ABSTRACT
The aim of this study was to determine the distribution of high-risk human papillomavirus genotypes (HR-HPV) in women from the general population of five West African countries. This was a cross-sectional descriptive study, involving 2133 women from nine cities of five West African countries: Benin, Burkina Faso, Côte d'Ivoire, Niger and Togo. Women were screened for precancerous cervical lesions and HR-HPV infection. The detection of HR-HPV was done by a multiplex real-time PCR on extracted viral DNA. The average age of the women in this study was 35.06 ± 10.00 years with a range of 15 to 65 years. The overall prevalence of high-risk HPV infection among general population sample of women in five West African countries was 33.61% (717/2133). The prevalence of dysplasia was 8.81%. In decreasing order of frequency, the genotypes found were: HPV 52 followed by HPV 31, 59, 51, 66, 45, 68, 56, 56, 58, 35, 39, 18, 33 and 16. The prevalence of HPV16/18 (bivalent vaccine types) was 7.02%. This study reveals a high prevalence of HPV 52 in West Africa. The extent and diversity of HR-HPV genotypes in these West African countries deserve special attention for prevention.
Key words: High-risk HPV, real time PCR, genotypes, women, epidemiology, West Africa.
Human papillomavirus (HPV) infection and cervical cancer remain a major concern worldwide, especially in sub-Saharan Africa where cervical cancer, induced by high-risk HPV (HR-HPV), is the leading cause of cancer death in women. In addition, the slow and insidious evolution of this condition as well as the absence of systematic screening would explain why it is most often diagnosed at a late stage. HPV infection is the most common sexually transmitted infection (STI) in the world, with 660 million people infected according to the World Health Organization (WHO). The WHO estimates that the annual incidence of cervical cancer was 500,000 with more than 90% of cases in developing countries. In sub-Saharan Africa, invasive cervical cancer is the most common cancer in women with more than 75,000 new cases and more than 50,000 deaths per year (Ferlay, et al., 2010). In Africa, the prevalence of HPV infection reaches 21.3% with significant regional variations: 33.6% in East Africa, 21.5% in West Africa and 21% in Southern Africa (Ferlay et al., 2010)countries, both nationally and internationally, especially in developing countries, cancer has a negative impact on the general health of the family and results in a loss of income and huge health expenditures, as it mainly affects the economically productive age group. In Burkina Faso, annual number of cervical cancer cases is estimated at 2.517 and cervical cancer deaths are found to be 2.081 per year (ICO/IARC, 2018).
The best means of control and prevention through the use of prophylactic vaccination against HPV, is not available to all populations, both urban and rural. In addition, 12 years after the release of the first two HPV vaccines (2006), despite GAVI's efforts, they remain expensive and are not yet accessible to the entire population of the West African sub-region. Some pharmaceutical companies have made efforts to reduce the cost of the vaccine in order to expand HPV vaccination campaigns for girls in some African countries. However, the HPV vaccines available on the market only cover two HR-HPV genotypes, HPV16 and HPV18. HPV16 and 18 genotypes are believed to be the most prevalent in Europe and the rest of the world, while preliminary studies by Djigma et al. (2011); Ouédraogo et al. (2011); Zohoncon et al. (2013) and Ouedraogo et al. (2015) and Rahimy et al. 2015) in Burkina Faso have rather shown a high prevalence of the HPV 30 and 50 family. A deep knowledge of circulating HPV genotypes is of a high interest in specific populations for the development of effective HPV vaccine covering the predominant genotypes in these populations. A large sample study is therefore crucial to determine the circulating genotypes in the general population on the one hand and in cervical cancer cases on the other hand. For effective control of cervical cancer, a preventable malignant tumor through prophylactic vaccination is done. This study aims to describe the molecular epidemiology of high-risk HPV genotypes in women without cervical lesions in nine cities of five West African countries.
Study type and population
This was a cross-sectional, descriptive study that collected 2133 endocervical samples from the cervix of women in the general population without cervical lesions. The samples came from five West African countries: Benin, Burkina Faso, Côte d'Ivoire, Niger and Togo.
Inclusion criteria
Included were all non-pregnant women and girls who freely consented after receiving information on the study.
Criteria for non-inclusion
Not included in the study were women or girls who were virgins or pregnant or who had a total hysterectomy.
Collection of samples
After sensitization on HPV infection prevention and cervical cancer risk, and after obtaining free and informed consent of women, a questionnaire was administered to women to collect socio-demographic, behavioural and clinical information, and an endocervical swab was performed at the cervix of women; followed by screening for precancerous cervical lesions by IVA/VILI. The samples collected were sent to the CERBA/LABIOGENE molecular biology and genetics laboratory, University Joseph Ki-Zerbo, Burkina Faso, for molecular analyses.
Extraction of viral DNA from HR-HPV
The DNA extraction was done using DNA-Sorb-A kit (Sacace Biotechnologies, Como, Italy) by following the protocol supplied by the manufacturer.
Real-time HR-HPV detection by multiplex PCR
Detection of high-risk HPV genotypes was made by real-time PCR using "HPV Genotypes 14 Real-TM Quant" kit (Sacace Biotechnologies, Como, Italy) and Sacycler-96 Real time PCR v.7.3 (SACACE Biotechnologies, Como, Italy). This genotyping is based on multiplex real time PCR amplification for each sample and the β- globin gene was used as internal control. The “HPV Genotypes 14 Real-TM Quant" kit allowed to detect the following 14 high-risk HPV genotypes such as HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, HPV66 and HPV68. Each sample was subjected to multiplex amplification in 4 tubes and each tube contained primers of the target regions (L1 gene and oncoproteins E6 and E7) of three or four types of HPV-HR and of the human beta-globin gene as control internal. For each sample we had respectively for the 4 tubes: PCR-mix-1 16, 18, 31, IC; PCR-mix-1 39, 45, 59, IC; PCR-mix-1 33, 35, 56, 68; PCR-mix-1 51, 52, 58, 66. The pre-PCR steps consisted in: preparing the Mix solution (PCR-buffer-FRT + Hot Start DNA Polymerase) and the Reaction Mix solution (Mix solution + each PCR- mix-1).
For each sample, 15 μL of the Reaction Mix solution was introduced, into the 4 tubes and add 10 μL of the extracted DNA. The total volume of the reaction was 25 μL. This PCR reaction mixture contained in sterile 0.2 mL microtubes was introduced onto the plate of the SaCycler-96 Real Time PCR v.7.3 (Sacace Biotechnologie, Italy) for amplification. The PCR program used was as follows: 1 cycle of 95°C for 15 min; 5 cycles of 95°C for 05 s, 60°C for 20 s, 72°C for 15 s; 40 cycles of 95°C for 05 s, 60°C for 30 s and 72°C for 15 s.
Ethics approval and consent to participate
This study was approved by the Health Research Ethics Committee of Burkina Faso with the reference number 2016-02-0012 on 03/02/2016. All study participants gave their free written and informed consent according to the Helsinki Declarations.
Data analysis
Data were entered and analyzed using the IBM SPSS software in its 21 version and Epi Info 6. The Chi-square test was used for comparisons with a significant difference for p Ë‚ 0.05.
Sociodemographic, behavioral and clinical characteristics of the study population
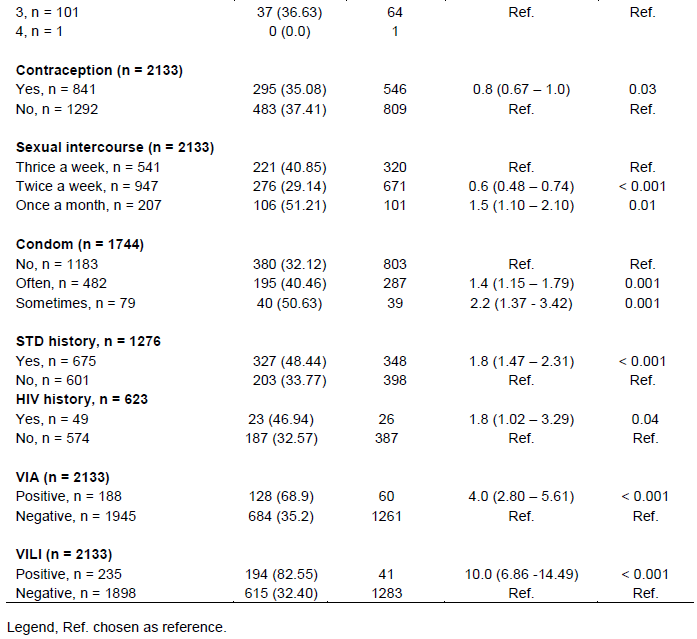
The average age of the women in this study was 35.06 ± 10.0 years a range of 15 to 65 years. The median age was 34 years. The 25 - 34 years age group was in the majority with 39.05% (833/2133) of women in the study population. Married women accounted for 73.18% (1561/2133) of the population; and secondary education was the majority at 38.44%. The average age at first intercourse was 18.5 ± 3.28 years with extremes of 6 to 30 years. Women reported having only one sexual partner in 83.87% (1789/2133) of cases. The frequency of sexual intercourse was on average twice a week in 51.90% of cases. Among the women in the study, 67.83% (1183/1744) did not use condoms; 60.57% (1292/2133) did not use a contraceptive method; 31.65% (675/2133) had a history of sexually transmitted infection (STI) and 2.30% (49/2133) reported being HIV positive. Screening for precancerous and cancerous cervical lesions by visual inspection with acetic acid (VIA) and visual inspection with lugol (VILI) among the women in the study had a dysplasia prevalence of 8.81% or 188 positive VIA/VILI. Table 1 shows the characteristics of the study population.
Prevalence of high-risk HPV infection among women in the general population
The overall prevalence of high-risk HPV infection among women in the general population of the five West African countries was 33.61% (717/2133). By country, the prevalence of high-risk HPV infection was 34.78% (160/460) in Benin; 37.09% (171/461) in Burkina Faso; 39.67% (192/484) in Côte d'Ivoire; 12% (30/250) in Niger and 34.31% (164/478) in Togo. Figure 1 shows the prevalence of HR-HPV infection by city, with nine cities in the five West African countries.
Frequency of HR-HPV genotypes in women in the general population of the five West African countries
Cumulative total number of genotypes identified in HPV-infected women was 1068 genotypes. HPV52 was the most common genotype (Table 2). Figure 2 shows the frequencies of the 14 high-risk HPV genotypes detected in our study.
The presence of the different high-risk oncogenic HPV genotypes in the women in our study is shown in Figure 3. Figure 3 shows the mapping of high-risk HPV genotypes in the five countries of our study: Benin, Burkina Faso, Cote d'Ivoire, Niger and Togo. Among women in our study, without cervical lesions and infected with HR-HPV, the prevalence of HPV16/18 (bivalent vaccine types) was 7.02% (Figure 4).
Multiple and isolated infections
Of the 717 women infected with HPV, 250 or 34.87% had a high-risk multiple HPV infection and 467 women (65.13%) had an isolated infection. The number of HR-HPV genotypes per woman ranged from 1 to 6. Multiple infections with 2 and 3 genotypes were in the majority, with 161/250 (64.40%) and 70/250 (28.00%) of cases respectively. Among the two-genotype combinations, one woman was infected with HPV16/18; while among the three-genotype co-infections, two women were infected with HPV16/18/31. Table 3 presents the different combinations of multiple infection with 4, 5 and 6 high-risk HPV genotypes obtained in this study.
HPV and risk factors
Age, marital status, education level, occupation, number of sexual partners, frequency of sexual intercourse, condom non-use and STI history, contraceptive use, HIV infection and presence of VIA/VILI dysplasia positive, were significantly associated with HR-HPV infection. Table 4 presents the risk factors for HPV infection in women in our study.
In our study, the overall prevalence of high-risk HPV infection among women in nine (09) cities in five (05) West African countries was 33.61% (717/2133). The prevalence of high-risk HPV infection in each country varied. According to the literature, the prevalence of HPV infection varies according to region; in West Africa this prevalence is estimated at 21.5% (WHO/ICO, 2009), which is lower than those in our study: either the general prevalence or those found in each of the five countries in our study except the prevalence found in Niger. It should be noted, however, that the data in this study are 9 years old and that the prevalence of HPV has certainly changed positively since 2010. This is worrying in the sense that the prevalence of cervical cancer could also change in parallel.
Our prevalence are consistent with those of other African authors such as: 42.6% in Ghana (Obiri-Yeboah et al. 2017), 54% in South Africa (Adler et al., 2013), 76% in Tanzania (Watson-Jones et al. 2013). In contrast, Wang et al. (2018) reported a general prevalence of HPV infection of 14.5% in China, which is lower than in our study. In our study, the 25 to 34 year of age group was 39.05% or 813/2133 and was the age group of women most affected by HPV infection. These infected young women are the most vulnerable layer, and therefore at risk of developing cervical cancer later on. According to some authors, with regard to age-specific prevalence, young women in the 20-25 age group have the highest prevalence (>20%). Prevalence then declines rapidly with age, reflecting the most often transient nature of HPV infection. According to these authors, this decrease is much more pronounced in countries with high socio-economic levels and in these countries, prevalence is less than 10% beyond the 30 to 35 age group. In addition, they report that a re-augmentation is generally observed in women of menopausal age, without the causes of this increase being clearly established (De Sanjosé et al., 2007; Louie et al., 2008).

Another study on the carrying of HPV infection in women in the general population reported extremes of age from 17 to 68 years in China, which is similar to ours, that is 15 to 65 years (Wang et al., 2018). The fourteen high-risk HPV genotypes investigated in our study were all identified. The cumulative number of genotypes identified in high-risk HPV-infected women was 1068 genotypes in total. HPV52 was the most common genotype followed by HPV31, HPV59, HPV51, HPV66, HPV45, HPV68, HPV56, HPV58, HPV35, HPV39, HPV18, HPV33 and HPV16. All these genotypes are at high oncogenic risk. Wang et al. (2018) reported the presence of HPV16, HPV58, and HPV52 genotypes as the most common genotypes among women in China. Other authors such as Yuan et al. (2019) reported that HPV52 and HPV58 infection are as common as HPV16 infection.
The importance of knowing the high-risk HPV genotypes circulating in our countries lies in the fact that these genotypes are oncogenic and their involvement in cervical cancer is well established. The presence of these high-risk HPVs in the West African population in our study merits preventive action. The presence of high-risk HPV genotypes in cervical cancers historically confirmed in the West African region must also be taken into account, but genotypes present in the general population should not be overlooked. However, some studies in Benin and Burkina Faso on high-risk HPV genotypes involved in cervical cancer and histologically confirmed precancerous lesions in anatomy and pathological
cytology had reported the presence of HPV genotypes 18, 31, 39, 45, 16, 35, 52 and 58 (Zohoncon et al., 2016a; Zohoncon et al., 2016b). Chen et al. (2018) had identified HPV16, 18, 58, 52, 33, 31, 68, 45, 66 and HPV 39 in invasive cervical cancers and the most predominant were HPV16, 18, 58 and 52. Other studies have reported the presence of HPV genotypes 35, 52, 52, 31, 58, 58, 59, 39, 39, 51, 51, 56, 16, 18, 33, 45, 66 in women in the general population (Zohoncon et al., 2013; Ouedraogo et al., 2015; Traore et al., 2016; Obiri-Yeboah et al., 2017, Ouedraogo et al., 2018). In women with normal cytology in Pakistan, Aziz et al. (2018) reported the presence of high-risk HPV genotypes such as HPV45 (12.5%), HPV33 (8.33%), HPV18 (6.25%) and HPV16 (4.16%). The prevalence of HPV16/18 (bivalent vaccine types) in our study was 7.02% in women of the general population in West Africa. ICO/IARC reported that prevalence of HPV 16 and/or HPV 18 among women with normal cytology in subregion Western Africa was 4.3% (ICO/IARC, 2018). These studies report a relatively low frequency of HPV16 and 18 and a predominance of other high-risk HPV genotypes.
When considering the two types of female population such as women in the general population or women without cervical lesions and women with cervical cancer, the finding seems to be that the accumulation of other high-risk HPV genotypes is higher compared to the accumulation of HPV16 and 18 except in Europe or in some countries of the world where HPV16 and 18 are found in 70% of cervical cancer cases (ICO/IARC, 2018). Cervical cancer is one of the few cancers that can be prevented by controlling HPV infection through prophylactic vaccination and screening / early diagnosis / treatment. The currently available vaccines such as Cervarix (bivalent HPV 16 and 18), Gardasil 4 (quadrivalent HPV16, 18, 6 and 11) and Gardasil 9 (nonavalent HPV 16, 18, 31, 33, 45, 52, 58, 6 and 11) are the prophylactic vaccines that different countries use. HPV vaccines have the potential to reduce the incidence of cervical and other anogenital cancers. But the choice of vaccine should be directed towards effective control of this scourge.
In addition, in our study, 34.87% of women infected with HPV had multiple infections and the number of high-risk HPV genotypes per woman ranged from 1 to 6. Multiple infections with 2 and 3 genotypes were in the majority, with 161/250 (64.40%) and 70/250 (28.00%) respectively. The frequency of high-risk multiple HPV infection in our study is higher than those reported by other authors: 14.9% in China (Wang et al., 2018), 19.8% in the United States (Monsonego et al., 2015), 24.3% in Italy (Panatto et al., 2013)and lower than the 48.1% reported by Kavanagh et al. (2013). These differences can be explained by the size of the study populations, the type of population, the number of genotypes sought and the risk factors. Several multiple infections in this study (for example HPV16/31/39/59/58/68; HPV16/51/52/56/66; HPV31/35/45/52/68; HPV16/39/45/59; HPV16/18/45/52; HPV18/45/52/58) are combinations of HPV genotypes found in invasive cervical cancer, hence the importance of focusing on genotypes found in the general population.
In this study, risk factors were significantly associated with HPV infection among women. Other studies have also noted these risk factors that influence or increase the risk (Monsonego et al., 2015; Aziz, et al., 2018; Ouedraogo et al., 2018). This mapping of high-risk HPV genotypes circulating in women in the general population shows diversity in the distribution of genotypes and raises questions about effective prophylactic actions to control HPV. However, mapping high-risk HPV genotypes in invasive cervical cancer in West Africa would strengthen this control.
This study provides us with a mapping of high-risk HPV among sexually active women in the general population in nine cities in five West African countries. It shows a predominance of the HPV 52 genotype followed by HPV 31, 59, 51, 66, 45, 68, 56, 58, 35, 39, 18, 33 and 16 and a prevalence of high-risk HPV infection ranging from 12 to 50%. The HPV genotypes predominant in the general population in West Africa are not HPV16 and 18. Is it due to viral clearance or genetic mechanisms? As cervical cancer is one of the few preventable cancers, it is crucial to place emphasis on prophylactic vaccination against broad spectrum HPV, adapted to the African context.
The authors wish to thank the "University Agency of Francophony" (AUF) and the CERBA/LABIOGENE, University Joseph Ki-Zerbo. They express their deep gratitude to the Italian Episcopal Conference (IEC).
The authors have not declared any conflict of interests.
REFERENCES
|
Adler D, Laher F, Wallace M, Grzesik K, Jaspan H, Bekker LG, Gray G, Valley-Omar Z, Allan B, Williamson AL (2013). "High rate of multiple concurrent Human Papillomavirus infections among HIV-uninfected South African Adolescents." Journal of Immunological Techniques in Infectious Diseases 2(1):1000106.
Crossref
|
|
|
|
Aziz H, Iqbal H, Mahmood H, Fatima S, Faheem M, Sattar AA, Tabassum S, Napper S, Batool S, Rasheed N (2018). "Human papillomavirus infection in females with normal cervical cytology: Genotyping and phylogenetic analysis among women in Punjab, Pakistan." International Journal of Infectious Diseases 66:83-89.
Crossref
|
|
|
|
|
Chen Z, Zhou J, Chen Y, Zhu J (2018). "Distribution of human papillomavirus genotypes and its relationship to clinicopathology in invasive cervical carcinoma in Zhejiang Province, China." Journal of Cancer Research and Therapeutics 14(4):780-784.
Crossref
|
|
|
|
|
De Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX (2007). "Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis." The Lancet infectious diseases 7(7):453-459.
Crossref
|
|
|
|
|
Djigma FW, Ouedraogo C, Karou DS, Sagna T, Bisseye C, Zeba M, Ouermi D, Gnoula C, Pietra V, Ghilat-Avoid-Belem NW, Sanogo K, Sempore J, Pignatelli S, Ferri AM, Nikiema JB, Simpore J (2011). "Prevalence and genotype characterization of human papillomaviruses among HIV-seropositive in Ouagadougou, Burkina Faso." Acta Tropica 117(3):202-206.
Crossref
|
|
|
|
|
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010). "Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008." International journal of cancer 127(12):2893-2917.
Crossref
|
|
|
|
|
ICO/IARC (Initial Coin Offering /International Agency for Research on Cancer) (2018). Human Papillomavirus and Related Diseases in Burkina Faso, ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre): 70.
|
|
|
|
|
Kavanagh K, Sinka K, Cuschieri K, Love J, Potts A, Pollock KG, Cubie H, Donaghy M, Robertson C (2013). "Estimation of HPV prevalence in young women in Scotland; monitoring of future vaccine impact." BMC Infectious Diseases 13(1):519.
Crossref
|
|
|
|
|
Louie K, Didelot MN, Damay A, Nagot N, Mayaud P, Segondy M (2008). "Papillomavirus humains (HPV) et cancers associés: aspects épidémiologiques." Revue francophone des laboratoires 405:27-34.
Crossref
|
|
|
|
|
Monsonego J, Cox JT, Behrens C, Sandri M, Franco EL, Yap PS, Huh W (2015). "Prevalence of high-risk human papilloma virus genotypes and associated risk of cervical precancerous lesions in a large US screening population: data from the ATHENA trial". Gynecologic Oncology 137(1):47-54.
Crossref
|
|
|
|
|
Obiri-Yeboah D, Akakpo PK, Mutocheluh M, Adjei-Danso E, Allornuvor G, Amoako-Sakyi D, Adu-Sarkodie Y, Mayaud P (2017). "Epidemiology of cervical human papillomavirus (HPV) infection and squamous intraepithelial lesions (SIL) among a cohort of HIV-infected and uninfected Ghanaian women." BMC Cancer 17(1):688.
Crossref
|
|
|
|
|
Ouedraogo CM, Djigma FW, Bisseye C, Sagna T, Zeba M, Ouermi D, Karou SD, Pietra V, Buelli F, Ghilat-Avoid-Belem NW, Sanogo K, Sempore J, Moret R, Pignatelli S, Nikiema JB, Simpore J (2011). "[Epidemiology, characterization of genotypes of human papillomavirus in a population of women in Ouagadougou]." Journal de gynécologie obstétrique et biologie de la reproduction (Paris) 40(7):633-638.
Crossref
|
|
|
|
|
Ouedraogo CM, Rahimy RM, Zohoncon TM, Djigma FW, Yonli AT, Ouermi D, Sanni A, Lankoande J, Simpore J (2015). "[Epidemiology and characterization of high-risk genotypes of human Papillomavirus in a population of sexually active adolescents in Ouagadougou]." Journal de gynécologie obstétrique et biologie de la reproduction (Paris) 44(8):715-722.
Crossref
|
|
|
|
|
Ouedraogo RA, Zohoncon TM, Guigma SP, Angele Traore IM, Ouattara AK, Ouedraogo M, Djigma FW, Obiri-Yeboah D, Ouedraogo C, Simpore J (2018). "Oncogenic human papillomavirus infection and genotypes characterization among sexually active women in Tenkodogo at Burkina Faso, West Africa." Papillomavirus Research 6:22-26.
Crossref
|
|
|
|
|
Panatto D, Amicizia D, Tanzi E, Bianchi S, Frati ER, Zotti CM, Lai PL, Bechini A, Rossi S, Gasparini R (2013). "Prevalence of human papillomavirus in young Italian women with normal cytology: how should we adapt the national vaccination policy?" BMC Infectious Diseases 13(1):575.
Crossref
|
|
|
|
|
Traore IM, Zohoncon TM, Dembele A, Djigma FW, Obiri-Yeboah D, Traore G, Bambara M, Ouedraogo C, Traore Y, Simpore J (2016). "Molecular Characterization of High-Risk Human Papillomavirus in Women in Bobo-Dioulasso, Burkina Faso." BioMed Research International, 7092583.
Crossref
|
|
|
|
|
Traore IMA, Zohoncon TM, Ndo O, Djigma FW, Obiri-Yeboah D, Compaore TR, Guigma SP, Yonli AT, Traore G, Ouedraogo P, Ouedraogo CMR, Traore Y, Simpore J (2016). "Oncogenic Human Papillomavirus Infection and Genotype Characterization among Women in Orodara, Western Burkina Faso." Pakistan Journal of Biological Sciences 19(7):306-311.
Crossref
|
|
|
|
|
Wang X, Ji Y, Li J, Dong H, Zhu B, Zhou Y, Wang J, Zhou X, Wang Y, Peppelenbosch MP, Pan Q, Ji X, Liu D (2018). "Prevalence of human papillomavirus infection in women in the Autonomous Region of Inner Mongolia: A population-based study of a Chinese ethnic minority." Journal of Medical Virology 90(1):148-156.
Crossref
|
|
|
|
|
Watson-Jones D, Baisley K, Brown J, Kavishe B, Andreasen A, Changalucha J, Mayaud P, Kapiga S, Gumodoka B, Hayes RJ, de Sanjose S (2013). "High prevalence and incidence of human papillomavirus in a cohort of healthy young African female subjects." Sexually Transmitted Infections 89(5):358-365.
Crossref
|
|
|
|
|
WHO/ICO World Health Organization/Initial Coin Offering (2009). Human papilloma virus and related cancers in Ethiopia.
|
|
|
|
|
Yuan XW, Li YJ, Qiu Q, Luo ZY, Zhao XF(2019). "Prevalence and genotype distribution of human papillomavirus among 9945 women from the Nanhai area of Foshan". BMC infectious diseases 19(1):1-6.
Crossref
|
|
|
|
|
Zohoncon TM, Bado P, Ouermi D, Traoré E, Djigma F, Traore IMA, Yonli AT, Ouédraogo C, Akpona SA, Lompo O (2016a). "Human papillomavirus genotypes involved in invasive cervical cancer from formalin-fixed, paraffin embedded tissues in Ouagadougou, Burkina Faso." International Journal of Current Research 8(9):39314-39318.
|
|
|
|
|
Zohoncon TM, Bisseye C, Djigma FW, Yonli AT, Compaore TR, Sagna T, Ouermi D, Ouedraogo CM, Pietra V, Nikiema JB, Akpona SA, Simpore J (2013). "Prevalence of HPV High-Risk Genotypes in Three Cohorts of Women in Ouagadougou (Burkina Faso)" Mediterranean Journal of Hematology and Infectious Diseases 5(1):e2013059.
Crossref
|
|
|
|
|
Zohoncon TM, Ouedraogo TC, Brun LVC, Obiri-Yeboah D, Djigma WF, Kabibou S, Ouattara S, Gomina M, Yonli AT, Bazie V, Ouedraogo C, Lompo O, Akpona SA, Simpore J (2016b). "Molecular Epidemiology of High-Risk Human Papillomavirus in High-Grade Cervical Intraepithelial Neoplasia and in Cervical Cancer in Parakou, Republic of Benin." Pakistan Journal of Biological Sciences 19(2):49-56.
Crossref
|
|