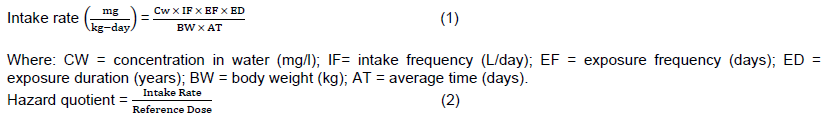ABSTRACT
Recent increases in the frequency and severity of toxic algae blooms in freshwater lakes has been a major concern for small communities that rely on them for drinking water supply. A hazard quotient approach to risk characterization is employed to analyze the effectiveness of five conventional treatment methods for removal of cyanotoxins. The application of the method for risk assessment and mitigation is demonstrated for five case studies, including Lake Champlain (Quebec), Coal Lake (Alberta), Butte Lake (Alberta), Kubbani Lake (Nigeria) and Bomo Lake (Nigeria).
Key words: Cyanotoxins, human health, hazard quotient, lake water intake, water quality.
Agricultural non-point source pollution combined with global warming have caused major algal blooms in our freshwater rivers and lakes (Asnaashari et al., 2015; Stang et al., 2016; Gazendam et al., 2016). Cyanobacteria are photosynthetic prokaryotes that thrive well in various kinds of habitats ranging from freshwater and marine environments to hot springs and deserts (Duy et al., 2000; Ballot et al., 2003, 2010; Baxa et al., 2010; Bogialli et al., 2013; Chia et al., 2009, Chia and Kwaghe, 2015). They are popularly referred to as blue-green algae but their physiological, morphological and metabolic structures clearly identify them as bacteria. The photosynthetic origin of cyanobacteria is similar to that of algae but the pigments are located in the thylakoids, which is in the cytoplasm (Chorus et al., 2000; Codd et al., 2005; Drabkova and Marsalek, 2007; Echenique et al., 2014; Ostermaier and Kurmayer, 2010; Sayyad et al., 2015).One of the basic “metabolic processes of cyanobacteria” is the fixation of di-nitrogen in aerobic conditions using nitogenase, an enzyme that converts di-nitrogen to ammonium. This process enhances a bloom of cyanobacteria in surface waters (Ernst et al., 2006; Fastner et al., 2007; Fawell et al., 1999; Fischer et al., 2000; Graham et al., 2010; Gilroy et al., 2000; Harvey et al., 2015). Cyanobacteria have many properties which result in their relative success and predominance during the blooming season. The following factors are responsible for cyanobacteria blooms in aquatic habitat: aquatic temperatures above 25°C, low light intensity in water, and low nitrogen-to-phosphorous ratios (Hans and Timothy, 2013; Heisler et al., 2008; Hrudey et al., 1999; Griffiths and Saker, 2003; Kaushik and Balasubramanian, 2013; Paerl et al., 2011; Tencalla and Dietrich, 1997).
Presently,there about 3000 known species of cyanobacteria but not all produce toxins. Due to the increasing numbers of cases of cyanobacteria blooms, the occurrence of several harmful cyano-toxins in water supplies has also increased. There is growing concern about the potential for negative health effects on humans due to these toxins (Keijola et al., 1989; Baker et al., 2015; Makarewicz and Lewis, 2015; Merel et al., 2012; Mohamed et al., 2015).
Notwithstanding the recent scientific and technical advances in drinking water treatment plants around the globe, the concentrations of cyanobacterial-toxins have been reported to increase in treated drinking relative to raw water source for small water treatment plants. These toxins enter water supplies after lysis of cyano-bacterial cells, as induced by water collection and treatment activities resulting in subsequent release of toxins in finished treated waters. The toxins released by blue-green algae cannot be removed by conventional treatment methods (Merel et al., 2010; Newcombe and Nicholson, 2002; Nicholson et al., 2003; Szlag et al., 2015; Westrick et al., 2010; Zamyadi et al., 2012).
Drinking water treatment processes may cause breakthrough of toxins into treated drinking water as demonstrated by Zamyadi et al. (2012) in a full-scale water treatment plant system. The passage of toxins, toxic cells and cell debris through filtration systems inhibited chlorination that resulted in the breakthrough of microcystins, resulting in exceedance of Canadian and WHO water quality standards for treated drinking water. Long-term consumption of water contaminated by cyanobacteria and algae is known to cause liver failure, cardiac arrhythmia, dysfunction of the nervous system and skin tumors (Ontario Health Unit, 2014; Farrer et al., 2015).
The objective of this study is to evaluate the use of conventional treatment options and riverbank filtration for managing cyano-toxins, to assess the vulnerability of existing municipal drinking water facilities using surface water sources.
The application of the hazard index is demonstrated for five case studies, including Lake Champlain (Quebec), Coal Lake (Alberta), Butte Lake (Alberta), Kubbani Lake (Nigeria) and Bomo Lake (Nigeria).
Lake Champlain’s Missisquoi Bay (Quebec)
The population is about 330,000. The Missisquoi River is 130 km long and a tributary of Lake Champlain. The Missisquoi River cuts across Vermont in the United States and southern Quebec in Canada located between Latitude 45° and 0’ E and longitude 72° and 35’ due west. The Missisquoi River catchment area covers the Green mountains along the US-Canada border (Lake Champlain and Eastern townships in Quebec before emptying into Lake Champlain’s Missisquoi Bay at Richford (Zamyadi et al., 2012).
Coal Lake, City of Wetaskiwin (Alberta)
The population is about 12,525. Wetaskiwin is a city in the Province of Alberta Canada (coordinates 52°N and 113°W). Wetaskiwin is located 70 km south of Edmonton; it sits on what was “formerly a coast of the large sea that covered much of Alberta, millions of years ago”. Wetaskiwin sits at an elevation of 760 m. Coal Lake, a reservoir developed on the Battle River, is on the east side of the City (Zurawell, 2002).
Butte Lake, Town of Picture Butte (Alberta)
The population is about 1650. Picture Butte is a city in southern Alberta (coordinates 49° 52’ N and 112° 46’ W). Picture Butte has a landmass of 2.9 km2 and sits at an elevation of 900 m, 27 km north of the City of Lethbridge. Old-man River and Picture Butte Lake is the sources of drinking water for this town (Zurawell, 2002).
Kubanni Lake (Nigeria)
The population of this community is about 560,000. The coordinates of this water body are 11° 08’N and 07° 43’E. It has two major tributaries: Kampagi River and Kubanni River. Kubanni Lake is the major source of water to Ahmadu Bello University community and its environs. Surrounding the Kubanni Lake catchment is agricultural land; the farmers in this location utilize agricultural practices that are not environmentally sustainable for the lake (Chia and Kwaghe, 2015).
Bomo Lake (Nigeria)
The population is about 300,000. This lake is 6 km north west of ABU Zaria; it is located 11° 12’N and 07° 38’E and about 671 m above sea level (Chia et al., 2009).
Due to variations in the species, concentrations and locations of cyanotoxins, appropriate analytical methods are required in order to understand the abundance and occurrence and the potential toxicity of cyanobacterial populations, and to effectively assess the risk of cyanotoxins to humans in drinking water supplies (Kutovaya and Watson, 2014).
Data for small drinking water treatment plants in Eastern Township Quebec, Wetaskiwin Alberta, Picture Butte Alberta, Kubanni and Bomo Lake in Zaira Nigeria are adapted from different literature before, and after conventional treatment. Data are collected and monitored on a weekly basis for spring and summer seasons for the raw water samples from these plants; the changing characteristic of the toxins in the raw water and treated drinking water are the major foci for the drinking water data collected.
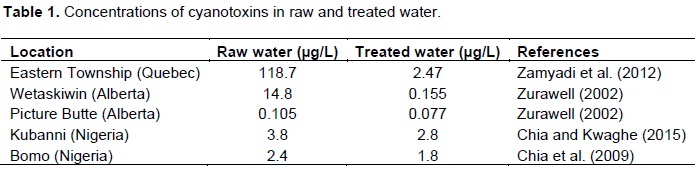
Table 1 lists the concentrations of cyanotoxins in raw and treated water for the selected study regions. The data in Table 1 are in agreement with the removal efficiencies of Cyanotoxins using the conventional treatment range from 25 to 99%. Apart from Picture Butte in Table 1, the concentrations of cyanotoxins in the raw water for all other locations are above the Canadian guidelines for microcystins in drinking water. Further, the concentrations of cyanotoxins in the treated waters, with the exception of Wetaskiwin and Picture Butte, remained above WHO and Canadian guideline.
Risk assessment of cyanotoxins
A hazard quotient is the ratio of the potential exposure to a substance and the level at which no adverse effects are expected. Risk assessment using the intake rate, hazard quotient and hazard indices using the equations presented below, are described to determine the risk associated with drinking water contaminated with cyano-bacterial toxins.
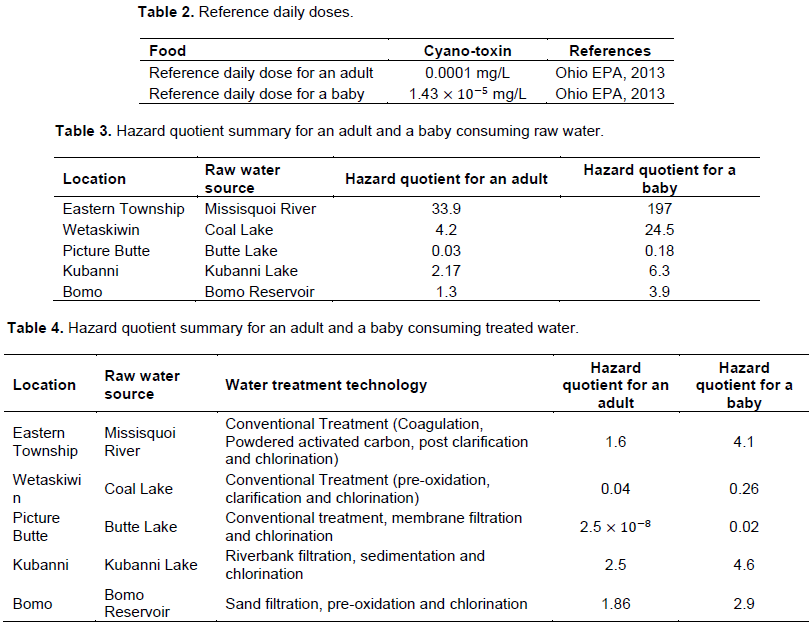
The intake rate and hazard quotients for consuming raw water, water after conventional treatment in the two geographical locations are calculated using Equations 1 and 2 using the data presented in Table 2. The calculation conditions below are used in deriving Tables 3 and 4.
Table 3 summarizes the hazard quotient (the ratio of the potential exposure to a substance and the level at which no adverse effects are expected) of raw water and cow milk for an adult and a baby drinking raw water. From the hazard quotient values presented in Table 3, the results indicate that the Butte Lake is the only safe drinking water in terms of microcystins.
Table 4 presents a summary that relates the hazard index of treated drinking water for an adult and a baby to the water treatment technology used drinking water treatment plants; from the hazard quotient values presented in Table 4, the results indicate that a combination of conventional treatment and membrane filtration which is used in Picture Butte is an efficient technique for removing cyanotoxins.
Table 1 was used together with Equations 1, 2, and 3 to calculate the non-carcinogenic risk for a person that weighs 70 kg and drinks 2 L (Canada) and 4 L (Nigeria) of water daily. The individual drank directly from the contaminated lake. Furthermore, the same approach is performed for a baby that weighs 10 kg and drinks 0.24 L of water per day.
A hazard quotient is the ratio of the potential exposure to a substance and the level at which no adverse effects are expected. The hazard quotients for an adult and a baby consuming raw water are summarized in Table 3. The hazard quotient for an adult consuming raw water in four communities (study areas in Canada and Nigeria) except Picture Butte exceeded unity. This was similar to the hazard quotient for a baby consuming raw untreated water for all the communities under study with the exception of Picture Butte.
The hazard quotients for an adult and a baby consuming treated drinking water is presented in Table 4; for an adult in Eastern Township, Kubanni and Bomo consuming treated drinking water the hazard quotient is greater than one, while for an adult in Wetaskiwin and Picture Butte consuming treated drinking water, the hazard quotient is less than one. Similarly, the hazard quotient result for a baby consuming treated drinking water in Eastern Township, Kubanni and Bomo exceeds 1, while for a baby consuming treated water in Alberta (Wetaskiwin and Picture Butte), the hazard quotient is less than one.
The presences of cyanotoxins in treated drinking water supply from small water treatment plants reaffirms the need to assess the risk of cyanotoxins from these small water treatment plants. In this study, the hazard quotients for an adult and a baby were calculated for a single exposure of treated water for the five treatment plants. The summary calculations are shown in Table 3. The results indicate that a combination of conventional treatment and membrane filtration used in Picture Butte (Table 4) is an efficient technique for removing cyanotoxins. The other Canadian water treatment plants that use combination of coagulation, activated carbon, oxidation, clarification and chlorination have shown a high percentage of removal of cyanotoxins (greater than 97%). This highlights the importance of adopting a more efficient water treatment technologies in affected areas to minimize the risk to a human receptor. On the other hand, in Nigeria, a simple sand filtration or riverbank filtration combined with only chlorination was found to be ineffective in reducing the cyanotoxins concentration to a safe and tolerable level. The efficiency of cyanotoxins removal for both treatment systems in Nigeria were found to around 25%. The people living in these two communities are exposed to elevated concentration of toxin in their drinking water supply.
The results in the different communities included in this study show clearly that hazard quotient for babies are much higher than that for adults. Excluding Picture Butte community, the increased risk for babies compared to adults is between 1.6 and 4.1 to all other four communities. Moreover, all the results above were calculated based on a single exposure (drinking water). Therefore, it is expected that the risk index, which is defined as the sum of hazard quotients due to different exposures, is also alarming. Other exposures that were not considered in this study are food crops irrigated with cyanotoxins-contaminated water, inhalation of water aerosols, and other water sports like swimming and surfing (Queensland Health, 2001).
The assessment of the cyanotoxin removal capacity for small water treatment plants shows that the concentrations of cyanobacterial toxins in treated drinking water in Quebec and northern Nigeria are of concern; the hazard quotient derived from a combination of treated or untreated water from drinking water treatment plants in Quebec and northern Nigeria exceeded unity which indicates the need for appropriate remedial action to be taken or an alternative water supply developed. Also, the intake rate exceeded the recommended World Health Organization intake guideline of 1 μg/L.
Applicable strategies to eliminate cyanobacteria blooms would be appropriate to adopt for water treatment facilities in Eastern Township (Quebec) and northern Nigeria in order to protect consumer’s health. A more sustainable strategy that consists of reducing the introduction of nutrients in surface waters is ideal for a long-term solution. Advanced water treatment techniques such as membrane filtration should be adopted since they have been proven to be very effective in treating all kinds of organic contaminants. Small drinking water treatment plants can be upgraded to include membrane filtration and ozonation. This will help to achieve high removal efficiency.
The authors have not declared any conflict of interests.
REFERENCES
|
Asnaashari A, Gharabaghi B, McBean E, Mahboubi AA (2015). Reservoir Management Under Predictable Climate Variability and Change. J. Water Climate Change 6(3):472-485.
Crossref
|
|
|
|
Baker LC, Manassaram-Baptiste D, LePrell R, Bolton B (2015). Cyanobacterial and algae blooms: Review of health and environmental data from the harmful algal bloom-related illness surveillance system (HABISS) 2007–2011. Toxins 7:1048-1064.
Crossref
|
|
|
|
|
Ballot A, Fastern J, Wiedner C (2010). Paralytic shellfish toxin-producing Cyanobacterium. Alphanizomenon gracile in Noeast Germany. Appl. Environ. Microbiol. 76:1173-180.
Crossref
|
|
|
|
|
Ballot A, Pflugmacher S, Wiegand C, Kotut K, Krienitz L (2003). Cyanobacterial toxins in Lake Baringo, Kenya. Limnologica. Tech. Sci. J. 33:2-9.
Crossref
|
|
|
|
|
Baxa DV, Kurobe T, Ger KA, Lehman PW (2010). Estimating the abundance of toxic microcystins in San Francisco Esturay using qualitative real-time PCR. Harmful algae 9:342-349.
Crossref
|
|
|
|
|
Bogialli S, Nigro Di Gregorio F, Lucentini L, Ferretti E, Ottaviani M, Ungaro N, Abis PP, Cannarozzi De Grazia M (2013). Management of toxic Cyanobacterium bloom (Planktothrix rubescens) affecting an Italian drinking water basin: A case study. Environ. Sci. Technol. 47:574-583.
Crossref
|
|
|
|
|
Chia A, Abolude D, Laden Z, Akanbi O, Kalabonus A (2009). The presence of Microcyctins in Aquatic ecosystems in Northern Nigeria: Zaria as a Case Study. Resour. Environ. 3(4):170-178.
|
|
|
|
|
Chia MA, Kwaghe MJ (2015). Microcyctins Microcystins contamination of surface water supply sources in Zaria-Nigeria. Environ. Monitor. Assess. 187:10.1007/s10661-015-4829-3.
Crossref
|
|
|
|
|
Chorus I, Falconer IR, Salas HJ, Bartram J (2000). Health Risks Caused By Freshwater Cyanobacteria In Recreational Waters. J. Toxicol. Environ. Health Part B 3:323-347.
|
|
|
|
|
Codd GA, Lindsay J, Young FM Morrison LF, Metcalf JS (2005). Harmful cyanobacteria: from mass mortalities to management measures. Springer: pp. 1-23.
Crossref
|
|
|
|
|
Drabkova M, Marsalek B (2007). A review of in-lake methods of cyanobacterial blooms control and management. CyanoData – The Glogal Database of Methods for Cyanobacterial Blooms Management, Centre for Cyanobacteria and their Toxins.
|
|
|
|
|
Duy TN, Lam PKS, Shaw GR, Connell DW (2000). Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev. Environ. Contam. Toxicol. 163:113-186.
Crossref
|
|
|
|
|
Echenique RO, Aguilera A, Giannuzzi L (2014). Problems on drinking water related to toxigenic Cyanobacteria: Some cases studied in Argentina. Adv. Limnol. (65):431-444.
Crossref
|
|
|
|
|
Ernst B, Hoeger SJ, O' Brien E, Dietrich DR (2006). Oral toxicity of the microcystins-containing cyanobacterium planktothrix rubescens in European whitefish (Coregonus lavaretus). Aquat. Toxicol. 79:31-40.
Crossref
|
|
|
|
|
Farrer D, Counter M, Hillwig R, Cude C (2015). Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins 7:457-477.
Crossref
|
|
|
|
|
Fastner J, Rucker J, Stuken A, Preubel K, Nixdorf B, Chorus I (2007). Occurrence of cyanobacterial toxin cylindrosperospin in northeast Germany. Environ. Toxicol. 22:26-32.
Crossref
|
|
|
|
|
Fawell JK, Mitchell RE, Evertt DJ, Hill RE (1999). The toxicity of Cyanotoxins in the Mouse: microcystins exposure in human. Exp. Toxicol. 181:162-167.
Crossref
|
|
|
|
|
Fischer WJ, Hiltzfield BC, Tencalla F, Eriksson JE, Mikhailov A, Deietrich DR (2000). Microcystin-LR Toxicodynamics, induced pathology and immunohistochemical localization in livers of blue-green algea exposed rainbow trout (Oncorhynchus mykiss). Toxicol. Sci. 54:365-373.
Crossref
|
|
|
|
|
Gazendam E, Gharabaghi B, Ackerman J, Whiteley H (2016). Integrative Neural Networks Models for Stream Assessment in Restoration Projects. J. Hydrol. 536:339-350.
Crossref
|
|
|
|
|
Gilroy DJ, Kauffman KW, Hall RA, Huang X, Chu FS (2000). Assessing Potential Health Risk from Microcystin Toxins in Blue-Green Algae Dietary Supplements. Environ. Health Perspective 108:435-439.
Crossref
|
|
|
|
|
Graham J, Loftin K, Meyer M, Ziegler A (2010). Cyanotoxin mixtures and taste and odor compounds in cyanobacterial blooms from Midwest United States. Environ. Sci. Technol. 44:7361-7368.
Crossref
|
|
|
|
|
Griffiths DJ, Saker ML (2003). The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. 18:78-93.
Crossref
|
|
|
|
|
Hans WP, Timothy GO (2013). Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Environ. Microbiol. DOI 10.1007/s00248-012-0159.
|
|
|
|
|
Harvey R, McBean EA, Murphy HM, Gharabaghi B (2015). Using data mining to understand Drinking Water Advisories in small water systems: A case study of Ontario First Nations drinking water supplies. Water Resour. Manage. 29(14):5129-5139.
Crossref
|
|
|
|
|
Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, Dortch Q, Gobler CJ, Heil CA, Humphries E, Lewitus A, Magnien R, Marshall HG, Sellner K, Stockwell DA, Stoecker DK, Suddleson M (2008). Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8:3-13.
Crossref
|
|
|
|
|
Hrudey S, Burch MD, Drikas M, Gregory R (1999). Remedial measures. In Toxic cyanobacteria in water: a guide to their public health consequences, monitoring, and management. Edited by Chorus I, Bartram JE, Spon FN New York. pp. 275-312.
|
|
|
|
|
Kaushik R, Balasubramanian R (2013). Methods and Approaches for Detection of Cyanotoxins in Environmental samples: A review: Critical Rev. Environ. Sci. Technol. 43:1349-1383.
Crossref
|
|
|
|
|
Keijola A, Himberg K, Esala A, Sivonen K, Kiisvirata L (1989). Removal of cyanobacterial toxins in water treatment processes: laboratory and pilot plant experiments. Toxic. Assess. 3:643-656.
Crossref
|
|
|
|
|
Kutovaya OA, Watson SB (2014). Development and application of a molecular assay to detect and monitor geosmin-producing cyanobacteria and actinomycetes in the Great Lakes. J. Great Lakes Res. 40:404-414.
Crossref
|
|
|
|
|
Makarewicz JC, Lewis TW (2015). Exploring spatial trends and causes in Lake Ontario coastal chemistry: Nutrients and pigments. J. Great Lakes Res. 3:794-800.
Crossref
|
|
|
|
|
Merel S, Clément M, Thomas O (2010). State of the art on Cyanotoxins in water and their behaviour towards chlorine. Toxicon 55:677-691.
Crossref
|
|
|
|
|
Merel S, Walker D, Chicana R, Snyder S, Baures E, Thomas O (2012). State of Knowledge and concerns on Cyanobacterial blooms. Environ. Int. 59:303-327.
Crossref
|
|
|
|
|
Mohamed ZA, Deyab MA, Abou-Dobara MI, El-Sayed AK, El-Raghi WM (2015). Occurrence of cyanobacteria and microcystin toxins in raw and treated waters of the Nile River, Egypt: implication for water treatment and human health. Environ. Sci. Pollution Res. 22:11716-11727.
Crossref
|
|
|
|
|
Mouchet P, Bonnélye V (1998). Solving algae problems: French expertise and worldwide applications. AQUA 47(3):125–141.
|
|
|
|
|
Newcombe G, Nicholson BC (2002). Treatment options for the saxitoxin class of Cyanotoxins. Water Sci. Technol. Water Supply 2(5-6):271-275.
|
|
|
|
|
Nicholson BC, Shaw GR, Morrall J, Senogles PJ, Woods TA, Papageorgiou J, Kapralos C, Wickramasinghe W, Davis BC, Eaglesham GK, Moore MR (2003). Chlorination for degrad- ingsaxitoxins (paralytic shellfish poisons) in water. Environ. Technol. 24(11):1341-1348.
Crossref
|
|
|
|
|
Ohio State Environmental Protection Agency (2013). Public Water System Harmful Algal Bloom Response Strategy 1:1-47.
|
|
|
|
|
Ontario Health Unit (2014). Beach management guidance document; Ministry of health and long-term care pp. 1-16.
|
|
|
|
|
Ostermaier V, Kurmayer R (2010). Application of real-time PCR to estimate toxin production by the cyanobacterium Planktothrix sp. Appl. Environ. Microbiol. 76:3495-3502.
Crossref
|
|
|
|
|
Paerl HW, Hall NS, Calandrino ES (2011). Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 409:1739-1745.
Crossref
|
|
|
|
|
Queensland Health (2001). Environmental health assessment guidelines: Cyanobacteria in recreational and drinking wa-ters. Environmental Health Unit Bris- bane, Australia.
|
|
|
|
|
Sayyad G, Vasel L, Besalatpour A, Gharabaghi B, Golmohammadi G (2015). Modeling Blue and Green Water Resources Availability in an Iranian Data Scare Watershed Using SWAT. J. Water Manage. Modeling doi: 10.14796/JWMM.C391.
Crossref
|
|
|
|
|
Stang C, Gharabaghi B, Rudra R, Mahboubi A, Ahmed S (2016). Conservation management practices - success story of the Hog Creek and Sturgeon River Watersheds, Ontario, Canada. J. Soil Water Conserv. 71(3):237-248.
Crossref
|
|
|
|
|
Szlag D, Sinclair J, Southwell B, Westrick J (2015). Cyanobacteria and Cyanotoxins Occurrence and Removal from five High-risk conventional Treatment drinking water plants. Toxins 7:2198-2220.
Crossref
|
|
|
|
|
Tencalla F, Dietrich D (1997). Biochemical Characterization of Microcystin toxicity in trout (Oncorhyncnchus mykiss). Toxicon 35:583-595.
Crossref
|
|
|
|
|
Westrick JA, Szlag DC, Southwell BJ, Sinclair J (2010). A review of cyanobacteria and Cyanotoxins removal /inactivation in drinking water treatment. Anal. Bioanal. Chem. 397:1705-1714.
Crossref
|
|
|
|
|
Zamyadi A, Sherri L, Yan F, McQuaid N, Dorner S, Sauve S, Prevost M (2012). Toxic cyanobacterial breakthrough and accumulation in a drinking water treatment plant: A monitoring and treatment Challenge. Water Res. 46:1511-1523.
Crossref
|
|
|
|
|
Zurawell R (2002). An initial Assessement of Microcystin in Raw and Treated Municipal drinking water derived from Eutrophic surface waters in Alberta. 1-15:
|
|