation was done in hatching tanks (1.0 m × 1.0 m × 0.5 m) of 50 L capacity (Akankali et al., 2011). The fertilization rates were determined after the eggs were thoroughly mixed with the sperm by the assessment of eggs through simple observation of the morula (many-celled) developmental stage of the embryo after 12 h of fertilization under the stereo microscope (Omitogun et al., 2010).
The percentage fertilization and hatchability rates were calculated as shown in the following equation:
The fertilization rate was determined using this formula =

The hatchability rate was determined using this formula=

Where; NF is the number of fertilised eggs; NH is the number of larvae hatched; Ns is the total number of eggs set/incubated.
Determination of gonadotrophin profile of catfish induced with GnRH analogue, fresh pituitary gland and cultured pituitary cells
Blood samples from the injected female catfish were taken with 2 ml syringe from the caudal veins into EDTA bottles at 0 h (before injection), 3, 6, 9 and 12 h after injection with the Ovaprim®, fresh pituitary gland and cultured pituitary cells of a set of 4 catfishes for each treatment(Weil et al., 2003). Two milliliter of the blood samples were collected at each time interval and centrifuged at 3000 rpm for 10 min to get blood plasma which was preserved under -20°C refrigeration in eppendorf tubes until analysed for gonadotrophin (FSH and LH) using enzyme-linked immunosorbent assay (ELISA) test kits (Preferred Med, USA). The FSH and LH quantitative test kits (Preferred Med, USA) were based on a solid-phase enzyme- linked immunosorbent assay. The assay system utilized one anti FSH or anti LH antibody for solid phase (microtiter wells) inmmoblization and another mouse monoclonal anti-FSH or anti-LH antibody in the antibody-enzyme conjugate solution. The test sample was allowed to react simultaneously with the antibodies resulting in the FSH or LH molecules being sandwiched between the solid phase and enzyme-linked antibodies.
Data analysis
Data generated were subjected to analysis of variance, level of significance was determined at P>0.05 and means were separated and compared using Duncan multiple range test and Tukey's Studentized range (HSD) test.
Effects of culture media and serum on growth and proliferation of pituitary cells
Increased cell count occurred after 3-h of culture in all the culture media. The highest increase was first observed in the McCoy 5a medium (80.4 ± 6.48 × 106 cells/ml) at 3-h of culture. The final count after 12-h of culture showed that RPMI 1640 had 315.1+17.5 × 106 cells/ml, 233+ 18.5 × 106 cells/ml in M2 and 196.1 + 16.23 ×106 cells /ml in McCoy 5a. Cell count differed significantly among the three culture media used (Table 1). Sigmoid patterns of growth curves were observed in the three culture media (Figure 1).The highest proliferation rate was recorded in RPMI 1640 medium (0.71 cells /h) and lowest in M2 medium (0.59 cells/h) after 12 h of culture (P<0.05). At 12-h of culture, proliferation in the RPMI medium was significantly different (P <0.05) from the two other media. Two phases were noticed in the growth curves of the three media; the lag phase which was the period of gradual growth and adaptation to the in vitro conditions and the exponential phase which was the period of rapid growth and cell multiplication. The rate of multiplication was highest in RPMI 1640. This implies that the medium is rich enough to support cell growth and division.
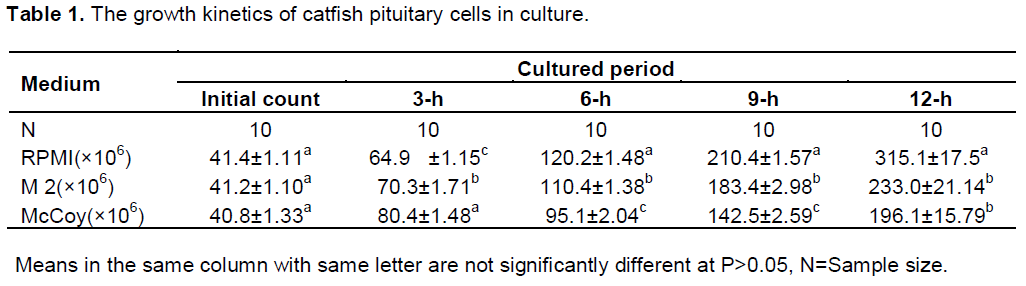
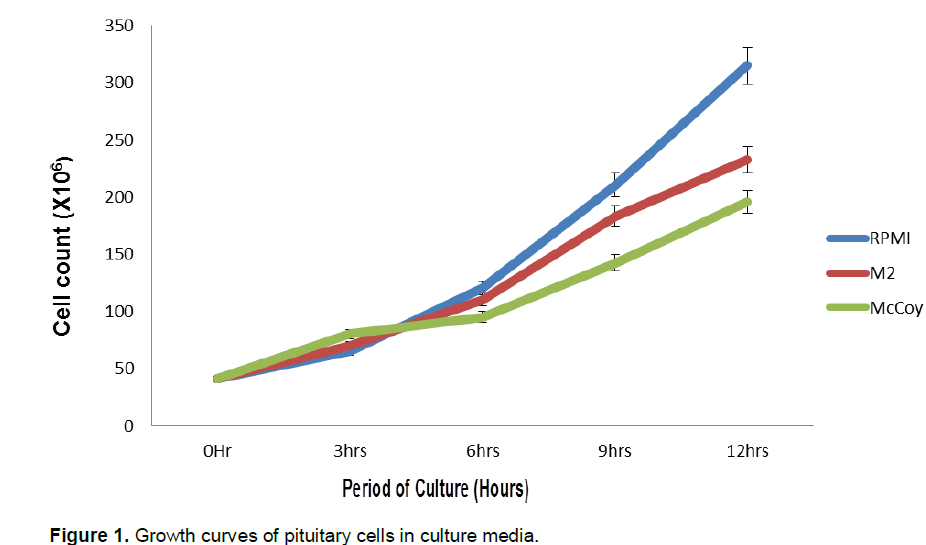
Effects of injected primary culture of pituitary cells on quantities, fertility and hatchability of catfish oocytes spawned
RPMI 1640 culture medium was selected to induce spawning because of its superior performance in terms of cell counts and shortest population doubling time. The three inducers used were gonadotrophin releasing hormone analogue (Ovaprim), cultured pituitary cells (CPC) and fresh pituitary gland (FPG). The GnRH analogue induced female catfish spawned 157±13.66 g/kg of mature eggs, CPC spawned 97.5±12.76 g/kg of mature oocytes and FPG spawned 127.5±11.54 g/kg of mature eggs (Table 2). The fertilization rates of eggs from the three inducers used were in the order of Ovaprim>FPG>CPC with significant differences (P<0.05) among them. The results with ovaprim group were also in line with Gbemisola (2014) results. There was no difference in hatchability among eggs ovulated using the three inducers. An aliquot of 1 ml of cultured pituitary cells caused a free release of eggs from the genital papilla of female broodstock after 10 h of inducement at 27°C. In vitro cultured pituitary cells stimulated the release of hormones into the blood circulatory system which induced ovulation and production of 97 g/kg of mature eggs. Differences in fertilization rates were attributed to varying levels of developmental competence of the oocytes released (Nagahama and Yamashita, 2008). There was no significant difference in the hatchability rates of the oocytes from the three inducers used considering the uniform condition during incubation period. Further study is however necessary to improve on the potency and efficiency of the hormones released by cultured pituitary cells to induce ovulation of sufficient quantities of fertile eggs.
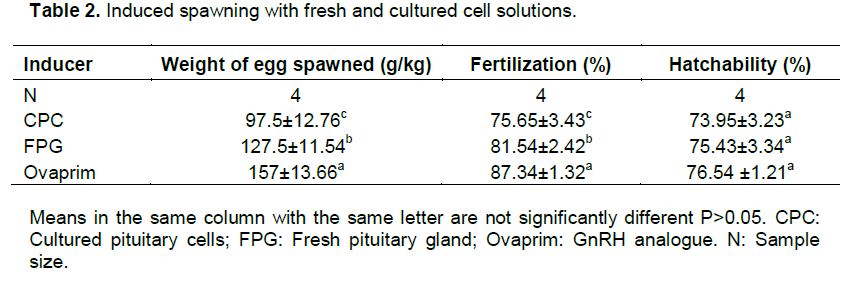
Effects of hormones secreted in vitro in the primary culture of pituitary cells on plasma gonadotrophins
Plasma Luteinizing hormone concentrations at 0 hour before injection were at low levels (0.010 mlU/mL in CPC group, 0.011 mlU/mL in FPG group and 0.012 mlU/mL Ovaprim group) in broodstock selected for hormone determination. After 3-h of injection, FPG, CPC and GnRH analogue (Ovaprim) gave significant rise (P < 0.05) in the LH plasma levels in all the female broodstock induced (0.470 in CPC, 0.052 in FPG and 0.048 in GnRH) over the initial values. There was no significant difference (P>0.05) in the LH levels in all the three inducers used. The levels of plasma LH remained constant still 9-h when the levels rose to 0.127 mlU/ml in CPC, 0.066 mlU/ml in FPG and 0.149 mlU/mL in Ovaprim (Table 3). The highest level of plasma LH was observed at 12-h in ovaprim injected group (0.25 mlU/mL).
Plasma FSH concentrations at 0-h before injection were not detected for all the experimental female catfish used (this was indicated by the negative value). The plasma FSH rose from undetectable level to 0.058 mlU /ml in CPC, 0.03 mlU/mL in FPG and 0.06 mlU/mL in GnRH after 3-h of post-injection. Gradual increase was observed at 9-h post injection over that of the 3-h in all the three inducers used and these peaked at 12h after injection with significant difference (P<0.05) in the levels. The peak value was found in cultured pituitary cells injected group with 0.128 mlU/mL (Table 4).
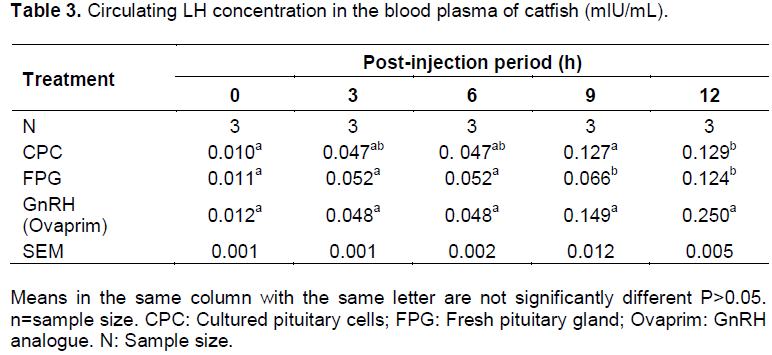
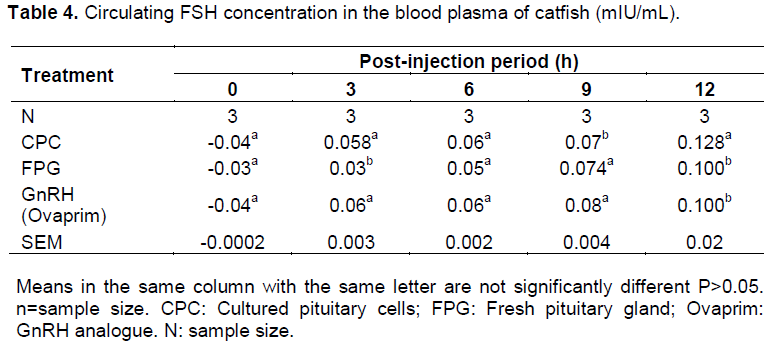
Differential patterns of secretions were observed in the two hormones under consideration after injection of cultured pituitary cells, fresh pituitary gland and ovaprim (Figure 2). It is assumed that FSH controls the early stages of oogenesis and at the later stages, FSH is reduced and atresia sets in (Ge, 2005; Gharib et al., 1990). Oocyte expulsion from the granulosa layer is hampered as FSH level is lowered (Smitz and Cortvrindt, 2002).
The plasma LH level from cultured pituitary cells had the same value with that of ovaprim at 3-h post injection and hence the initiation of ovulation. The increase in the LH plasma level is characterized by termination of vitellogenesis and progression to meiosis resumption which is oocyte maturation (Prat et al., 1996). FSH rise
occurred from 3-h after injection in all the three groups. In fish administered with cultured pituitary cells, the gradual rise was observed from 3-h to the 12-h period of ovulation. The pattern of secretions was similar to that of fresh gland and that of the GnRH analogue as the significant level was only observed at the 12-h (Figure 2). It was observed that cultured pituitary cells contained highest amount of plasma FSH among the three inducers used. Increase in FSH was probably responsible for overcoming or breaking the ovarian atresia caused by the rearing environment (Ola et al., 2008; Breton et al., 1998). The three processes were noticed occurring simultaneously and sequentially after induction; these were yolk deposition (vitellogenesis), occyte maturation and ovulation or spawning (Schmitz et al., 2005). From this study, it can be inferred that the surges in the plasma levels of these two hormones are responsible for initiation of ovulation, completion of ovulation and spawning of the mature oocytes as observed in Figure 2(a, b and c). The observation was in line with the findings of Levavi-Sivan et al. (2010).
The study has attempted to provide an alternative and basic tissue culture solution to the expensive imported gonadotrophin releasing hormone analogue. The culture of pituitary cells under 5% CO2 and 30°C in RPMI 1640 medium with 10% FBS has been identified as a good culture condition for cell division and proliferation of catfish pituitary gland. The rate of multiplication was significantly highest in RPMI 1640 among the three culture media used. The in vitro cultured pituitary cells were potent enough to stimulate spawning of 97.5 g/kg of oocytes. The use of cultured pituitary cells can serve as a good alternative to synthetic hormone analogues if it is further improved upon. The study has also provided useful information on the plasma gonadotrophin level of African catfish which is important to our understanding of responsiveness of the gonads to injection of gonadotrophin and natural stimulation which is very useful in planning breeding and hypophysation.
The authors hereby declare that no conflict of interest exists among them.
This work was substantially supported by the grants from Awolowo University, Ile-Ife, Nigeria and Chinese Academy of Science. The technical inputs of Mr Akin Babatunde, Mr Adesoji Adeyosoye and Mrs O. A. Adeloye are greatly acknowledged.
REFERENCES
|
Adebayo OT, Fagbenro OA (2004).Induced ovulation and spawning of pond raised African giant catfish, Heterobranchus bidorsalis by exogenous hormones. Aquaculture 242:229-236.
Crossref
|
|
|
|
Adewumi AA, Olaleye VF (2011). Catfish culture in Nigeria: Progress, prospects and problems. Afr. J. Agric. Res. 6(6):1281-1285.
|
|
|
|
Akankali JA, Seiyaboh EI, Abowei JFN (2011). Fish hatchery management in Nigeria. Adv. J. Food Sci. Technol. 3(2):144-154.
|
|
|
|
Breton B, Govoroun M, Mikolajczyk T (1998). GTH I and GTH II secretion profiles during the reproductive cycle in female rainbow trout; relationship with pituitary responsiveness to GnRH-A stimulation. Gen. Comp. Endocrinol. 111:38-50.
Crossref
|
|
|
|
Chen MJ, Chiou PP, Liao Y, Lin C, Chen TT (2010). Development and characterisation of five rainbow trout pituitary single-cell clone lines capable of pituitary hormones. J. Endocrinol. 205:69-78.
Crossref
|
|
|
|
Fagbenro OA, Salami AA, Sydenham DHL (1998). Induced ovulation and spawning in the catfish, Clarias isheriensis, using pituitary extracts from non-piscine sources. J. Appl. Aquact. 1(4):15-20.
Crossref
|
|
|
|
Gbemisola OB (2014). Sperm Quality and reproductive performance of male Clarias gariepinus induced with synthetic hormones (Ovatide and Ovaprim). Int. J. Fish. Aquact. 6(1):9-15.
Crossref
|
|
|
|
Ge W (2005). Intrafollicular paracrine communication in the zebrafish ovary: The state of the art of an emerging model for the study of vertebrate folliculogenesis. Mol. Cell Endocrinol. 237:1-10.
Crossref
|
|
|
|
Gharib SD, Wierman ME, Shupnik MA, Chin WW (1990). Molecular biology of pituitary gonadotropins. Endocrinol. Reserve 11:177-199.
Crossref
|
|
|
|
Gupta HP, Saxena VB (1999). Influence of foetal calf serum on the storage resistance of crossbred bull spermatozoa. Indian Vet. J. 76(7):633-635.
|
|
|
|
Gupta SC, Gupta N, Ahlawat SPS, Kumar A, Taneja R, Sharma R, Sunder S, Tantia MS (2005). In Vitro Culture of Skin Fibroblast Cells for Potential Cloning by Nuclear Transfer. In Applications of Gene-Based Technologies for Improving Animal Production and Health in Developing Countries. pp. 631-640. Springer Netherlands.
Crossref
|
|
|
|
Huhtaniemi I (2000). Mutations of gonadotrophin and gonadotrophin receptor genes: What do they teach us about reproductive physiology? J. Reprod. Fertil. 119:173-186.
Crossref
|
|
|
|
Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ (2010). Perspectives on fish gonadotrophins and their receptors. Gen. Comp. Endocrinol. 105:412-437.
Crossref
|
|
|
|
Lubzens E, Young G, Bobe J, Cerdà J (2010). Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 165(3):367-389.
Crossref
|
|
|
|
Lugo JM, Rodriguez A, Helguera Y, Morales R, Gonzalez O, Acosta J, Besada V, Sanchez A, Estrada MP (2008). Recombinant novel pituitary adenylatecyclase-activating polypeptide from African catfish (Clarias gariepinus) authenticates its biological function as a growth- promoting factor in low vertebrates. J. Endocrinol. 197:583-597.
Crossref
|
|
|
|
Makkar HPS, Kumar V, Oyeleye OO, Akinleye OA, Angulo-Escalante MA, Berker K (2011). Jatrophaplatyphylla, a new non-toxic Jatropha species: Physical properties and chemical constituents including toxic and anti-nutritional factors of seeds. Food Chem. 125:63-71.
Crossref
|
|
|
|
Nagahama Y, Yamashita M (2008). Regulation of oocyte maturation in fish. Dev. Growth Diff. 50(s1):S195-S219.
Crossref
|
|
|
|
Ola SI, AI J, Liu J, Wang Q, Wang Z, Chen D, Sun Q (2008). Effects of gonadotrophins, growth hormone, and activina on enzymatically isolated follicle growth, oocyte chromatin organisation, and steroid secretion. Mol. Reprod. Dev. 75:89-96.
Crossref
|
|
|
|
Olaleye VF (2005). A review of reproduction and gamete management in the African catfish (Clarias gariepinus Burchell 1822). Ife J. Sci. 11:63-72.
|
|
|
|
Omitogun OG, Olaniyan OF, Oyeleye OO, Ojiokpota C, Aladele SE, Odofin WT (2010). Potentials of short term and long term cryopreserved sperm of the African giant catfish (Clarias gariepinus Burchell, 1822) for aquaculture. Afr. J. Biotechnol. 9(41):6973-6982.
|
|
|
|
Oyeleye OO, Omitogun OG (2007). Evaluation of the motility of the cryopreserved sperm of the African giant catfish (Clarias gariepinus, Burchell 1822). Ife J. Agric. 22:11-15.
|
|
|
|
Prat F, Sumpter JP, Tyler CR (1996). Validation of radioimmunoassays for two salmon gonadotrophins (GTH) and (GTH II) and their plasma concentrations throughout the reproductive cycle in male and female rainbow trout (Oncorhynchus mykiss). Biol. Reprod. 54:1375-1382.
Crossref
|
|
|
|
Salami AA, Fagbenro OA, Balogun AM, Atoyebi O, Olowoyeye MF (2006). Effective dose of amphibian pituitary extracts for the induced spawning of the Clariid Catfish, Clarias gariepinus (Burchell 1822). J. Aquac. Tropics 11:9-12.
|
|
|
|
Schmitz M, Aroua S, Vidal B, LeBelle N, Elie P, Dufour S (2005). Differential regulation of luteinizing hormone and follicle stimulating hormone expression during ovarian development and under sexual steroid feedback in the european eel. Neuroendocrinology 81:107-119.
Crossref
|
|
|
|
Smitz JEJ, Cortvrindt RG (2002). The earliest stage of follicullogenesis in vitro. Reproduction 612(123):185-202.
Crossref
|
|
|
|
Teugels GG (1986a). Taxonomy, Phylogeny, biogeography of catfishes (Ontario physi, Siluroidei): An Overview: Aquat. Living Resour. 9:34.
|
|
|
|
Weil C, Bail PY, Sabin N, Le Gac F (2003). In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 130:2-12.
Crossref
|
|
|
|
Yeung CM, Chan CB, Leung PS, Cheng CHK (2006). Cells of the anterior pituitary. Int. J. Biochem. Cell Biol. 38:1441-1449.
Crossref
|