Full Length Research Paper
ABSTRACT
Disorders of sex development (DSD) when diagnosed early is important as it pose a real public health problem in Senegal. Among the supporting tools, molecular ones, which are not available everywhere are very useful. In this context, cytogenetic and molecular analyses were implemented in cytology laboratory at the Aristide Le Dantec hospital to enhance the DSDs diagnosis as well as evaluate the impact of the parents' age on such abnormalities. 55 cases of DSD have been received in the cytology laboratory for which cytogenetic (Barr chromatin and GTG karyotype) and molecular (SRY gene research) techniques have been used to characterize these anomalies according to the standards described in the international nomenclature. Three categories of DSD were found, namely 46,XX DSD, 46,XY DSD and chromosomal DSD. SRY is present in 4 patients 46,XX and absent in 3 patients 46,XY and results showed that the diagnosis is made earlier than previously (about 07 years). The study thus suggests the importance of complementarity (cytogenetics and molecular biology) in the diagnosis of DSD but also and especially the importance of early diagnosis from birth. Analysis of the epidemiological data also showed a slight correlation between maternal age and DSD. This showed us that a better characterization of DSD via increasingly powerful tools helps understanding on such pathologies and allows good medical care for patients.
Key words: Disorders of sex development (DSD), karyotype, SRY, hermaphroditis.
INTRODUCTION
Disorders of sex development (DSD) are rare abnormalities that can affect 1 to 3 of 10,000 children at birth (Bashamboo et al., 2010; Goultaiene et al., 2016; Mastrandrea et al., 2012). They are defined as individual whose genitals are difficult or even impossible to describe. However for Guillot (2008), these anomalies account for more than 10% of the population because any person who does not correspond to the morphological standards is de facto considered as an intersex. Moreover, these anomalies constitute an inadequacy between the sex reported at birth and the real sexual identity of the individual (Azonbakin et al., 2016; Gueniche et al., 2008; Guillot, 2008; Querfani et al., 2007). Advances in biology have shown us that the sex definition is not only based on physical criteria but requires an integrative approach (Hersmus et al., 2012). After the anatomical criterion (presence of penis or vagina), we have the gonadic (testicular or ovarian), genetic (XY or XX) or even the social criteria (male or female) (Azonbakin et al., 2016; Hersmus et al., 2012; Poulat et al., 1992; Sultan et al., 2001). An absence of one of these criteria can therefore lead to one of the known forms of DSD.
These anomalies were the subject of a new nomenclature based on an international consensus in 2006 (Diakité et al., 2013; Kim and Kim, 2012; Wiesemann et al., 2010). They can thus be classified as 46,XX DSD, 46,XY DSD and sex chromosome DSD, corresponding respectively to the terminology of female pseudo-hermaphroditism, male pseudo-hermaphroditism and true pseudo-hermaphroditism, which are now proscribed as they have a pejorative connotation (Lee et al., 2006). These DSDs cover broad clinical phenotypes that are essential to identify regardless the period of their expression (Folligan et al., 2012; Idrissi, 2012). Indeed, individuals affected by true hermaphroditism (also named ovotesticular DSD) possess both testicular and ovarian tissues associated with karyotypes that can be 46,XX (60% of cases), mosaics (30%) or 46,XY (only 10%) (Querfani et al., 2007). Furthermore, in sex chromosome DSD category we can find pathologies such as Turner and Klinefelter syndromes (Lux et al., 2009; Öçal, 2011).
On the other hand, the 46,XY DSD individuals derived from an inadequate masculinization of a genetically male embryo (Diakité et al., 2013; Goultaiene et al., 2016). They are associated with male gonads, but external genitalia remain ambiguous due to a pronounced deficiency of the hormone derived from testosterone (Azonbakin et al., 2016; Idrissi, 2012; Lee and Houk, 2008; Lin et al., 2007) . The last category 46,XX DSD refers to the presence of ovaries with external genitalia ambiguous and virilized to varying degrees such as penniform clitoris. This may be due to early exposure to androgens related to an adrenal tumor or inappropriate hormone therapy in pregnant women (Diakité et al., 2013; Folligan et al., 2012). The impact of these anomalies is heavy all over the world with a prevalence of 0.1 to 2% (Creighton and Minto, 2001). However, this estimate is even more worrying in the most disadvantaged areas because of the lack of opportunities to diagnose these infections as soon as possible (Azonbakin et al., 2016; Diakité et al., 2013; Folligan et al., 2012).
In Senegal, public cytogenetic structures specializing in the diagnosis and/or screening of congenital malformations are rare despite the rapidly expanding techniques (Matejka and Cribiu, 1987; Popescu, 1975; Popescu et al., 2000) widely used in cytogenetic to facilitate diagnosis in sub-Saharan Africa area (Diakité et al., 2013). Moreover, molecular biology techniques tend to be important in diagnosis of these diseases due to genetic recombination with, for example the SRY gene case that can sometimes be found on X chromosome (Faye et al., 2007; Gao et al., 2013). In addition, other techniques such as the study of Barr chromatin is sometimes used to make a first-line diagnosis of these anomalies (Artois and Salmon, 2009). This is a very rapid medical test to determine the percentage of Barr’s corpuscles that correspond to the condensation of the second X chromosome in females, which range from 15 to 30% while it is between 0 and 5% for male epithelial cells. We can face a mosaic case where the percentage is found between 6 and 14% (Faye et al., 2007). Nevertheless, Barr’s chromatin results can be influenced by several factors (Gueye et al., 2014) and the integration of these techniques could be of great value in the diagnosis of DSDs.
Despite the remarkable human variability, our gender identities are heavily constructed, socially and culturally and yet there is little data about the impact of these DSD in Senegal. Similarly, the genetic aspects of these abnormalities are little studied in Africa and therefore in Senegal especially in the most disadvantaged areas because of the lack of adequate diagnostic tools (Ediati et al., 2015). Due to the high birth rate and inbreeding but also to the continuation of procreation until a late age, every year there are a large number of births of children with genetic abnormalities, especially in families from disadvantaged areas (Juniarto et al., 2016). In addition, the management of these sexual disorders faces local beliefs that consider these abnormalities abnormal and shameful (Warne and Raza, 2008; Ediati et al., 2015), thus, leading to secrecy, social isolation and stigma (Ediati et al., 2017). These patients are therefore faced a real problem of identity, which makes difficult to see if the term is applicable to obtain a national incidence rate and therefore does not really reflect the importance of this problem. In this context, we aim to identify and classify through genetic analysis (cytogenetic and molecular techniques) the DSDs faced in Laboratory of Clinical Cytology - Cytogenetic - Reproductive Biology at the Le DANTEC hospital for better orientation and management of patients.
METHODOLOGY
Patients and samples
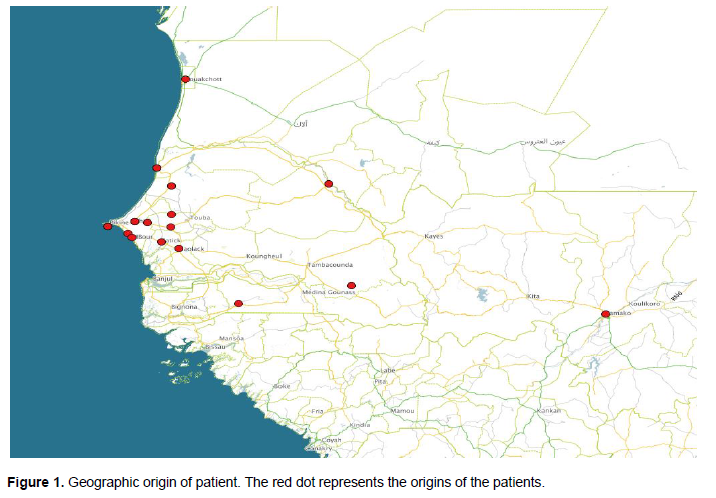
The study was carried out in the Cytogenetic Unit of the Clinical Cytology-Cytogenetic-Reproductive Biology Laboratory of the Aristide Le Dantec Hospital (HALD), where only external patients received for a DSD indication have been integrated to the study. Clinical examination was performed and various information were taken such as declared sex (at birth), age of patient and parents at the diagnostic time, ethnicity and geographic origin as shown in Figure 1 (Table 1). All these information are then compiled and analysed under R v3.1.1. (R Development Core Team, 2008)using Fisher's exact test (with a significance level of 0.05) to see how the pathology is related or not to the age of parents.
Genetic studies
Several analyses were carried out, namely the Barr chromatin test, the GTG karyotype and the amplification of the SRY gene using three types of sampling. The specific techniques are explained in the following.
The chromatin test of Barr
This test was carried out from epithelial cells taken with a spatula by scratching the internal mucosa of the cheek followed by a spread on a slide and then an instantaneous fixing using the lacquer. Subsequently, cytoplasm lysis with chloric acid 1N (1N) was carried out at 56°C for 7 min followed by a series of hydration and dehydration with alcohol and distilled water. Finally, GUARD coloration was made before examining on an optic Microscope at least 200 interphasic nucleic (Faye et al., 2007).
GTG Karyotype
Venous blood was taken on a heparin tube and the cell culture was made within 72 h following the sampling according to the protocol described by Dia (2015), Gao et al. (2013) and Tijo and Levan (1956).
This technique was based on a culture at 37°C in the presence of 5% CO2 and was done by inoculating 0.5 ml of blood. Before staining the Giemsa slides, enzymatic digestion was carried out in a trypsin solution for the labelling of G-band chromosomes followed by microscopic observation to analyse the metaphases and thus establish the GTG karyotype of the different patients concerned.
The establishment of the karyotypes was carried out using an imaging system composed of an epifluorescence microscope associated with the image capture and processing software "Leica CW 4000 cytogenetics".
Amplification of SRY gene
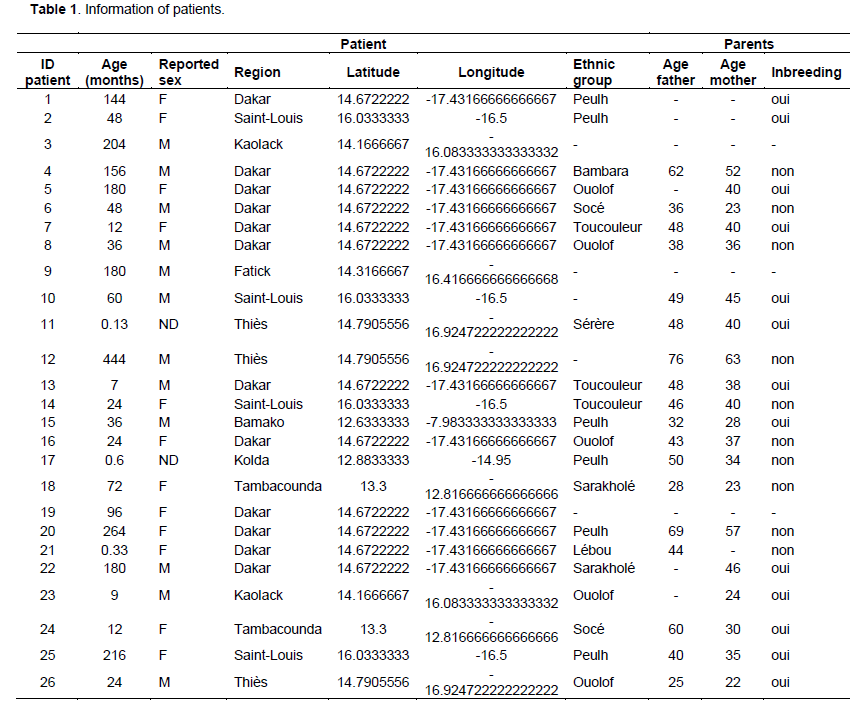
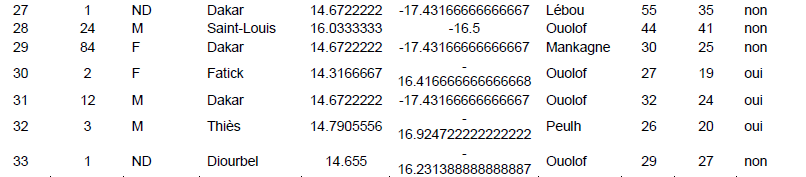
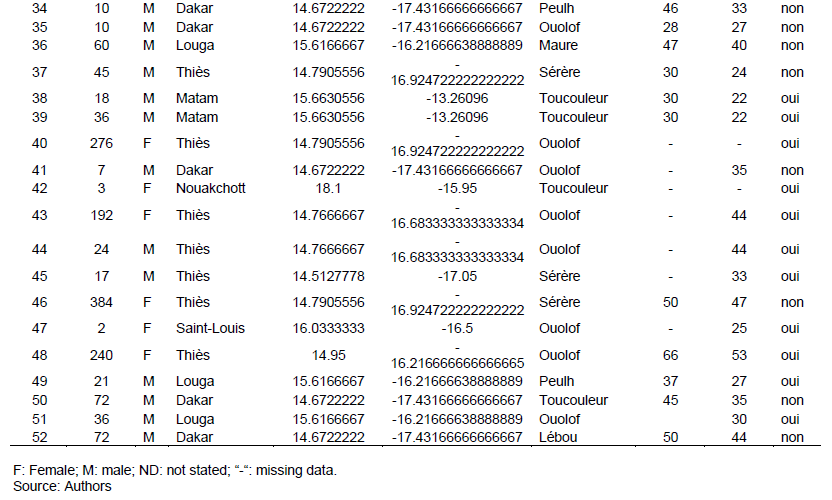
Blood was sampled on an EDTA tube and investigated the SRY gene using
RESULTS
Chromatin of Barr
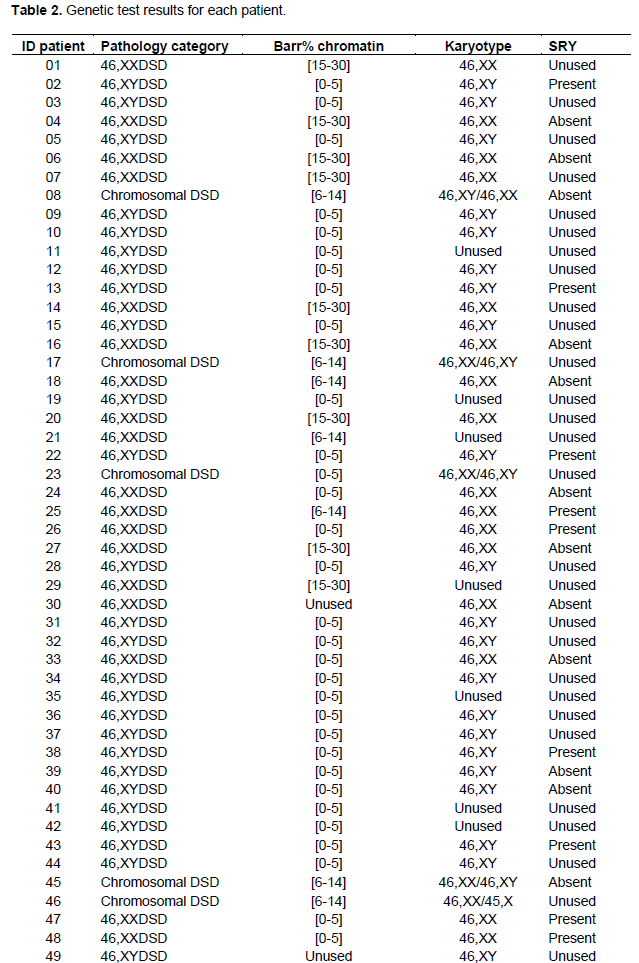
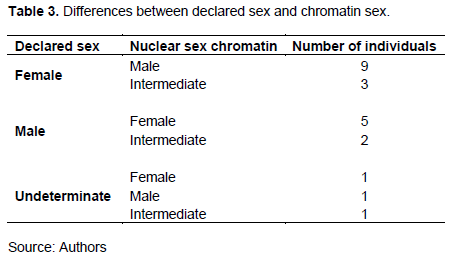
Only two individuals (3.64%) of all patients did not perform the Barr chromatin test (Table 2). The three known categories have been found, these were the male (61.82%), the female (21.82%) and the intermediate chromatin sex (12.73%). For all the patients who did this test, 58% showed congruent results with the sex reported at the birth. However, for 42% of the patients, the diagnosis was different from the declared sex with seven possibilities that have been encountered (Table 3).
Indeed, following the Barr chromatin analysis, for the 22 patients whose sex was declared different from the chromatin sex results, three sex groups were proposed (Feminine, Male, and Undetermined). The first group "Feminine" concerns twelve individuals declared female, nine of them have a male chromatin sex, while the remaining three have an intermediate chromatin sex. The second group, "Male", consists of seven declared male patients, five of them had female chromatin and two intermediate chromatin sexes. The last group named "Indeterminate" referred to three patients of undetermined and/or undeclared sex at birth. In the latter group, we found the three known chromatin sex categories (Female, Male and Indeterminate).
The karyotype GTG
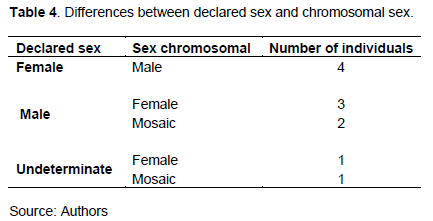
For the 55 patients studied, more than 85% performed karyotype tests. Five chromosomal formulas 46,XY (43.64%), 46,XX (29.09%), 46,XY / 46,XX (5.45%), 47,XXY (5,45%) and 46, XX / 45, X (1.82%) were found in the study population. After the analysis, 77% of the cases showed congruent results with the declared sex, while in 23% of the cases, the diagnoses were different with five possibilities encountered (Table 4). Indeed, in the "Feminine" group, we found four patients, all of them had a male chromosomal sex. In the group "Male" where we recorded five patients, three had a female chromosomal sex and 2 had a mosaic. In the last "Indeterminate" group, we obtained one female chromosomal sex patient and one chromosomal mosaic patient.
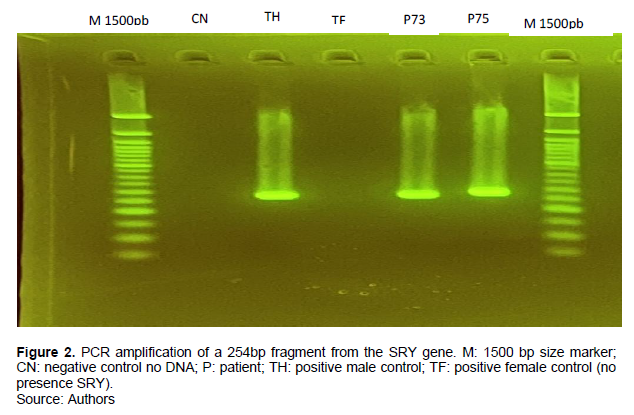
Search the SRY gene
The amplification of the SRY gene as shown in Figure 2 was based on the results of the karyotype and chromatin of Barr. For the 22 DNAs of patients thus analysed, SRY was found (SRY+) in 9 patients of them while absent (SRY-) in the remaining (Table 2).
Comparisons between genetic data and DSDs classification
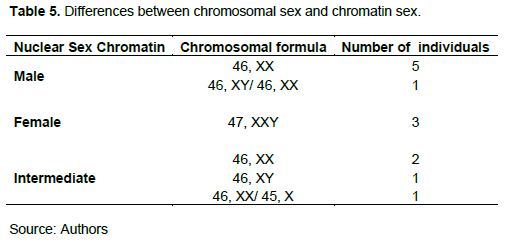
Results based on karyotype reveal that 72% are consistent with those of Barr chromatin and SRY gene search; that is to say, it refers to the same sex categories (Masculine or Feminine). On the other hand, for 28% of the cases the results are different from several cases of figures encountered (Table 5). Five patients showed an intermediate Barr chromatin level in the absence of the SRY gene and associated with a male (01), female (01) or mosaic (03) karyotype; other cases (08 patients) presented a male type of Barr chromatin associated with a karyotype of a female type (06 patients with the presence of the SRY gene in 03 of them), male (01 patient with no SRY) and mosaic (01). Finally, three patients had female type Barr chromatin levels associated with 47,XXY chromosomal formulas suggestive of Klinefelter syndrome.
The various pathologies listed are mainly in the anomalies of testicular development, anomalies of androgens and anomalies of ovarian development. The three classes defined in the international nomenclature, namely 46,XY DSD, 46,XX DSD and DSD chromosomes have all been found in our patients. Among these three classes, category 46,XY DSD is the most represented (p = 0.0007). We also note in the context of chromosomal DSDs the presence of Turner syndromes in mosaic and Klinefelter as well as three cases of ovotesticular DSD.
Impact of age on pathology
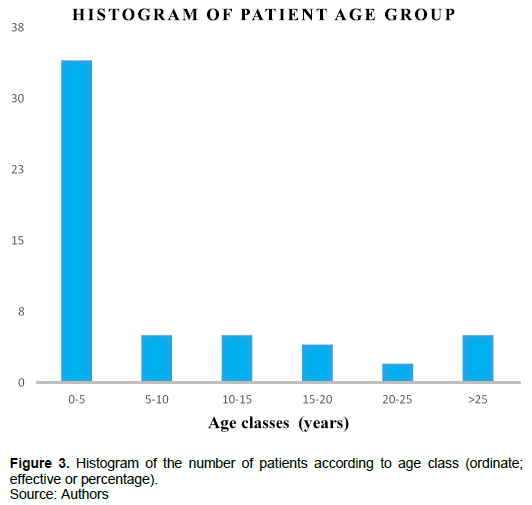
The average age of patients received is 9.7 years with extremities ranking from 4 days to 48 years. The results of the different age classes are as shown in Figure 3. The most significant age group (p = 1.239e-08) varies from 0 to 5 years with 67.28%, followed by the classes [6 to 10 years] and [11 to 15years] each representing 9.1%; followed by classes [16 to 20] with 7.28%; the class [>25 years] represented 5.45% and finally the class [21 to25 years] represented 1.82%. The Fisher test carried out between the age group and the pathology categories showed a very significant value (p = 1.239e-08). The parental age ranged from 25 to 76 years for fathers with an average of 39.39 years and between 19 and 63 years for mothers with an average of 32.29 years. The correlation between the pathology observed in the patient and the age of the parents is slightly significant for mothers (p = 0.02) compared to fathers (p = 0.08).
DISCUSSION
Involvement of age in DSDs patients
The importance of DSD compared to other types of pathologies can be explained by the fact that these abnormalities most often affect sexual chromosomes with the presence in most of these patients of a phenotype suggestive organ (penoclitoris, ovotestis, testicular or clitoral hypertrophy, android morphotype, among others). As it is known, during ovogenesis, each of the 23 pairs of chromosomes had an equal risk of error during segregation, but these risks are higher in sexual chromosomes (Kamiguchi et al., 1994). Thus, the results obtained showed an age of consultation relatively young for these patients (almost 10 years). This average compared to those obtained in European countries seems to be considerably higher. Indeed, in those countries, 60% of the children with DSD are diagnosed at birth or even during the prenatal period (Gueye et al., 2014; Mastrandrea et al., 2012). In our case, only 12 of the 55 cases of DSDs came for medical consultation before one year. However, it should be noted that the 3 oldest patients had Klinefelter's syndrome and therefore, came to consultation for primary sterility. The average age without the three later patients decreases to 7 years. This shows a great improvement of this average in Africa, if we just look back ten years ago in the studies carried out in the sub-Saharan area (an average: 14 years on the medical care of DSDs in Mali in 2003) (Kossi, 2003), 18.75 years on the sexual ambiguities in Dakar in 2001 (Ndiaye, 2001), 5 years concerning the surgical management of DSDs in Dantec Hospital HALD in 2004 (DIOUF, 2004). This delay in consultation (about 7 years) compared to developed countries may be due to the scarcity of specialized structures in these DSDs affections but also can be explained by several other reasons: lack of information and specific training that would lead to a rapid and early referral of patients for care (Folligan et al., 2012).
The socio-economic reasons are related to the fact that most of the patients are from the rural areas, which could cause inaccessibility to adequate services, the level of awareness but also the support (financial, logistic, etc). Furthermore, our data showed a slight correlation between maternal age (slightly increased) and the presence of these abnormalities in the patients studied.
Several studies have for long been interested in the impact of parental age on the occurrence of such pathologies. Maternal age is the only one that has an unequivocal link with number chromosomal abnormalities, especially trisomy 21 (Vekemans, 2003; Pellestor, 2004). On the other hand, and more recently, the advanced paternal age showed to be implicated in the occurrence of congenital anomalies due to the mutations that occur during spermatogenesis. Such mutations occurrence increases with age and should be checked in further studies later.
Contribution of genetic methods in the diagnosis of DSDs
Several chromosomal formulas have been found, highlighting both the importance of clinical diagnosis and the genetic methods used here (Barr Chromatin, Karyotype and SRY gene research). The results obtained by the karyotype, the Barr chromatin and the search for the SRY gene have allowed us to find a genetic sex congruent with the sex declared except for a few patients in whom the different analyses carried out one by one appear contradictory but interpreted together allow us to strengthen explanation of the phenotypes. Among these, three of them represented true cases of hermaphroditism corresponding to chromosomal DSDs. The presence of the two genotypes has the effect of diluting the percentage of Barr chromatin present in the patient, thus explaining the result obtained. Moreover, in four of the other patients we have evoked the translocation of the TDF on the X chromosome, which could be confirmed by molecular biology during analysis of SRY gene.
In the case of the few patients, the X chromosome (normally inactivated) had to be activated by the presence of this TDF, which allows it to behave like a Y chromosome and could therefore, explain the incongruence between the tests on the one hand, but also the presence of male external genitalia for these patients. Indeed, the SRY gene is often detected in 80% of XX men and 10% of true hermaphrodites XX (Barbaux et al., 1995) as shown in our study where the SRY gene was found only in individuals 46,XX. These different cases illustrated the fact that the Barr chromatin test must be done for any new born with an abnormality of the external genital organs but also must always be supplemented by a karyotype whenever possible to exactly know the chromosomal formula of the patient concerned (Ndiaye, 2001). Indeed, this examination already makes it possible to distinguish patients with more than one sexual chromosome X from those who have one or those who lack one. This does not mean that Barr chromatin is not useful when the karyotype has been performed as in some cases, Barr’s chromatin may be indicative or even indispensable (Gueye et al., 2014).
The results also show that we can never be satisfied with Barr Chromatin alone in a DSDs diagnosis.
Cytogenetic analysis must always include a karyotypes (Diakité et al., 2013; DIOUF, 2004) which makes it possible to know the chromosomal formula of an individual (Ndiaye, 2001) and may prove to be important in the mosaics cases (46,XX/46,XY) as found in three of our patients. Other cases of mosaics have also been encountered, the latter being rather due to non-homogeneous syndromes and corresponding to the Turner's syndrome in our case (46, XX /45, X0) which dilute the barr chromatin thus found.
Finally, we found the Klinefelter syndrome in three of our patients whose Barr Chromatin tests revealed a rate that refers to the female chromatin sex, which is explained by the presence of the second X chromosome set found in the karyotype. On the other hand, the male phenotype is due to the expression of the genes on the Y chromosome. Indeed, the Y chromosome plays a dominant role in the determinism of the testis. Independently of the number of X chromosomes, an individual with only one Y chromosome develops in the male direction (Poulat et al., 1992; Barbaux et al., 1995; Al Jurayyan, 2011).
CONCLUSION
Disorders of sex development constitute a real public health problem and malformations are the leading cause of infant mortality. Cytogenetic is of great value in the diagnosis and management of patients. The development and integration of the techniques of molecular biology via the research of the SRY thus made it possible to reinforce the reliability of the results. Indeed, the karyotype and the Barr chromatin have limits (intermediate level of chromatin of Barr or resolution of micro-rearrangements to be detected), hence the necessity to use molecular cytogenetics to refine the diagnosis. Analysis of epidemiological data showed a slight correlation between maternal age and pathology. Of course, these results could be related to the small size of our study population and the missing data encountered during the analysis. The latest studies have clearly shown that the age of consultation is becoming increasingly younger over the years, which is a major advance mainly due to the development of cytogenetic techniques but also and especially for molecular biology. This study induces us to orient ourselves towards molecular cytogenetics, which would allow many cases to find an answer and therefore a suitable treatment.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Al Jurayyan NA (2011). Medical Sciences. Malaysian Journal of Medical Sciences 3(18):4-12. |
|
|
Artois M, Salmon D (2009). Détermination expérimentale du sexe et de l'âge chez le renard roux (Vulpes vulpes): Validité et reproductibilité des techniques choisies?: Mammalia. Mammalia 45(3). |
|
|
Azonbakin, S., Axede, B., Avakoudjo, J., Sissoko, S., Ouedraogo, A., Adjagba, M., Alao, M., Darboux, R., & Laleye A (2016). Anomalie du développement sexuel (DSD, 46 XY) par déficit en 17 β-Hydroxysteroïde deshydrogenase de type 3: Aspects clinique et biologique. Journal de La Société de Biologie Clinique Du Bénin 25:70-73. |
|
|
Barbaux S, Vilain E, McElreavey K, Fellous M (1995). Update on sex determinism in mammals. MS. medicine science 11(4):529-536. |
|
|
Bashamboo A, Ledig S, Wieacker P, Achermann JC, McElreavey K (2010). New Technologies for the Identification of Novel Genetic Markers of Disorders of Sex Development (DSD). Sexual Development 4(4-5):213-224. |
|
|
Creighton S, Minto C (2001). Managing intersex. BMJ?: British Medical Journal, 323(7324):1264-1265. |
|
|
Dia F (2015). Contribution de cytogénétique au diagnostic de la différenciation sexuelle et des aneuploïdies à l'hôpital Aristide le Dantec (Mémoire de Master En Biologie Animale 238 :47). Université Cheikh Anta Diop de Dakar. |
|
|
Diakité M L, Berthé H JG, Timbely A, Diallo M, Maiga M, Diakité A, Diallo M, Ouattara K, Faure A (2013). Problématique de la prise en charge des anomalies de la différenciation sexuelle dans le service d'urologie: CHU Point G. Progrès en Urologie 23(1):66-72. |
|
|
DIOUF AW (2004). [These doctorat en Médecine, Cheikh Anta Diop Dakar]. |
|
|
Ediati A, Juniarto AZ, Birnie E, Drop SLS, Faradz SMH, Dessens AB (2015). Body Image and Sexuality in Indonesian Adults with a Disorder of Sex Development (DSD). The Journal of Sex Research 52(1):15-29. |
|
|
Ediati A, Juniarto AZ, Birnie E, Okkerse J, Wisniewski A, Drop S, Faradz SMH, Dessens A (2017). Social stigmatisation in late identified patients with disorders of sex development in Indonesia. BMJ Paediatrics Open 1(1):e000130. |
|
|
Faye O, Azza S, Adil B, Doudou D, Berthé MA, Ndiaye M, Afoutou JM, Touré CT, Anthonioz P (2007). Diagnostic interest of Barr chromatin test in sex determination: About one case of male pseudohermaphrodism. Dakar Medical 52(3):204-208. |
|
|
Folligan K, Laleye A, Moumouni H, Koffi KS, Yao GV, Adjagba M, James YE, Anoukoum T, Akakpo-Numado G, Hazemdji-Nimtche H, Defolo A (2012). Anomalie de developpement sexuel: Un cas de pseudohermaphrodisme masculin ou anomalie de developpement sexuel XY. Journal de La Recherche Scientifique de l'Universite de Lome 14(1):51-54. |
|
|
Gao X, Chen G, Huang J, Bai Q, Zhao N, Shao M, Jiao L, Wei Y, Chang L, Li D, Yang L (2013). Clinical, cytogenetic, and molecular analysis with 46, XX male sex reversal syndrome: Case reports. Journal of Assisted Reproduction and Genetics 30(3):431-435. |
|
|
Goultaiene A, Elmortaji K, Sentissi R, Moataz A, Rabii R, Aboutaib R, Dakir M, Debbagh A, Meziane F (2016). Place de la laparoscopie dans la prise en charge des anomalies de différenciation sexuelle: À propos de 4 cas. Pan African Medical Journal 23(1). |
|
|
Gueniche K, Jacquot M, Thibaud E, Polak M (2008). L'identité sexuée en impasse…À propos de jeunes adultes au caryotypeXY nées avec une anomalie du développement des organes génitaux et élevées en fille. Neuropsychiatrie de l'Enfance et de l'Adolescence 56(6):377-385. |
|
|
Gueye MV, Faye O, Ndiaye A, Diop N, Diallo AS, Diallo MS (2014). Etude cytogénétique des anomalies chromosomiques par la chromatine de Barr et le caryotype au service d'histologie-embryologie-cytogénétique de Dakar: À propos de 100 cas. Journal de La Société de Bioloçgie Clinique Du Bénin 25:90-95. |
|
|
Guillot V (2008). Intersexes: Ne pas avoir le droit de dire ce que l'on ne nous a pas dit que nous étions. Nouvelles Questions Féministes 27(1):37. |
|
|
Hersmus R, Stoop H, White SJ, Drop SL, Oosterhuis JW, Incrocci L, Wolffenbuttel KP, Looijenga LH (2012). Delayed Recognition of Disorders of Sex Development (DSD): A Missed Opportunity for Early Diagnosis of Malignant Germ Cell Tumors. International Journal of Endocrinology pp. 1-9. |
|
|
Idrissi HK (2012, June 3). Hermaphodisme: Une anomalie congénitale très rare. L'Observateur du Maroc & d'Afrique | Hermaphrodisme. |
|
|
Juniarto AZ, van der Zwan YG, Santosa A, Ariani MD, Eggers S, Hersmus R, Themmen AP, Bruggenwirth HT, Wolffenbuttel KP, Sinclair A, White SJ (2016). Hormonal evaluation in relation to phenotype and genotype in 286 patients with a disorder of sex development from Indonesia. Clinical Endocrinology 85(2):247-257. |
|
|
Kamiguchi Y, Tateno H, Mikamo K (1994). Chromosomally Abnormal Gametes as a Cause of Developmental and Congenital Anomalies in Humans. Congenital Anomalies 34(1):1-12. |
|
|
Kim KS, Kim J (2012a). Disorders of Sex Development. Korean Journal of Urology 53(1):1. |
|
|
Kossi EK (2003). Les ambiguités sexuelles en service de médecine interne de l'Hôpital National du Point G A propos de douze cas [Doctorat medecine, UNIVERSITÉDEB AMAKO Faculté de Médecine de Pharmacie et D'Odonto-Stomatologie]. |
|
|
Lee PA, Houk CP (2008). Disorders of Sexual Differentiation in the Adolescent. Annals of the New York Academy of Sciences 1135(1):67-75. |
|
|
Lee PA, Houk CP, Ahmed SF, Hughes IA, in collaboration with the participants in the International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. (2006). Consensus Statement on Management of Intersex Disorders. PEDIATRICS, 118(2):e488-e500. |
|
|
Lin L, Philibert P, Ferraz-de-Souza B, Kelberman D, Homfray T, Albanese A, Molini V, Sebire NJ, Einaudi S, Conway GS, Hughes IA (2007). Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46, XY disorders of sex development with normal adrenal function. The Journal of Clinical Endocrinology and Metabolism 92(3):991-999. |
|
|
Lux A, Kropf S, Kleinemeier E, Jürgensen M, Thyen U (2009). Clinical evaluation study of the German network of disorders of sex development (DSD)/intersexuality: Study design, description of the study population, and data quality. BMC Public Health 9(1):1-7. |
|
|
MacLean HE, Warne G, Zajac JD (1997). Intersex disorders: Shedding light on male sexual differentiation beyond SRY. Clinical Molecular Endocrinology 46(1):101-108. |
|
|
Mastrandrea LD, Albini CH, Wynn RJ, Greenfield SP, Robinson LK, Mazur T (2012). Disorders of Sex Development: Management of Gender Assignment in a Preterm Infant with Intrauterine Growth Restriction. Case Reports in Medicine 1-4. |
|
|
Matejka M, Cribiu EP (1987). Idiogramme et représentation schématique des bandes G des chromosomes du mouton domestique (Ovis aries L.). Genetique, Selection, Evolution 19(1):113. |
|
|
Ndiaye M (2001). Ambigüités Sexuelles: Prise en Charge à Dakar These doctorat en Pharmacie, Cheikh Anta Diop Dakar. |
|
|
Öçal G (2011). Current Concepts in Disorders of Sexual Development. Journel of Clinical Research in Pediatric Endocrinology 3(3):105-114. |
|
|
Pellestor F (2004). Âge maternel et anomalies chromosomiques dans les ovocytes humains. Médecine/sciences 20(6-7):691-696. |
|
|
Popescu CP (1975). L'étude du caryotype Bovin (Bos taurus) L.) par les méthodes de Bandes. Annales de Biologie Animale Biochimie Biophysique 4(15):751-756. |
|
|
Popescu CP, Hayes H, Dutrillaux B (2000). Techniques in animal cytogenetics. Springer-Verlag. http://prodinra.inra.fr/?locale=fr#!ConsultNotice:3224 |
|
|
Poulat F, Goze C, Boizet B, Berta P (1992). Gene SRY et anomalies de la determination genetique du sexe chez l'homme. Andrologie 2(2): 50. |
|
|
Querfani B, El Mhef S, Rabii R, Joual A, Bennani S, Meziane F (2007). Hermaphrodisme vrai (à propos d'un cas). Journal Marocain d'Urologie 1(6):24-27. |
|
|
R Development Core Team (2008). R: A Language and Environment for Statistical Computing. R foundation for statistical computing. |
|
|
Settin A, Elsobky E, Hammad A, Al-Erany A (2008). Rapid Sex Determination Using PCR Technique Compared to Classic Cytogenetics. International Journal of Health Sciences 2(1):49-52. |
|
|
Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, Lumbroso S, Nicolas JC (2001). Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Molecular and Cellular Endocrinology 178(1-2):99-105. |
|
|
Tijo JH, Levan A (1956). The chromosome number of man. Hereditas 42(1-2):1-6. |
|
|
Vekemans M (2003). Âge maternel et autres facteurs de risque de la trisomie 21. Annales de Biologie Clinique 61(4):497-499. |
|
|
Wiesemann C, Ude-Koeller S, Sinnecker GHG, Thyen U (2010). Ethical principles and recommendations for the medical management of differences of sex development (DSD)/intersex in children and adolescents. European Journal of Pediatrics 169(6):671-679. |
|
|
Wu QY, Li N, Li WW, Li TF, Zhang C, Cui YX, Xia XY, Zhai JS (2014). Clinical, molecular and cytogenetic analysis of 46, XX testicular disorder of sex development with SRY-positive. BMC Urology 14(1):1-5. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0