ABSTRACT
The recalcitrance of Theobroma cacao L. to somatic embryogenesis, due to non-adapted physiological and metabolical responses to environmental stress, limits its propagation. The present work aims to ameliorate somatic embryogenesis in T. cacao throughout a physiological approach. For this purpose, the influence of the position of flowers buds used as explants was evaluated. Flowers buds were collected from different parts of the tree: orthotropic main stem (OS), primary plagiotropic fan branch (FI) and secondary plagiotropic fan branch (FII). Evolution of some biochemical parameters such as phenolic compounds, soluble sugars, proteins contents and peroxidase activity was followed at different steps of somatic embryogenesis, considering the origin of the explants used. Results obtained show that callogenesis is induced on all explants independently of their origin, with an 80% average frequency. Embryogenesis frequencies were ca 2 fold higher in staminodes-derived calluses from FII and FI than OS. Meanwhile petals of FII do not differentiate embryos. Biochemical analysis shows that the content of phenol is low in calluses during somatic embryo establishment. Explants from FII present the lowest values (after 49th days of culture). Sugars content decrease during callogenesis. When embryos are established the sugars content decrease in explants from OS. During the same period, proteins’ and phenols contents increased in staminodes-derived calluses from all origin; while there was decrease in petals from FI and FII. Buds from fan branch are suitable for somatic embryogenesis process and this capacity correlate with peroxidase activity which decrease during embryos dedifferentiation phase.
Key words: Theobroma cacao L., somatic embryogenesis, proteins, phenols compounds, soluble sugars, peroxidase activity, microclimate.
Theobroma cacao L. (chocolate tree) is grown in the humid tropics and constitutes an important source of incomes for many countries of the West and Central Africa regions. This tree, when grown in wild state, can
reach 12 to 15 m of length (Wood et al., 1985). It is a diploid (2n=20) and preferentially allogamy plant. The root system has a growth dimorphism characterized by an orthotropic development axis, the pivot and plagiotropic lateral ramifications and lateral roots. Cocoa trunk is characterized by a vertical harbor (orthotropic), a phyllotaxis 3/8; long-stalked leaves, axillary orthotropic buds, a defined growth, differentiation of three to five plagiotropic buds under the apex, at the time of the degeneracy of the terminal bud. Cauliflorous tree, inflorescences cymes biparous with reduced inter-node develop at the flowering areas called flower cushions. The flowers are hermaphroditic, with small sizes (diameters ranging from 0.5 to 1 cm), regular and composed of 5 sepals, 5 petals, 5 staminodes, a pistil and an ovary. Pollination is predominantly entomophilous although it can be done manually in the experimental fields. Flowering in cocoa is manifested by the production of a minimum of 50 000 flowers during the term with less than 5% of production pods (Lass, 1999). Cocoa trees demonstrate a high degree of segregation for many traits when propagated by seeds. For this reason, clonal propagation systems such as rooted cuttings and grafting have been applied for multiplication of elite varieties but a vast majority of cocoa plants in production were derived from seeds (Eskes, 2005). The use of in vitro propagation methods for cocoa could potentially contribute to efforts at crop improvement, germplasm conservation, and rapid distribution of new improved varieties.
Somatic embryogenesis is a vegetative propagation method which permits the production of several embryos capable of generating plants similar to the initial one from non-sexual tissues. Somatic embryogenesis has been the most used method these last years for in vitro regeneration of elite genotypes of cocoa (Niemenak et al., 1998; Maximova et al., 2008; Noah et al., 2013). Somatic embryos germinate to yield entire plant with a pivoting root that we cannot obtain by cutting. Temporary immersion culture and suspension culture can enhance embryos multiplication of cocoa (Niemenak et al., 2008). The success of this method is mostly dependent of genotype and culture medium composition. Li et al. (1998) achieved better somatic response from many genotypes using DKW complex salt with floral explants.
Somatic embryogenesis plays an important role in clonal propagation. When integrated with conventional breeding programs and molecular plus cell biological techniques, somatic embryogenesis provides a valuable tool to enhance the pace of genetic improvement of commercial crop species (Stasolla and Yeung, 2003). There are evidences that the main metabolic and developmental processes occurring in the zygotic embryogenesis may be recapitulated in the somatic embryogenesis (Fehér et al., 2003). But the latter are less efficient in converting carbohydrates in lipids and storage proteins during the late developmental stages (Cangahuala et al., 2009). The levels of these substances change along the developmental stages of cells cultures, and their role has been ascribed to the transduction signal cascade or as substrate for cell growth and morphogenesis (Lulsdorf et al., 1992). Storage proteins are the source of amino acids for seed germination (Misra et al., 1993). Proteins could also be involved in the regulation of cell expansion and establishment of biophysical characteristics required for the morphogenesis (Jiménez, 2001). Soluble sugars, such as glucose and sucrose, are involved in the regulation of developmental processes occurring from embryo development to seed maturation (Gibson, 2005).
Generally, when used as source of plant materials for somatic embryogenesis, flowers buttons are collected from the tip of branches on cocoa trees, whereas their production is distributed on the whole plant and is extended all over the year. Due also to the low production and conversion of somatic embryos obtained from different genotypes of T. cacao, leading to important economic lost, a new physiological approach was developed. Assessment of somatic embryogenesis was done using floral explants (staminodes and petals) from different position on the tree: orthotropic main stem (OS), primary plagiotropic fan branch (starting from the first ramification) and secondary plagiotropic fan branch (starting from the second ramification). The present research work objectives were to investigate the mechanism that can help to solve the recalcitrance of T. cacao and to understand the influence of explant position on the tree on biochemical events underlying somatic embryogenesis in this specie.
Preparation of plant materials
The tissue system used in this investigation was the same as the system previously described by Minyaka et al. (2008). Studies were carried out on the cacao genotype “SCA 6”, which is included in the gene-bank of the Institute of Agricultural Research and Development at Nkolbisson (Yaounde, Cameroon). Flowers buds were collected at different positions on the same tree during all the experimentation, including orthotropic main stem (OS), primary plagiotropic fan branch (FI) and secondary plagiotropic fan branch (FII) early in the morning. They were surface sterilized by immersion for 20 min in 3% (w/v) sodium hypochlorite followed by three rinses in sterilized distilled water of 2 min each. Staminodes and petals were excised with scalpels and placed on culture media (in distinct set) into Petri dishes (Figure 1).
Culture medium preparation
All media were defined using Driver and Kuniyuki Walnut (DKW) medium basal salt of Driver and Kuniyuki (1984). The explants were first cultured in primary callus growth medium. Primary callus growth (PCG) medium was supplemented with 250 mgL-1 glutamine, 100 mgL-1 myo-inositol, 1 mLL-1 DKW vitamin stock (100 mgmL-1, 2 mgmL-1 thiamine-HCl, 1 mgmL-1 nicotinic acid and 2 mgmL-1 glycine), 20 gL-1 glucose, 18 μM 2,4-dichloro-phenoxyacetic acid (2,4-D) and 45.4 nM thidiazuron (TDZ). Media
were dispensed into sterilized Petri dishes after autoclaving for 20
min at 1 bar pressure and 121°C. Each Petri dish contains either 30 staminodes or 30 petals. Experiments were repeated 5 times with five replicate Petri dishes at each culture initiation. Petri dishes were incubated in dark at 25 ± 1°C for 14 days. After 14 days in PCG medium, explants were transferred to secondary callus growth (SCG) medium. SCG medium consisted of DKW basal salts, supplemented with 0.5 mLL-1 DKW vitamin, 20 gL-1 glucose, 9 μM 2,4-D, 250 μgL-1 kinetin and 0.22% (w/v) gelrite. Cultures were also incubated at 25 ± 1°C for 14 days in darkness. Cultures from SCG1 medium were transferred in embryo development (ED) medium. ED medium was made of DKW basal salts supplemented with 6.0 mM MgSO4, 1 mL DKW vitamin, 30 gL-1 sucrose, 1 g.L-1, glucose and 0.22% (w/v) gelrite. Cultures were incubated at 25 ± 1°C in darkness for 21 days. Two others sub-cultures of explants were made every 21 days in ED medium for the development of embryos.
Biochemical analysis
At the end (91st days) of each experience (a given culture), calluses of each characteristic development stages of somatic embryogenesis, that is, calluses aged 14, 28, 49, 70 and 91 days, respectively, were collected from different sources of explant and analyzed independently.
Estimation of phenolic contents
Phenolic compounds were extracted as described by El Hadrami (1995) and Macheix et al. (1990). Embryonic mass (100 mg) were ground in chilled mortars with 2 ml of 80% (v/v) methanol at 4°C. After incubation, tubes were centrifuged thrice at 7000 g for 30 min, supernatant were recuperated each time. Mixture of the three supernatants constituted the crude extract. Total phenols were quantified using the method described by Singleton and Rossi (1965). 15 μl of alcoholic extract were added to Folin-Ciocalteu reagent (250 μl), 2.5 mL of distilled water and 0.5 ml of sodium carbonate (20 %). The mixture was incubated at 40°C for 20 minutes and the blue color was determined at 760 nm. The content of soluble phenolic was expressed in mg-equivalent of gallic acid per fresh weight (FW).
Estimation of soluble sugars contents
Soluble sugars were extracted according to the modified method of Babu et al. (2002). Biological material (400 mg) was ground in mortar with 2 mL of 80% (v/v) ethanol and centrifuge at 6000 g for 20 min. The supernatant was collected and constituted the crude extract. 50 μL of this alcoholic extract was added to 5 mL of Anthron reagent, homogenized and incubated at 80°C for 20 min.
After cooling in melting ice, the absorbance of the green complex formed was determined at 620 nm. Glucose was use as standard.
Estimation of protein contents and POX activity
Calluses (500 mg) were ground in chilled mortar with 2 mL of TAMET buffer (0.5 M Tris, 0.3 M ascorbic acid, 0.2% (v/v) β-mercaptoethanol, 0.01 M EDTA and 0.02% (v/v) Triton X 100, pH 6.7); and 0.125 g polyvinylpyrrolidone was added in the medium. The crude homogenate was centrifuged for 15 min at 20000g and 4°C. The supernatant was removed and used as crude extract for proteins and enzymes assays. Proteins were quantified according to Bradford (1976) method. Peroxidase (POX) activity was measured spectrometrically at 420 nm by the guaiacol-H2O2 method
of Erdelsky and Fric (1979). The specific activity of the enzymes was expressed as the change in optical density per mg of fresh weight (FW).
Statistical analysis
Data were subjected to statistical analysis using SPSS software version 16.0. Analysis of variance was performed where applicable and differences between means were determined using LSD and Tukey Test.
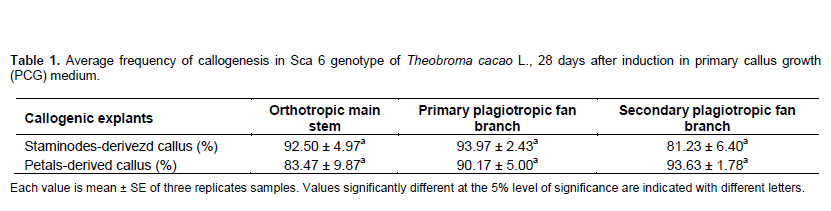
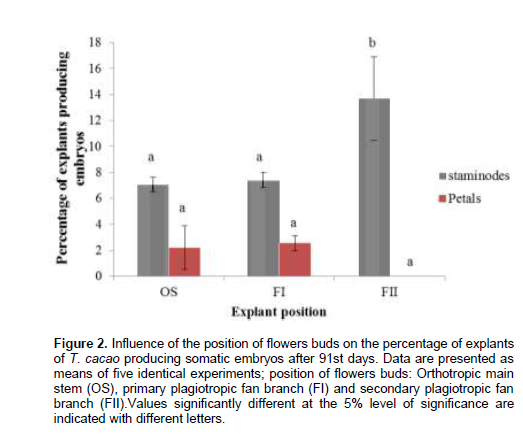
The results showed that calluses were distinguishable on floral explants after 4 to 5 days of cultivation in the primary growth medium. Callus development was well established in secondary callus growth medium, and was quite similar in tissues from different origins, according to their morphological aspects (Figure 1). There were no significant differences (pË‚0.05) among the average frequency of callogenesis (after 28 days) when explants were cultivated in DKW medium containing plant regulators. However, callus growth was most influenced by the type and origin of explant used. The average callogenesis frequency was up to 80% in T. cacao tissues, with the highest value obtained with staminodes explants collected on FI (93.97±2.43%) (Table 1). By the end of three-week in embryo development medium, some calluses differentiated roots or embryos. The earliest somatic embryos were observed between 49 and 55 day of culture on embryogenic calluses. Petals-derived calluses collected on secondary branches do not differentiate somatic embryos during all the experiment (91 days). The percentage of explants producing somatic embryos is highly influenced by the nature and the origin of explant cultivated in culture media (Figure 2). No statistical differences (pË‚0.05) were found among the rate of petals-derived calluses producing somatic embryos from different origin. Whereas, in staminodes-derived calluses, this rate decrease significantly when moving up from OS to FII. In fact, this rate of explants-derived calluses producing somatic embryos were approximately 3 times higher in staminodes tissues than petals after 91 days of culture, with the highest value obtained with staminodes-derived calluses from FII (13.70±3.21%). As observed, embryogenic and non-embryogenic tissues of T. cacao calluses were discernable based on coloration; embryogenic calluses were brown (phenolized) and friable, while non-embryogenic were white and rough. Somatic embryos undergo different developmental stages as shown by the result: globular, heart shaped, torpedo and cotyledonnary stage (Figure 1C, D, E and F). Cotyledonnary stage constituted the last development stage. At this stage, somatic embryos submitted to maturation treatment were able to germinate and generate plantlets (Figure 1G, H, and I).
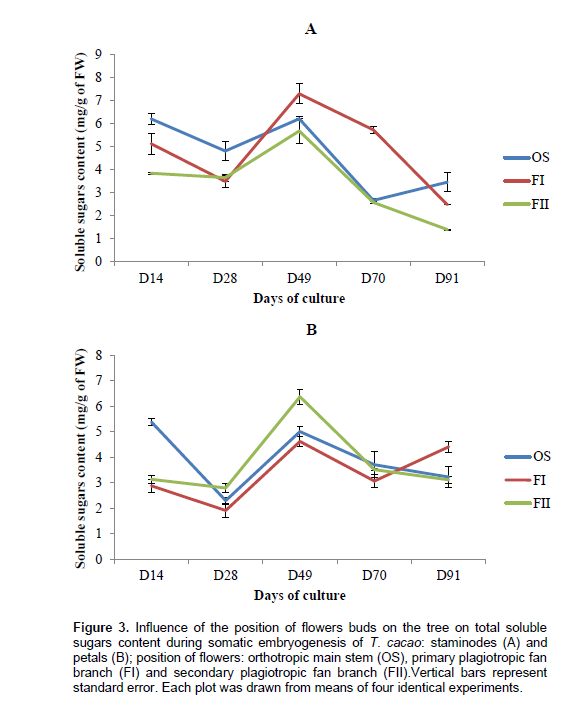
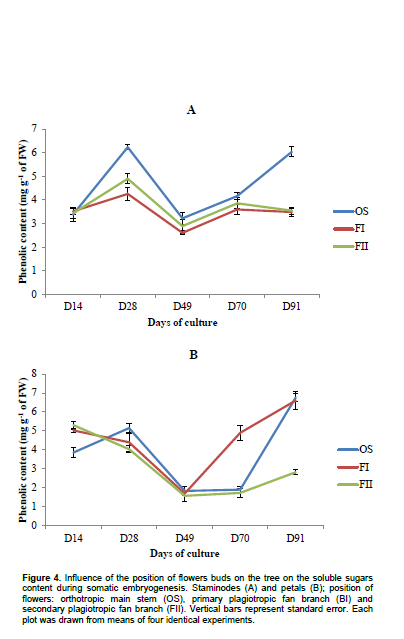
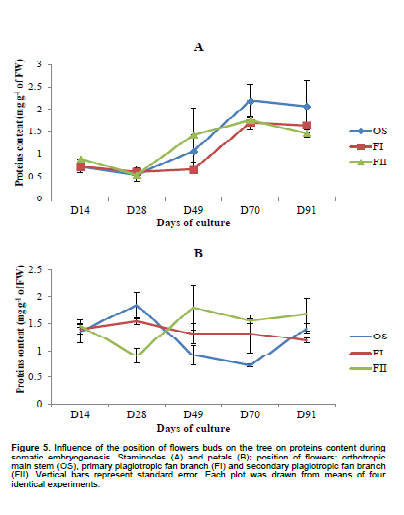
Biochemical analysis showed that the total content of polyphenols compounds in staminodes-derived calluses ranged from 2.16 ± 0.15 to 8.04 ± 0.22 mg g-1 of FW in OS, 1.69 ± 0.08 to 6.61± 0.49 mg g-1 of FW in FI and from 1.73± 0.23 to 4.9± 0.33 mg g-1 of FW in FII (Figure 3). There is no regular evolution of this determinant during somatic embryogenesis process in both types of explants (staminodes or petals) from the same position on the tree. After 28th days (corresponding to induction step), total phenols compounds increased in staminodes-derived calluses (84, 21 and 43%, for OS, FI and FII, respectively). Constitutively, soluble carbohydrates were low in calluses from all origins (Figure 4). Statistical differences (p<0.05) were found in the amount of carbohydrates between OS and FII calluses after 28th days. Whereas no statistical difference were found between the amount of sugars in calluses from FI and FII during this period, considering staminodes and petals tissues. Callogenesis in this case was characterized by a decrease in carbohydrates content. Among petals-derived calluses, those from FII displayed the highest amount of carbohydrates (6.37±0.3 mg g-1 of FW after 49 days), and OS the lowest (1.6±0.18 mg g-1 of FW after 28 days); While decreasing progressively in others stagesbetween 0.8 and 36% (in 70-day-old OS and 90-day-old FI, respectively). Except for calluses from FI and FII aged 14 days, the same observation was made in petals. The protein contents in staminodes-derived calluses varied from 0.537±0.178 mg g-1 (FII after 28 days) to 2.181±0.372 mg g-1 of FW (OS after 70 days), whereas in petals-derived calluses, it varied from 0.724± 0.03 mg g-1 (OS after 70 days) to 1.8495± 0.237 mg g-1 of FW (OS after 28 days). Protein content was significantly higher in petals-derived calluses from FII aged 49 days (1.809 ± 0.415 mg g-1 of FW) than in OS (0.911 ± 0.178 mg g-1 of FW) from the same source. It appears that, cells differentiation is characterized by high protein synthesis in staminodes tissues (Figure 5).
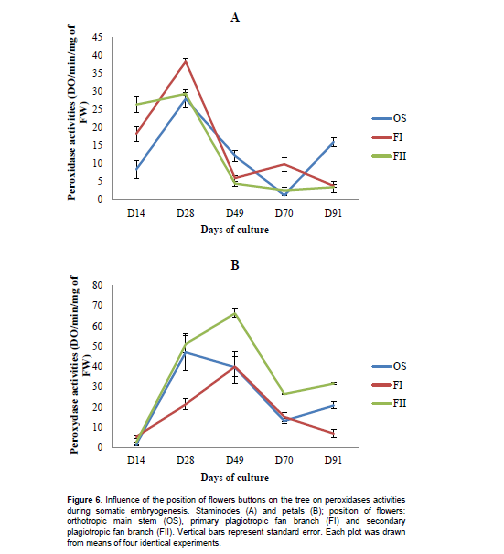
Peroxidases activities presented pattern related to a source of morphogenetic structure. The activity in staminodes-derived calluses after 49 days was 2.7 and 2.0 fold higher in OS than in FII and FI, respectively. There was no significant difference (p<0.05) among peroxidases activities in staminodes-derived calluses at any developmental stage of this experiment from each source of tissues. However, this value, after 28 days, was significantly higher in petals-derived calluses from BII as compared to OS and FI (Figure 6). Staminodes-derived calluses from OS presented the lowest activity (after 70 days).
Somatic embryogenesis is the process by which somatic cells, under inductive conditions, generate embryogenic cells, which undergo a series of morphological and biochemical changes resulting in the formation of somatic embryos (Schmidt et al., 1997; Komamine et al., 2005; Businge et al., 2013). Somatic embryogenesis forms the basis of cellular totipotency that is unique to higher plants. Currently, this clonal technique is considered to represent a prominent in vitro regeneration system for cocoa. Somatic embryogenesis allows rapid regeneration of elite genotypes, germplasm conservation and genetic transformation system (Maximova et al., 2002). Unfortunately, the recalcitrance of T. cacao to this technique and numerous factors that control it limit its systematical exploration. In this study, a comparative approach was applied to study physiological differences among T. cacao explants-derived calluses from different origins with the hope to understand the complexity of this phenomenon (recalcitrance). The influence of the position of flowers buds on the tree on somatic embryogenesis of “Sca 6” genotype, known as highly productive in farm was analyzed. The variation of phenols compounds, total soluble sugars, proteins contents and peroxidases activities was also performed in these conditions.
Results showed that both explants types used (staminodes and petals) are favorable to callogenesis with an average frequency above 80% in all callus-derived explants used in this experimentation. These results are in agreement with those previously obtained by Li et al. (1998) and Minyaka et al. (2009). These authors showed that callogenesis was effective in T. cacao using floral explants. The action of 2.4-dichloro-phenoxyacetic acid (2.4-D) and that of thidiazuron (TDZ) are responsible for this callogenesis. A synergic and/or a complementary effect of auxin/cytokinin action in induction of somatic embryogenesis process were reported in many species such as Ricinodendron heudelotti (Fotso et al., 2007) and Vitis vinifera (Olah et al., 2009). This callogenesis success could be due to the high mobilization of soluble sugars during this development step. This might consolidate the fact that reducing carbohydrates are important for calluses formation and cell differentiation (Ana et al., 1997). They regulate osmotic pressure (Blanc et al., 1999) and are major components of cell wall. In cocoa, the use of these metabolites is origin-dependent, as demonstrated by this study. And this could be justified by enzymatic equipment which varies in composition and/or function in cells of flowers buds according to their position on the tree. Study done by Alemano et al. (2003) showed that flowers buds of cocoa contain different types of phenolic compounds, and each type may be expressed qualitatively and quantitatively according to the developmental stage, indicating the key role played by these metabolites in the regulation of cell differentiation events.
Somatic embryogenesis in T. cacao is nature-and origin-dependent as shown by the present study data, but the last factor seems to be the most important. In fact, the average frequency of calluses producing somatic embryos increased when we move up from the orthotropic main stem to the secondary plagiotropic fan branch (with a maximum in staminodes-derived calluses from FII). Except for flowers buds from FII, the same observation is made for calluses of petals, as these last do not differentiate somatic embryos. Thus, somatic embryogenesis is an environmental stress-response Figure 4. Influence of the position of flowers buds on the tree on the soluble sugars content during somatic embryogenesis. Staminodes (A) and petals (B); position of flowers: orthotropic main stem (OS), primary plagiotropic fan branch (BI) and secondary plagiotropic fan branch (FII). Vertical bars represent standard error. Each plot was drawn from means of four identical experiments. Figure 5. Influence of the position of flowers buds on the tree on proteins content during somatic embryogenesis. Staminodes (A) and petals (B); position of flowers: orthotropic main stem (OS), primary plagiotropic fan branch (FI) and secondary plagiotropic fan branch (FII). Vertical bars represent standard error. Each plot was drawn from means of four identical experiments.
process, imposed on culture medium, and this response depends on the expression of genes present in tissues (Johnson et al., 1997). This low rate of somatic embryos produced can be explained by the recalcitrance of this crop to somatic embryogenesis process.
Soluble sugars content was high in calluses from all origins during embryos dedifferentiation step in primary and secondary plagiotropic fan branches. This increase could be due to the important role play by carbohydrates metabolism in organogenesis and morphogenesis. During embryos differentiation steps, this phenomenon is progressively inversed. Moreover during callogenesis, there is a decrease of carbohydrates content in flowers buds suggesting an important utilization of these metabolites. Zygotic and somatic embryos both highly accumulate enzymes of carbohydrate metabolism as demonstrated by several studies (Iraqi et al., 2001; Hendriks et al., 2003; Noah et al., 2013). The explanation for extensive carbohydrate metabolism is the heavy energy demand required for processes that occur during cell division and elongation. One of the factors generally considered as responsible for in vitro recalcitrance of cocoa is the high polyphenol compounds content and their oxidation. In this experiment, a high accumulation of these metabolites was particularly observed during callogenesis and embryos differentiation steps (from 70th to 91st day). Each type of explants used expressed a significant decrease in phenols compounds during embryos dedifferentiation stage (after 49th day) when grown in DKW medium supplemented with sulphate, with the lowest content reported in petals-derived calluses from secondary plagiotropic fan branch. There are several internal and external factors affecting the quality and/or the quantity of polyphenol compounds in plants. The quantitative differences registered within explants-derived calluses from different source could be explained, at least in part, by the interaction of several genetic, physiological, agronomic (position of flower bud on the tree), and environmental factors (microclimate) modifying the final concentration in each flower (Roubelakis-Angelakis and Kliewer, 1986). Besides light and temperature (Wang and Zheng, 2001), the availability of plant nutrients also has a great influence on the accumulation of polyphenols (Francis and Atwood, 1961; Doak and Miller, 1968; Piccaglia et al., 2002). Finally, a high accumulation of polyphenol compounds in floral explants during induction steps has been demonstrated to be not favorable to somatic embryogenesis process. A similar result was obtained in date palm (Phoenix dactylifera L.) by Zouine and El Hadrami (2004). Peroxidase activity was high in calluses during induction steps of somatic embryogenesis and low during dedifferentiation step (after 49th day of subculture). This result might underline the implication of this peroxidase in somatic embryogenesis. In fact, the controversy of the higher peroxidase activity during dedifferentiation step of somatic embryogenesis in calluses of petals could justify their low embryos production capacity as compared to their staminodes counterpart.
In conclusion, the data presented here clearly demonstrate the efficiency of floral explants from different position on the tree to somatic embryogenesis process. The comparison between flowers buds carried by the secondary fan branch, commonly used to regenerate T. cacao plantlets, and those from the main stem and primary fan branch showed many differences in physiological responses. The most pronounced difference among the tree types of explants-derived calluses concern with carbohydrates metabolism: FII and FI biochemical evaluation displayed a low utilization of carbohydrates, while OS explants are characterized by intensive glycolytic activities as documented by the exceptional decreased of sugars content during somatic embryogenesis. The absence of embryos dedifferentiation on petals-derived calluses from FII has been connected, at least in part, to changes in specific enzymes abundances. The results suggest that stress factors (microclimate) and genetic factors affect embryogenic capacity of floral explants in T. cacao and thereby reduce the regeneration frequency.
The authors have not declared any conflict of interests.
REFERENCES
|
Alemano L, Ramos T, Gargadenec A, Andary C, Ferriere N (2003). Localization and identification of phenolic compounds in Theobroma cacao L. somatic embryogenesis. Ann. Bot. 92:613-623.
Crossref
|
|
|
|
Ana BM, Yolanda C, Hilario G. Piedad G, Oscar H, Luisa M, Ana D, Nieves V (1997). Differences in the contents of total sugars, reducing sugars, starch and sucrose in embryogenic and non-embryogenic calli from Medicago arborea L. Plant. Sci. 154:143-151.
|
|
|
|
Babu S, Shareef M, Shetty P, Shetty T (2002). HPLC method for amino acids profile in biological fluids and inborn metabolic disorders of aminoacidopathies. Indian. J. Clin. Biochem. 17(2):7-26.
Crossref
|
|
|
|
Blanc G, Michaux- Ferriere N, Teison C, Carron MP (1999). Effect of carbohydrate addition on the induction of somatic embryogenesis in Hevea Brasiliensis. Plant Cell Tissue Organ Cult. 59:103-112.
Crossref
|
|
|
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72:248-254.
Crossref
|
|
|
|
Businge E, Bygdell J, Wingsle G, Moritz T, Egertsdotter (2013). The effect of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol. Planta. 149:273-285.
Crossref
|
|
|
|
Cangahuala-Innocente GC, Steiner N, Maldonado SB, Guerra MP (2009). Patterns of protein and carbohydrates accumulation during somatic embryogenesis of Acca sellowiana. Pesq. agropec.
Crossref
|
|
|
|
Doak KD, Miller PR (1968). Influence of mineral nutrition on pigmentation in sorghum. Agro. J. 60:430-432.
Crossref
|
|
|
|
Driver JA, Kuniyuki AH (1984). In vitro propagation of paradox walnut root stock. Hort. Sci. 19:507-509.
|
|
|
|
El Hadrami I (1995). L'embryogenèse somatique chez Phoenix dactylifera L.: quelques facteurs limitant et marqueurs biochimiques. Thèse de Doctorat, d'Etat Université Cadi Ayyad, Faculté des Sciences-Semlalia, Marrakech, P 227.
|
|
|
|
Erdelsky´ K, and Fricˇ F (1979). Practical and analytical methods in plant physiology. SPN, Bratislava.
|
|
|
|
Fehér A, Pasternak TP, Dudits D (2003). Transition of somatic plant cells to embryogenic state. Plant Cell Tissue Org. Cult. 74:201-228.
|
|
|
|
Fotso, Donfagsiteli TN, Sanonne, Omokolo ND (2007). Effet des phytohormones exogènes sur l'évolution de certains paramètres biochimiques au cours de l'embryogenèse de Ricinodendron heudolotii. Baill. Fruits 62:302-315.
|
|
|
|
Francis FJ, Atwood WM (1961). The effect of fertilizer treatment on the pigment content of cranberries. Proc. Am. Hort. Sci. 77:351-358.
|
|
|
|
Gibson SI (2005). Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 8:93-102.
Crossref
|
|
|
|
Hendriks JHM, Kolbe A, Gibon Y, Stitt M, Geigenberger P (2003). ADP-Glucose pyrophosphorylase is activated by posttranslational redox modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 133:838-849.
Crossref
|
|
|
|
Iraqi D, Tremblay FM (2001). Analysis of carbohydrate metabolism enzymes and cellular contents of sugar and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J. Exp. Bot. 52:2301-2311.
Crossref
|
|
|
|
Jiménez VM (2001). Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev. bras. Fisiol. Vegetal 13:196-223.
Crossref
|
|
|
|
Komamine A, Murata N, Nomura K (2005). Mechanisms of somatic embryogenesis in carrot suspension cultures – morphology, physiology, biochemistry, and molecular biology. In vitro Cell. Dev. Biol. Plant 41:6-10.
Crossref
|
|
|
|
Lass RA (1999). Cacao growing and harvesting practices. In: Knight, I. (Ed.), Chocolate and Cocoa Health and Nutrition. Blackwell Science Ltd., Oxford, London, pp. 11-42.
|
|
|
|
Li Z, Traore A, Maximova S, Guiltinan MJ (1998). Somatic embryogenesis and plant regeneration from floral explant of cacao (Theobroma cacao L.) using thidiazuron. In vitro Cell. Dev. Biol. Plant 34:293-299.
Crossref
|
|
|
|
Lulsdorf MM, Tautorus TE, Kikcio SI, Dunstan DI (1992). Growth parameter of somatic embryogenic suspension culture of interior spruce (Picea glauca-engelmannii complex) and black spruce (Picea mariana Mill.). Plant Sci. 82:227-234.
Crossref
|
|
|
|
Macheix JJ, Fleuriet A, Billot J (1990). Fruit Phenolics. Florida: CRC press. Inc. Boca Ranton, P 378.
|
|
|
|
Maximova SN (2008). Field performance of Theobroma cacao L. plants propagated via somatic embryogenesis. In vitro Cell. Dev. Biol. Plant 44:487-493.
Crossref
|
|
|
|
Maximova SN, Alemano L, Young A, Ferriere N, Traore A, Guiltinan MJ (2002). Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma cacao L. In Vitro Cell. Dev. Biol. Plant 38:252-259.
Crossref
|
|
|
|
Minyaka E (2009). Métabolisme du soufre et embryogenèse somatique chez Theobroma cacao L. (Malvaceae). Thèse de Doctorat/Ph.D, Université de Yaoundé I, Université de Cocody/Abidjan, 132 p.
|
|
|
|
Minyaka E, Niemenak N, Fotso, Sangare A, Omokolo ND (2008). Effect of MgSO4 and K2SO4 on somatic embryo differentiation of Theobroma cacao L. Plant Cell Tissue Organ Cult. 94:149-160. Minyaka E, Niemenak N, Issali EA, Sangare A, Omokolo ND (2010). Sulphur depletion altered somatic embryogenesis in Theobroma cacao L. Biochemical difference related to sulphur metabolism between embryogenic and non-embryogenic calli. Afr. J. Biotechnol. 9(35):5665-5675.
|
|
|
|
Niemenak N (1998). Recherche des marqueurs biochimiques et modification histologique au cours de la callogenèse et de l'embryogenèse somatique in vitro chez Theobroma cacao L. Thèse de doctorat 3ème cycle, Université de Yaoundé, P 138.
|
|
|
|
Niemenak N, Saare-Surminski K, Rohsius C, Omokolo ND, Lieberei R (2008). Regeneration of somatic embryo in Theobroma cacao L. in Temporary immersion bioreactor and analyses of free amino acids in different tissues. Plant Cell Rep. 27:667-676.
Crossref
|
|
|
|
Noah MA, Niemenak N, Sunderhaus S, Haase C, Omokolo ND, Winkelmann T, Braun HP (2013). Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J. Prot. 78:123-133.
Crossref
|
|
|
|
Olah R, Zok A, Pedryc S, Howard, Kovacs LG (2009). Somatic embryogenesis in a broad spectrum of grapes genotypes. Sci. Hort. 120:134-137.
Crossref
|
|
|
|
Piccaglia R, Marotti M, Baldoni G (2002). Factors influencing anthocyanin content in red cabbage (Brassica oleracea var capitata L f rubra (L) Thell). J. Sci. Food Agric. 82(13):1504-1509.
Crossref
|
|
|
|
Roubelakis-Angelakis KA, Kliewer WM (1986). Effect of exogenous factors on anthocyanins and total phenolics in grape berries. Am. J. Enol Vitic. 37:275-280.
|
|
|
|
Schmidt EDL, Guzzo F, Toonen MAJ, De Vries SC (1997). A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124(10):2049-2062.
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimeter of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticulture 16:144-158.
|
|
|
|
Wang SY, Zheng W (2001). Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 49:4977- 4982.
Crossref
|
|
|
|
Wood GAR, Lass RA (1985). Cacao. Longman Scientific and Technical (ed, England), P 620.
|
|
|
|
Zouine L, El Hadrami I (2004). Somatic embryogenesis in Phoenix dactylifera L.: effect of exogenous supply of sucrose on Proteins, Sugars and Peroxidases activities during the Embryogenic Cell Suspension Culture. Biotechnol. 3(2):114-118.
Crossref
|