ABSTRACT
Antiretroviral (ART) regimen switch is a common occurrence in resource-limited settings where patients present late for care or with an AIDS-defining event. ART regimen switch can be attributed to several factors emanating from either the individual, program or facility level. This retrospective study was carried out in a resource-limited comprehensive facility in North-central Nigeria. Treatment records of 4,206 Adult HIV/AIDS patients initiated on first line ART regimen from 2006 to 2013 were extracted and examined for switch to second line ART regimen for the purpose of this study after ethical clearance had been sought. Absolute CD4 count, World Health Organisation (WHO) clinical stage and viral load results at treatment initiation, point of switch to second line and at the end of 2014 (end-point for the study) were obtained. About 75% of the 4,206 patients initiated on first line highly active antiretroviral therapy (HAART) were retained in care and were still on first line HAART at the end of 2014 with a significant difference in median CD4 count, BMI, WHO clinical staging and viral load at baseline compared to the end of the study period. Out of the 4,206 adults patients initiated on first line HAART, 71 were later switched to second line regimen due to either first-line immunological or virological failures although only 57 patients without traces of co-infection were included in this study. At end-point, a very high (87.7%) WHO defined immunological response was achieved. The study revealed that although immunologic and virologic response in patients before ART regimen switch was low, it improved tremendously after switch to second line regimen in all patients including those with available viral load results. The study results showed a 96.5% retention rate of patients switched to second line HAART and a correlation between virological suppression and immunological response.
Key words: Immunological assessment, virologic response, HIV diagnosis, first line regimen, second line regimen.
Human Immunodeficiency Virus (HIV) testing coverage in most parts of the world at the beginning of the millennium was suboptimal, particularly among infants, adolescents and key populations (Antinori et al., 2011). Limited access to HIV diagnosis and treatment resulted in substantial numbers of patients presenting for care only when they have reached an advanced stage of disease.
With the announcement of the U.S President’s Emergency Plan for Acquired Immunodeficiency Disease Syndrome Relief in 2003 (Zeitz, 2017), access to HIV testing and Anti-retroviral therapy (ART) was made more available (WHO, 2016). As of 2006, WHO recommended a standard initial drug treatment option of two nucleoside reverse transcriptase inhibitors (NRTIs) and a non-nucleoside reverse transcriptase inhibitor (Gilks et al., 2006) to maximally suppress the HIV virus and stop the progression of HIV disease. In 2012 an estimated 1.6 million people died of HIV-related causes, and HIV/AIDS still ranks in the top five global causes of disability adjusted life years (Nathan et al., 2013). Huge reductions have been seen in rates of death and morbidity when use is made of a potent Anti-retroviral (ARV) regimen, particularly in the early stages of HIV disease (WHO, 2013).
In resource-limited settings, patients present far late for care, on average, with a CD4 cell count of <200 cells/mm3 or with an AIDS-defining event (Geng et al., 2011). Some of them are initiated on second line regimen even though late presentation is associated with lower survival. However, in high-income settings, a late presenter refers to any patient who presents with a CD4 cell count of < 350 cells/mm3 (Antinori et al., 2011). In 2007, it was estimated that a small proportion (4% adults and 1% children) of patients on ART were on second-line therapy (Renauld-Thery et al., 2007). The benefits of early access to HIV treatment led the WHO in 2013 to raise the CD4 threshold for initiating HIV treatment from 350 cells/mm3 to 500 cells/mm3 and to recommend ARV use for the prevention of HIV infection, particularly for pregnant women, young children, and key populations exposed to HIV risk. This recommendation increased the number of people eligible for treatment in low- and middle- income countries to 25.9 million even though it is estimated that 9.7 million people living with HIV are receiving treatment as against less than half a million people a decade ago (WHO, 2013).
Africa and Nigeria in particular continually face an increasing burden of HIV related death rates. For example, HIV related annual deaths in Nigeria increased from 192,000 deaths in 2008 to 217,148 deaths in 2012. Out of 1.5 million Nigerians confirmed to be HIV positive in 2012, only 500,000 had access to antiretroviral drugs (Chioma et al., 2012), however, as at December 2014, 747,382 individuals have been placed on ART (NASCP, 2014).
Study population and data collection
The existing database from the beginning of HIV/AIDS care and treatment clinic in 2006 at Asokoro district hospital - a comprehensive health facility was searched and data for 4206 adult patients initially placed on first line ART regimen but later switched to a second line ART regimen was retrieved. Asokoro district Hospital which is located in North central part of Nigeria serves the health care needs of the residents of Abuja and seven other surrounding states.
Eligibility criteria
Only patients initiated on first line ART, enrolled for treatment at the facility or transferred into the facility were included in the study. Patients with records of co-infection with tuberculosis and children were excluded from the study.
Data and statistical analysis
Basic demographic information such as sex, weight and age were obtained for patients who fulfilled the eligibility criteria. The number of months a patient had been on second line ART regimen was determined with respect to the cut-off point (end of 2014).
Data cleaning and descriptive data analysis of the patients’ characteristics were computed and stratified based on WHO clinical staging, CD4 count, duration patients remained in care and other variables at the initiation of HAART, point of switch and cut-off point using SAS propriety software 9.2. Comparisons of proportions between the groups of patients (at initiation of HAART, point of switch and cut-off point) were computed using chi-squared test for categorical variables and Student’s -test and the comparison was considered significant at P-value less than 0.05.
Ethical statement
The data was extracted from the treatment centre database with the privacy of the patients protected using only the assigned US President’s Emergency Plan For AIDS Relief (PEPFAR) identifiers. In addition, the data extraction and analysis were carried out anonymously. Medical ethics review was not required as the data obtained from the data base was retrospective from the previously provided treatment programme. However, the approval of the centre’s management and the Institute of human virology Nigeria health research ethics review committee were obtained in order to access the data.
Characteristics of the patients who remained on first line at HAART throughout the study
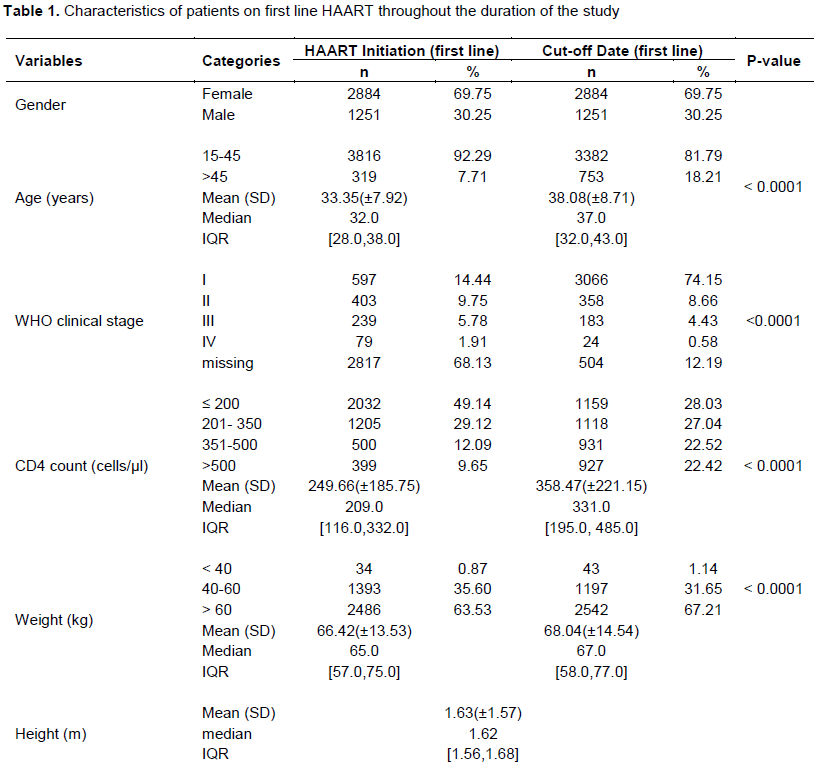
The baseline characteristics of the patients who remained on first line HAART regimen (n=4206) throughout the study period as depicted in Table 1 shows that a majority of them (92.0%) are between 15-45 years while just about 8% are older than 45 years with a preponderance of females (70%) compared to males (30%) and an average body mass index (BMI) of 24.77 kg/m2. The WHO clinical profile of the patients before ART initiation which was available for only a third of them showed that 14% of the patients were at clinical WHO stage I, while the rest were at WHO clinical stages II (10%), III (6%) and IV (2%) respectively. The CD4 count profile showed 50% of them with a CD4 count of ≤ 200 cells/µl, 30% with ≤ 350 cells/µl, while the remaining had a CD4 count of between 351-500 cells/µl. The median (IQR) viral load was 41739.00 (41501.0, 41828.0).
Changes in characteristics of patients still on first line ART throughout the duration of the study
The immunological and clinical characteristics of the patients on first line HAART regimen at the cut-off point when compared to baseline revealed a significant and tremendous improvement as depicted in Table 1. It showed that the median age of the patients increased from 32 years (IQR: 28-38 years) at initiation of ART to 38 years (IQR: 32-43 years) at the cut-off point (p<0.001). Changes were also noticed in the median (IQR) CD4 count from 209 (116-332) cells/ µl at baseline to 331 (195-485) cells/µl (p<0.001) with a reduction in the proportion of patients at baseline compared to cut-off date with CD4 count levels of ≤ 200 cells/µl (49 to 28%) and 201-305 cells/µl (29 to 27%). However, there was an increase in the proportion of patients at the baseline compared to the cut-off point with CD4 count levels of 351-500 cells/µl (12 to 22%) and >500 cells/µl (10 to 22%).
Similarly, there was an increased change in the median (IQR) weight from 65 kg (57-75 kg) at baseline to 67 kg (58-77 kg) at cut-off point ( p<0.001) while the mean BMI of patients increased from 24.77kg/m2 at baseline to 25.33 kg/m2 at the cut-off point. The WHO clinical stage profile of the patients showed that there was a general marked improvement in clinical response irrespective of the level of the clinical stage at the cut–off point when compared to the baseline. The proportion of patients with clinical stage II, III and IV at baseline reduced from 10 to 9%, 6 to 4% and 2 to less than 1% respectively at the cut of point while the proportion of clients with clinical stage I increased from about 14 to 74% (p<0.001).
Although only about 3% of the patients had a viral load count result, there was a significant difference between baseline and the cut-off point results (p<0.001). A 50% reduction in the proportion of patients with viral load >1000 copies/ml at baseline was recorded at the cut-off point. Also more than 100% point increase in the proportion of patients having viral load of <400 copies/ml was noticed at the cut-off point. However, despite these significant improvements in clinical, immunological and relative virological profile of the patients only about 75% of them were retained in care while the remaining were lost to follow up.
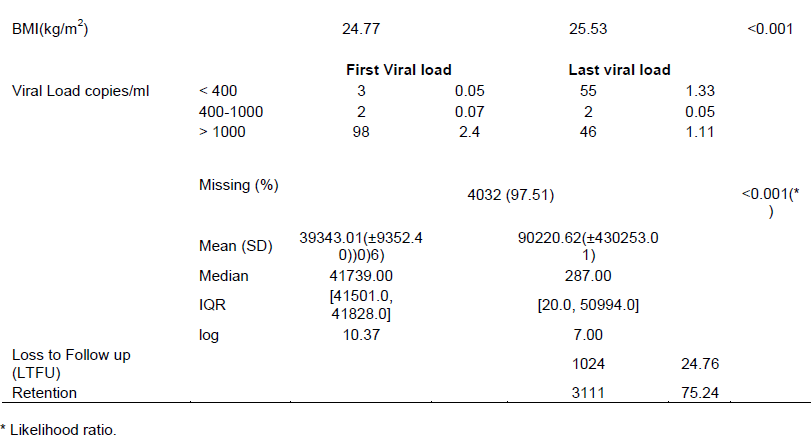
The changes in CD4 count as seen in Figure 1 between ART initiation and the cut-off point indicated that 33.3% of the patients had <50 cells/µl CD4 count increase (immunological non-responders), 46.5% of patients had an increase of between 50 to 350 cells/µl, 19.3% had an increase of between 351 to 950 cells/µl while about 1.6% of the patients had an increase of between 951 to 2450 cells/µl CD4 counts.
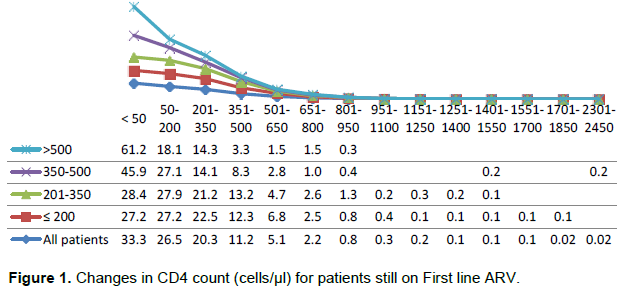
The least and highest time patients spent on first line ART were less than 1 month and 116 months (9.7 years) respectively as shown in Figure 2. It was observed that about 12.12% of patients spent less than 10 months on treatment while 1.35% of the patients spent 9.7 years on treatment.
Characteristics of patients switched to second line regimen at HAART initiation
From the data, the patients were initiated on a WHO recommended two NRTI-based and one NNRTI-based
first-line ART regimen. The second line regimen were Protease Inhibitors (PI)-based (included two NRTI and atanavir-rotonavir or lopinavir-ritonavir based). The baseline characteristics of the patients switched to second line HAART regimen is as depicted in Table 2. The age distribution of the patients shows that a majority of them (93.0%) were between 15-45 years while just about 7% were older than 45 years with a preponderance of females (64.9%) compared to males (35.1%). The WHO clinical stage profile of the patients before ART initiation showed that slightly above half of the patients were initiated while at clinical WHO stage I (54.4%) while the rest were at WHO clinical stages II (15.8%), III (19.3%) and IV (10.5%) in that order.
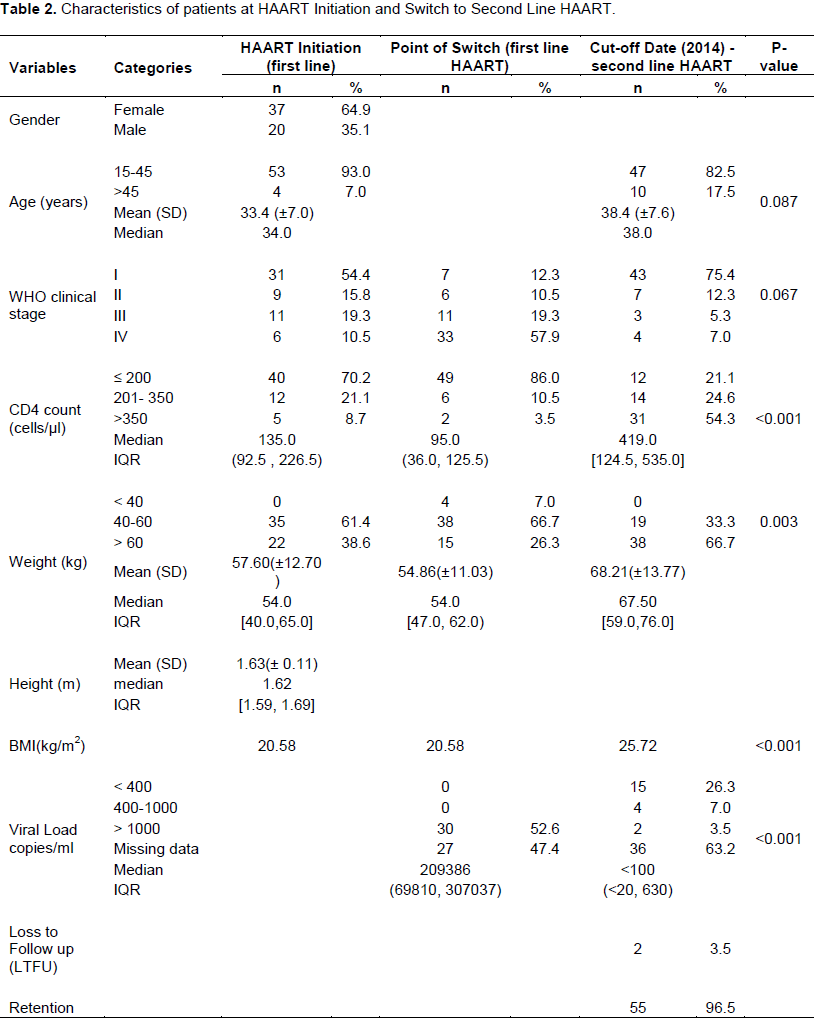
Results of the CD4 cells/µl count indicated that a majority of the patients (70.2%) had ≤ 200 cells/µl while 21.1 and 8.7% of the patients had absolute CD4 counts of 201-350 and >350 cells/µl respectively. The median (IQR) CD4 count for all the patients was 135.0 (92.5, 226.5) cells/µl. The weights of the patients fell mostly in the 40-60 kg (61.4%) range while only 38.6% of patients were >60 kg. None of the patients weighed less than 40 kg at the time of ART initiation. There were no viral load results available when patients were initiated on treatment.
Changes in characteristics after ART initiation to point of ART Switch
Patients spent an average (SD) and median (IQR) of 34.1 (±25.4) and 34.5 (12.0, 55.0) months respectively on first line regimen before the switch to second line regimen (p < 0.0001). The WHO clinical stage profile of patients declined with 77.4% on stage I and 33.3% on stage II that have either moved to stage III or stage IV. There was an evidence of immunological failure just before ART switch which was indicated by a drop in the median (IQR) CD4 cells/µl count from 135.0 to 95.0 cells/µl (36.0, 125.5) for all patients. In addition, the proportion of patients with CD4 absolute count of ≤ 200 cells/µl, increased from 70.2 to 86.0% while the proportion of patients with 201-350 and >350 cells/µl at ART initiation declined by about half as depicted in Table 2.
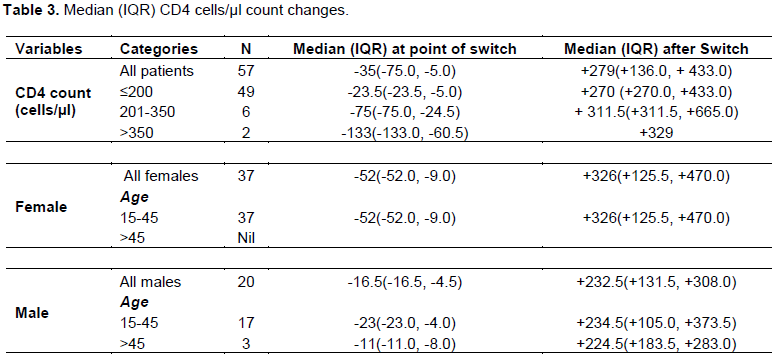
Changes were also noticed in the median (IQR) CD4 count for sex and age categories. There was a drop in the median CD4 absolute count by 52 points for females and 16.5 points for males just before ART switch compared to the baseline profile as shown in Table 3.
In a similar manner, there were changes in the weights of patients. The number of patients at baseline with body weight >60 kg reduced from 22 to 15 whereas there was a marginal increase in the number of patients that weighed 40-60 or <40 kg.
Changes in characteristics after ART switch to end of 2014
The median (IQR) and mean (SD) duration of time in care and treatment after switch to second-line treatment were 23.0 (18.0, 29.0) and 25.2 (±12.7) months respectively. The least and highest time patients spent on second line ART as at end of 2014 depicted in Figure 3 were 7 and 87 months. By the 87th month, patient with the longest time spent on second HAART was still active and on treatment.
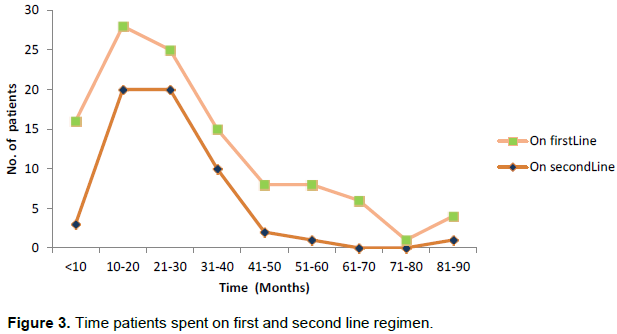
Two patients were lost to follow up (LTFU) - a male after 7 months on second line ART and a female after she was on second line ART for 14 months. Out of 57 patients that were on second line ART, 55 (96.5%) patients made up of 36 females and 19 males were still in care and on treatment at the end of 2014 as shown in Table 2. At the cut-off point (end of 2014), records of all 57 patients that were on second line ART including those who were LTFU showed that a very high WHO defined immunological response was achieved in 50 patients (87.7%) post ART switch. The number is comprised of forty-three (74.4%) and seven patients (12.3%) on clinical stages I and II respectively. Only seven (12.3%) patients experienced low immunological response made up of three (5.0%) patients on clinical stage III and four (7.0%) on stage IV.
Furthermore, although only 30 out of 57 patients on second line ART had a viral load result before the ART switch, at the cut-off point only 21 patients’ results could be accessed. Out of the 21 patients, 15 (74.4%) showed significant virological suppression with drop in viral load from >1000 copies/µl at ART switch to <400 copies/µl at the cut- off point (p<0.001). The proportion of patients with absolute CD4 cells/µl count of ≤ 200 cells/µl reduced from 86% at ART switch to 21.1% at the cut-off point. That of patients with CD4 cells/µl >350 cells/µl increased from 3.5 to 54.3% while there was about 10% points increase in the proportion of patients with 201-350 cells/µ at ART switch compared to that at the cut-off point. Also the median (IQR) CD4 count for all patients improved at cut-off point to 419.0 (124.5, 535.0) from 95.0 (36.0, 125.5) before ART switch (Figure 4). This increased change in median CD4 count was also noticed irrespective of the CD4 level, age or sex of the patient as seen in Table 3.
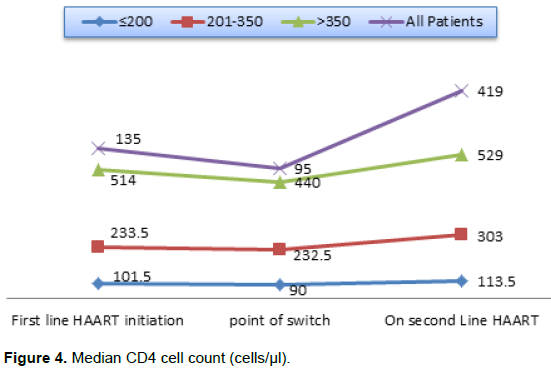
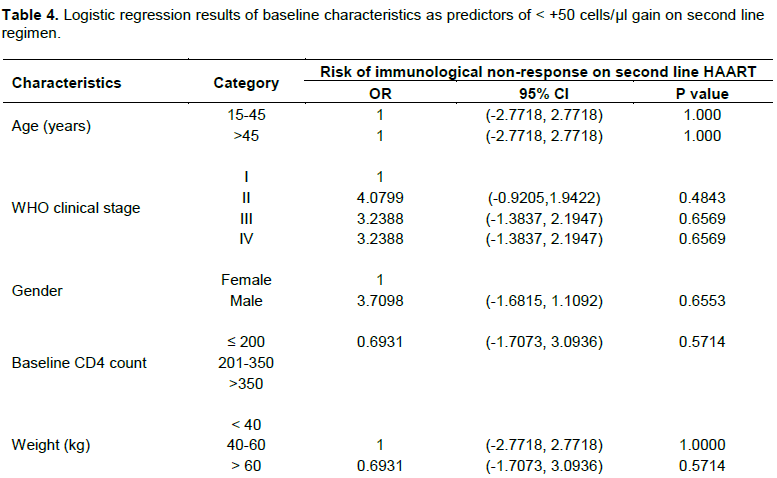
All these changes are evidences of marked improvement in immunological response from ART switch to post ART switch at the cut-off point. However Table 4 showed that baseline characteristics; age, WHO clinical stage, sex, baseline CD4 count and weight were not predictors of CD4 gain on second line regimen.
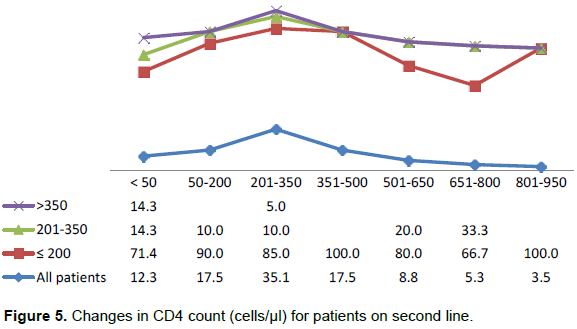
The changes in CD4 count after patients had been on second line regimen as seen in Figure 5 indicated that 12.3% of the patients had <50 cells/µl CD4 count increase (immunological non-responders). About 70% of the patients had an increase of between 50 to 350 cells/µl while 17.7% of the patients had an increase of between 351 cells/µl to 950 cells/µl. The increased change in CD4 count that was noticed for all patients was substantially contributed by patients with CD4 count of ≤200 cells/µl irrespective of the magnitude of the change.
The findings of this study aimed at assessing the performance of patients on second line HAART after failing on first line HAART showed a potential correlation between virological suppression and immunological response.
In addition, the contribution of the patients’ baseline characteristics to CD4 count improvement was investigated and the results did not show any significant influence of the factors as risks to immunological failure. However, a study by Addisu et al. (2015) found that very low CD4 count before ART initiation influences immune reconstitution after patients are placed on ART which is similar to our findings. This is in contrast to another study which reported that treatment outcomes in adult HIV naïve patients were not based on the baseline CD4 cell count (Kyaw et al., 2013). Specifically in terms of gender, some studies dissociated sex as a risk factor of immunological failure (Lawn et al., 2006; Smith et al., 2004) which is in tandem with our results.
It was observed in this study that the patients appeared to have had late enrolment into HIV care with 70.2% of the patients having absolute CD4 count of ≤ 200 cells/µl (median CD4 <140.0 cells/µl) at ART initiation. This aligns with a statement that “in resource-limited settings, patients present far late for care on average with a CD4 cell count of <200 cells/mm3 or with an AIDS-defining event” (Geng et al., 2011).
Furthermore, the above findings agree with a similar study which posited that substantial numbers of patients continue to present for care only when they have reached an advanced stage of disease despite progressive improvements in HIV diagnosis and access to treatment and care (Nathan et al., 2013).
In this study we excluded patients with co-infections in order to allow for common characteristics of the patients to determine their retention on second line treatment and to prevent bias in treatment outcomes as a result of the risk of early mortality for HIV patients co-infected with tuberculosis (Attia et al., 2001; Marshall et al., 2013)
The results of this study revealed that patients with depleted CD4 count of ≤200 cells/mm3 after failing first line ART had highest improvement in CD4 count rates as compared to other patients with higher CD4 count categories when switched to second line HAART. This is in concordance with a study which reported similar results for HIV patients with very advanced immunodeficiency starting ART in sub-Sahara Africa (Lawn et al., 2006). Other factors such as drug resistance or level of adherence by patients might be responsible for switching as pointed out in a previous study by Ford et al. (2012) which our study could not ascertain based on the limitation of the data.
Also, our study revealed that only the patients with ≤200 cells/mm3 after failing on first line regimen attained absolute CD4 cell count of >1000 cells/mm3. This is somehow contrary to the observation that patients with CD cell counts of ≤200 cells/mm3 as a result of treatment delay do not achieve a normal CD4 cell count even after a decade of effective therapy (Kelley et al., 2009) or are likely to die early in HIV care and treatment when there is delay in enrolling them in care (Boulle et al., 2010).
However, in our study only about 4.1% of the patients with ≤200 cells/mm3 had CD4 cell count >1000 cells/mm3, 24.5% achieved 500-1000 cells/mm3, 36.7% improved to between 300- 499 cells/mm3 and 24.5% still remained in the same stratum. Our results seems to differ with a report by (PAGAA, 2015) which stated that patients commenced on HAART with CD4 count below 350 cells/mm3 are very unlikely to improve to a high CD4 cell count compared to those with ≥350 cells/mm3.
Results of this study also indicated that patients despite spending close to 3 years on first line HAART, still had median CD4 count <100 cells/µl (Immunological non-response) before ART switch to second line HAART. The drop in absolute CD4 count was as high as >500 cells/µl with median CD4 count drop of >50 cells/µl in females and about 20 cells/µl in males. There was pronounced weight loss in most of the patients while on the first line regimen and the available viral load results indicated all the patients had virological non-suppression.
Treatment effectiveness was not closely monitored after ART initiation while ART switch was unnecessarily delayed. It was noticed that there was rapid immunological response and virological suppression within a short interval after patients had been initiated on second line HAART. It goes to show that when patients with signs of immunological non-response on first line regimen are early detected and switched to second line regimen, there is a positive tendency of them regaining immunological response and virologic suppression. Our findings revealed that patients with absolute CD4 count of >350 cells/µl at baseline performed better on first line regimen than the patients with ≤ 200 cells/µl at baseline. It also indicated that patients with 201-350 cells/µl at baseline responded faster to immunologic build up effort than those with ≤ 200 cells/µl absolute CD4 count.
The study findings was limited because of the inability of the facility in which our study was carried to conduct viral load test (which is vital in evaluating patients after being initiated on ART) for all patients due to financial resource constraint. The absence of the viral load results at ART initiation and in slightly above half of the population after ART switch is an indication that the patients at this centre were initiated on treatment based only on CD4 count results in addition to WHO clinical staging criteria. Also changes in WHO guidelines for initiating ARV within the period 2006 to 2014 (CD4≤200 – recommended since 2003; CD4≤350 – recommended since 2010; CD4≤350 +TasP – recommended since 2012 and CD4≤500 +TasP – recommended since 2013) was also a limitation affecting the outcomes of the study.
Based on the aforementioned findings we recommend that whenever patients in resource-limited settings are started on ART, funds should be made available for viral load test at baseline and subsequently to track regimen failure early and to monitor patients’ performance. This would aid in avoiding the tendency of CD4 cells count depletion which may amount to an AIDS defining situation. Improved access to viral load test and availability of results is sine qua non for effective treatment monitoring and early diagnosis of treatment failure in patients. It is important to note that delayed ART switch for patients who failed on first line regimen can result in non-responsiveness to treatment and potential fatality.
The authors have not declared any conflict of interests.
REFERENCES
|
Addisu A, Dagim A, Tadele E, Adissu A, Mussie A, Filmon K (2015) . CD4 Cell Count Trends after Commencement of Antiretroviral Therapy among HIV-Infected Patients in Tigray, Northern Ethiopia: A Retrospective Cross-Sectional Study. PLoS One 10(3):1-9 e0122583.
|
|
|
|
Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, Girardi E, Johnson M, Kirk O, Lundgren J, Mocroft A, D'Arminio Monforte A, Phillips A, Raben D, Rockstroh JK, Sabin C, Sönnerborg A, De Wolf F (2011). Late presentation of HIV infection: a consensus definition. HIV Med.12:61-64.
Crossref
|
|
|
|
Attia A, Huët C, Anglaret X, Toure S, Ouassa T, Gourvellec G, Menan H, Dakoury-Dogbo N, Combe P, Chêne G, N'Dri-Yoman T, Salamon R (2001). HIV-1-related morbidity in adults, Abidjan, Cote d'Ivoire: a nidus for bacterial diseases. J. Acquir. Immune Defic. Syndr. 28:478-486.
Crossref
|
|
|
|
Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, Ford N, Knight L, Osler M, Myers J, Goemaere E, Coetzee D, Maartens G (2010). Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South African. AIDS 24:563-572.
Crossref
|
|
|
|
Chioma O, Vincent U, Samuel Oyadongha (2012). World Aids Day: Nigeria records decline in new HIV infections.
|
|
|
|
Ford N, Roberts T, Calmy A (2012). Viral load monitoring in resource-limited settings: a medical and public health priority. AIDS 26(13):1719-1720.
Crossref
|
|
|
|
Geng EH, Hunt PW, Diero LO, Kimaiyo S, Somi GR, Okong P, Bangsberg DR, Bwana MB, Cohen CR, Otieno JA, Wabwire D, Elul B, Nash D, Easterbrook PJ, Braitstein P, Musick BS, Martin JN, Yiannoutsos CT, Wools-Kaloustian K (2011). Trends in the clinical characteristics of HIV-infected patients initiating antiretroviral therapy in Kenya, Uganda and Tanzania between 2002 and 2009. J. Int. AIDS Soc. 14:46.
Crossref
|
|
|
|
Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, Sutherland D, Vitoria M, Guerma T, De Cock K (2006) . The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet 368(9534):505-510.
Crossref
|
|
|
|
Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, Crane HM, Willig J, Mugavero M, Saag M, Martin JN, Deeks SG (2009). Incomplete Peripheral CD4+ Cell Count Restoration in HIV-Infected patients Receiving Long-Term Antiretroviral Treatment. Clin. Infect. Dis. 48:787-794.
Crossref
|
|
|
|
Kyaw NL, Thanachartwet V, Kiertiburanakul S, Desakorn V, Chamnanchanunt S, Chierakul W, Manosuthi W, Pitisuttithum P, Sungkanuparph S (2013). Baseline CD4 cell counts and outcomes among adult treatment naïve HIV patients after taking fixed dose combination GBO-VIR-S and GBO-VIR-Z in Thailand, Southeast Asian J. Trop. Med. Public Health 44:232-243.
|
|
|
|
Lawn SD, Myer L, Bekker L, Wood R (2006). CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Sahara Africa. BMC infect. Dis. 6:59.
Crossref
|
|
|
|
Marshall CS, Curtis A, Spelman T, O'Brien DP, Greig J, Shanks L, du Cros P, Casas EC, da Fonseca MS, Athan E, Elliott JH (2013). Impact of HIV-associated conditions on mortality in people commencing antiretroviral therapy in resource limited settings. PLoS One 8:e68445.
Crossref
|
|
|
|
Nathan F, Marco V, Gottfried H, Meg D (2013). Getting to zero HIV deaths: progress, challenges and ways forward. J. Int. AIDS Soc. 16:18927.
|
|
|
|
National AIDS & STI Control Programme (NASCP) (2014). 2014 Annual report on HIV/AIDS Health Sector response in Nigeria. Federal Ministry of Health.
|
|
|
|
PAGAA (Panel on Antiretroviral Guidelines for Adults and Adolescents) (2015) .Guidelines for the use of Antiretroviral agents in HIV1 infected
|
|
|
|
Renauld-Thery F, Nguimfack BD, Vitoria M, Lee E, Graaff P, Samb B, Perriëns J (2007). Use of antiretroviral therapy in resource-limited countries in 2006: distribution and uptake of first- and second-line regimens. AIDS 21(Suppl)4:S89-95.
|
|
|
|
Smith CJ, Sabin CA, Youle MS, Loes SK, Lampe FC, Madge S, Cropley I, Johnson MA, Phillips AN (2004). Factors influencing increases in CD4 cell counts of HIV positive persons receiving long-term highly active antiretroviral therapy. J. Infect. Dis. 190:1860-1868.
Crossref
|
|
|
|
WHO (World Health Organisation) (2013). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach.
|
|
|
|
WHO (World Health Organisation) (2016). Prevent HIV, Test and Treat All. WHO Support for Country Impact.
|
|
|
|
Zeitz PS (2017). President's Emergency Plan for AIDS Relief's (PEPFAR) Use of Geospatial Data. U.N. World Data Forum.
|