Full Length Research Paper
ABSTRACT
Enteric fever is a severe public health threat because of the rising antibiotic resistance of Salmonella species in developing countries, especially in its endemic areas like Bangladesh. This retrospective study was aimed to assess the effectiveness of a range of 17 commonly used antimicrobials against Salmonella Typhi and Salmonella Paratyphi A isolated from 601 enteric fever cases in Dhaka, Bangladesh. Conventional biochemical tests were used to identify Salmonella strains and the Kirby-Bauer disc diffusion method to perform the antibiotic sensitivity in SAIC Digital Diagnostic Lab, Dhaka. The 2017 Clinical Laboratory Standard Institute (CLSI) guideline was employed to interpret the antibiogram results, and statistical software SPSS (version 22.0) to analyze the obtained data. The number of male patients (54.74%) dominated over their female counterparts (45.26%). The patients aged from 1 month to 75 years, with a mean of 19.74±12.79 years. Among 601 Salmonella spp. isolates, S. Typhi (56.57%) prevailed over S. Paratyphi A (43.42%). Both strains showed >85% antimicrobial insusceptibility to three major antibiotics: ciprofloxacin, gentamicin, and amikacin. S. Typhi (65.29%) showed significantly greater resistance to azithromycin compared to S. Paratyhi A (14.9%) (p<0.001). Both pathogens reported over 95% sensitivity to ceftriaxone, cefixime, ceftazidime, amoxiclav, cephalexin, aztreonam, imipenem, and cefuroxime. To conclude, this study found an increased antibiotic resistance of Salmonella spp. to commonly prescribed antibiotics. These findings would help physicians and policymakers make informed decisions and provide better treatment to the affected patients.
Key words: Salmonella, antimicrobials, antimicrobial insusceptibility, antibiotic sensitivity, Dhaka.
INTRODUCTION
Enteric fever is a life-threatening systemic illness caused by Gram-negative Salmonella Typhi and Salmonella Paratyphi A (Crump and Mintz, 2010). It attacks almost 16 million people each year and causes over 153,000 deaths worldwide; notably, most of them belong to South Asia and sub-Saharan Africa. In 2017, nearly 17 million people worldwide got infected, and 117,000 patients lost their valuable lives with a heightened mortality of 4 to 5% (Global Burden of Disease Study, 2017). Its widespread prevalence in the developing and tropical regions like Asia and Africa is primarily due to the existing inadequate food and water safety. Likewise, this contagious fatal disease has also become endemic in Bangladesh (Crump and Mintz, 2010; Kirk et al., 2015). Between 2003 and 2004, Bangladesh reported enteric fever incidence as 200 episodes per 100,000 individuals each year compared to 394.2 episodes per 100,000 individuals in South Asia (Saha et al., 2018). One recent study by Ahmed et al. (2017) explored the bacterial etiology of bloodstream infections and found S. Typhi and S. Paratyphi A as the most frequently isolated organism with a high percentage of multidrug-resistant (MDR) strains (Ahmed et al., 2017). Worryingly, younger children in Bangladesh have experienced the highest incidence of enteric fever compared to Vietnam and other comparable regions (Brooks et al., 2005).
This deadly infection is regarded as “typhoid” when caused by S. Typhi and “paratyphoid” fever when by S. Paratyphi. These pathogens can transmit through the oral or fecal routes of patients and manifest morbidity through multiple signs: fever, abdominal pain, and non-specific symptoms, including nausea, vomiting, headache, and anorexia (Connor and Schwartz, 2005; Sur et al., 2007). When ingested, these Salmonella species bacteria colonize the small and large intestines, invade the gastrointestinal barrier, and then spread to the vital organs such as the liver, spleen and bone marrow (Raffatellu et al., 2008). However, due to increasing resistance of S. Typhi, the available antibiotics that can be considered for effective treatment are decreasing day by day (Das et al., 2017; Saha et al., 1997). This situation has been deteriorating abruptly in low and middle-income countries because of the higher antimicrobial resistance of S. Typhi and S. Paratyphi A strains. Multiple factors like incomplete treatment, overuse, and over-the-counter sales of antibiotics may contribute to this public health concern of antimicrobial resistance. Several studies confirmed that S. Typhi was first reported MDR against ampicillin, chloramphenicol, and cotrimoxazole in the early 1970s and ciprofloxacin in the early 1990s (Olarte and Galindo, 1973). Nowadays, roughly 90% clinical isolates from the urban settings of endemic regions showed decreased sensitivity to ciprofloxacin (Das et al., 2017; Iyer et al., 2017). Later, this trend also shifted to other classes of antibiotics such as azithromycin and ceftriaxone (Das et al., 2017). A recent study from Pakistan also revealed that S. Typhi induced extensive drug-resistance to ciprofloxacin and ceftriaxone (Klemm et al., 2018). Therefore, this study was carried out to investigate the current antibiotic susceptibility patterns of S. Typhi and S. Paratyphi A. Its findings would benefit healthcare professionals in making informed decisions and providing better treatment for enteric fever patients in the coming days.
METHODOLOGY
Study design and setting
A retrospective study spanning approximately one year (January 2019 to November 2019) was conducted based on the laboratory records of the SAIC Digital Diagnostic Lab database, Dhaka. In total, 601 blood culture-positive samples collected from the enteric fever patients were assigned for the study. A semi-structured checklist was used to extract all cultures and antimicrobial sensitivity test results of patients from the laboratory records notebook.
Isolation and identification of Salmonella spp.
Gram-staining and conventional biochemical methods were used to identify the Salmonella isolates (Figure 1). A culture media enriched with Selenite broth was used to support the likely growth of pathogens (Figure 2). Following the inoculation, the media was incubated overnight at 37°C and sub-cultured into Salmonella-Shigella agar, blood agar, and Mac-Conkey agar. Triple sugar iron (TSI) agar was initially used to differentiate the isolated Salmonella strains, resulting in alkaline slant, acidic butt, and H2S production. S. Typhi produced H2S but not gas, whereas S. Paratyphi A produced gas and some S. Paratyphi A produced H2S after 72 h. Both strains were motile but showed negative reactions in indole, citrate, and urea tests.
Antimicrobial susceptibility test (AST)
To determine the antibiotic susceptibility of Salmonella isolates, the Kirby-Bauer disc-diffusion method was performed on Muller-Hinton agar plates shown in Figure 2, (Bauer et al., 1966). Antibiotics used were selected based on their 2017 Clinical Laboratory Standard Institute (CLSI) guideline (CLSI, 2017), local prescription by physicians, and availability in the market. All isolates were tested against 17 different types of antibiotics from 8 classes: β–lactamases (Ampicillin-10 µg, Aztreonam-30 µg, Amoxicillin-Clavulanic acid- 30 µg), Carbapenem (Imepenem-10 µg), Aminoglycosides (Gentamycin-10 µg, Amikacin-30 µg), Co- trimoxazole (Co- trimoxazole-25 µg), Cephalosporin (Cefepime 30 μg, Ceftriaxon 30 μg, Cefixime 5 μg, Ceftazidime 30 μg, Piperacillin 75 μg, Cephalexin 30 μg, Cefuroxime 30 μg), Fluoroquinolone (Ciprofloxacin 5 μg), Tetracycline (Tetracycline-30 µg), and Macrolide (Azithromycin-10 µg). Subsequently, the results of AST were interpreted according to the CLSI 2017 guideline.
Statistical analysis
The data were tabulated and illustrated graphically using Microsoft Excel-2019 and subsequently analyzed by the statistical software, SPSS-22. The descriptive results were represented as a percentage, relative frequency, mean ± standard deviation (SD). At last, to find the association between the types of Salmonella spp. infection with patients’ attributes, and antibiotic sensitivity against the tested antibiotics, Chi-square tests and Independent Sample t-test were applied.
Ethical considerations
The Institutional Review Board and chairperson of the SAIC Digital Diagnostic Lab, Dhaka, acknowledged the required ethical approval for the study. It was ensured that the patients selected for the study had not received any antibiotics before 8 h of their sample collection.


RESULTS
Among 601 Salmonella isolates, 340 (56.57%) and 261 (43.42%) were confirmed as S. Typhi and S. Paratyphi A, respectively. The number of male patients (54.74%) predominated their female counterparts (45.26%). But, the distribution of male and female patients based on their infections either by S. Typhi or S. Paratyphi A was similar (p>0.05). Males and females suffered more from S. Typhi than S. Paratyphi A; about 60% males and 57% females tested positive for S. Typhi. The patients aged from 1 month to 75 years, with a mean of 19.74±12.79 years. The average age of the patients infected by S. Typhi and S. Paratyphi A was nearly the same: 19.64±13.39, and 19.87±11.97 years, respectively. The majority of the patients, almost 83%, were 5-40 years old. Patients of the 5-20 years group accounted for the highest, 47.42%, among all enteric fever cases, followed by the adult group, 21-40 years, contributing to 35.77% enteric fever cases. The least number of patients (1.5%) belonged to the oldest age group, >60 years. When S. Typhi and S. Paratyphi cases were distributed within different age groups, the number of typhoid patients outnumbered the paratyphoid patients in each age group. Within the groups of 41-60 and >60 years, the typhoid patients nearly doubled that of paratyphoid. The infection by both pathogens was most common among the age groups of 5 to 20 years, followed by 21-40 years (Table 1).
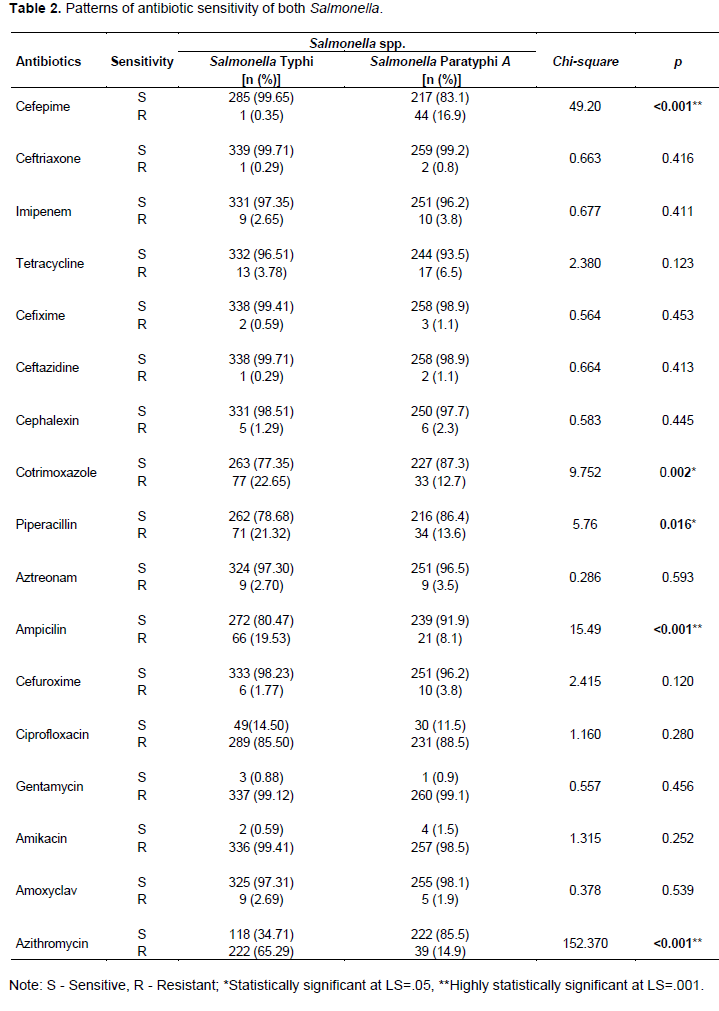
As shown in Figure 3, S. typhi and S. paratyphi A were highly insensitive (>85%) to ciprofloxacin, gentamycin, and amikacin. On the other hand, nearly 10-20% cases by both pathogens had developed resistance to cotrimoxazole, piperacillin, and ampicillin. Interestingly, 5 out of 17 antimicrobials tested showed invariable efficacy against nearly all typhoid and paratyphoid cases: cefixime, ceftazidime, cephalexin, aztreonam, and amoxicillin. Ten antibiotics were highly sensitive against S. Typhi; they all showed over 95% susceptibility (ceftriaxone 99.71%, ceftazidime 99.71%, cefepime 99.65%, cefixime 99.41%, cephalexin 98.51%, cefuroxime 98.23%, imipenem 97.35%, amoxiclav 397.31%, aztreonam 97.30% and tetracycline 96.51%). In striking resemblance with S. Typhi, 8 out of those 10 antimicrobials had over 95% efficacy against S. Paratyphi A as follows: ceftriaxone 99.2%, cefixime 98.9%, ceftazidime 98.9%, amoxiclav 98.1%, cephalexin 97.7%, aztreonam 96.5%, imipenem 96.2%, and cefuroxime 96.2%. On the other hand, S. Typhi demonstrated as high as over 85% resistance to the following antibiotics (gentamycin 99.12%, amikacin 99.41%, and ciprofloxacin 85.50%); however, S. Typhi showed lower resistance against other remaining antimicrobials (azithromycin 65.29%, cotrimoxazole 22.65%, piperacillin 21.32%, and ampicillin 19.53%) (Table 2).
Similar to the resistance shown by S. typhi, S. paratyphi A was found to be sensitive to cefepime 83.1%, tetracycline 93.5%, cotrimoxazole 87.3%, piperacillin 86.4%, and amikacin 91.9%. Likewise, S. Paratyphi A too showed over 85% insensitivity to the antibiotics (gentamycin 99.1%, amikacin 98.5%, and ciprofloxacin 88.5%, followed by cotrimoxazole 12.7%, piperacillin 13.6%, and azithromycin 14.9%) (Table 2). When the sensitivity of each antibiotic was distributed against the type of Salmonella spp., several significant variations (p<0.05) were observed in their sensitivity. Cefepime showed significantly uneven resistance to S. Typhi (.35%) and S. Paratyphi A (16.9%) (p<0.001). Cotrimoxazole was two times more resistant against S. Typhi (22.65%) compared to S. Paratyphi A (12.7%) (p=0.002). S. Typhi (19.53%) showed almost double insensitivity to ampicillin compared to S. Paratyphi A (8.1%) (p<0.001). Overwhelmingly, S. Typhi (65.29%) was about five times more resistant to azithromycin than S. Paratyphi A (14.9%) (p<0.001).

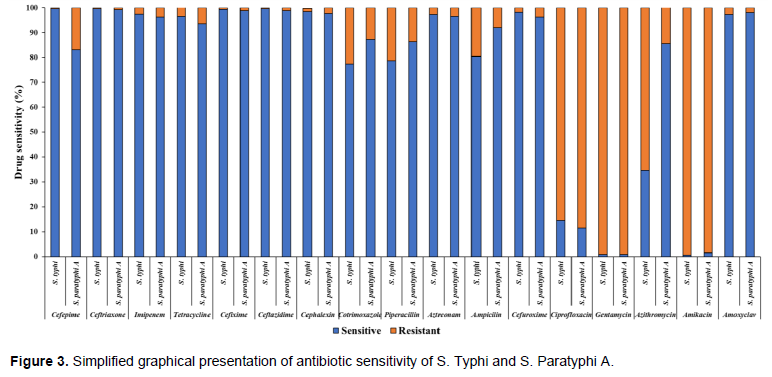
DISCUSSION
Enteric fever is a growing public health concern in developing and tropical countries, including Bangladesh. The indiscriminate use of antibiotics has intensified the problem by converting the previously sensitive drugs into resistant ones against the causative agent, Salmonella spp. In the present study, the existing susceptibility of S. Typhi and S. Paratyphi A were tried to investigate against some common antibiotics used to treat enteric fever.
This study showed, S. Typhi affected more enteric fever cases slightly compared to S. Paratyphi A, which is consistent with a previous study conducted by Ahmed et al. (2017). Likewise, Raza et al. (2012)also found that 55.8% of the enteric fever cases were diseased by S. Typhi and 44.2% with S. Paratyphi A. However, S. Typhi (66.6%) affected the number of enteric fever patients two times more than S. Paratyphi A (33.3%) (Guha et al., 2005). As far as the number of patients infected by both Salmonella infections, male patients dominated the females, with a proportion of 1.20:1. Accordingly, several studies presented that males were increasingly more susceptible to Salmonella spp. over females (Chowta and Chowta, 2005; Kumar et al., 2008).
In this study, patients aged 5-20 years accounted for the maximum enteric fever cases, whereas children under-5 years were less vulnerable than their older peers. Likewise, an earlier study revealed the majority of selected patients (63.8%) were 6-15 years, followed by the 16-25 years age group (22.41%) (Sattar et al., 2017). Again, Brooks et al. (2005) found that above-5 years children were more susceptible to enteric fever than those under-5 years, which is also comparable to our findings. Under-5-year cases, in this study, had slightly more chance to be affected by typhoid relative to paratyphoid fever. Some studies also found under-5-year children were more frequently affected by typhoid in comparison with paratyphoid fever (Naheed et al., 2010; Sinha et al., 1999). Although, some studies suggested that young children are less prone to typhoid fever (Ferreccio et al., 1984; Khanam et al., 2015).
In this study, S. Typhi was highly sensitive to cefepime, ceftriaxone, tetracycline cefixime, ceftazidime, cephalexin, cotrimoxazole, piperacillin, aztreonam, amoxiclav, and cefuroxime. Similarly, Ahmed et al. (2019) showed Salmonella spp. was highly effective against cefixime and ceftriaxone (Ahmed et al., 2019). Greater sensitivity of ceftriaxone to S. Typhi was also earlier found by another study (Britto et al., 2018). But, in sheer contrast to ours finding, a relevant Bangladeshi study in 2015 found higher resistance of S. Typhi for cotrimoxazole, cefixime, tetracycline, and ceftriaxone (Rahman, 2015). S. Typhi was highly sensitive to imipenem. Accordingly, imipenem (carbapenem) maintained high sensitivity to S. Typhi in many past studies. Rahman et al. (2015) reported increased sensitivity of S. Typhi to imipenem.
Two studies in Indonesia and China also noticed decreased resistance of S. Typhi to imipenem (Lugito and Cucunawangsih, 2017; Yaxian et al., 2015). However, we found alarmingly heightened resistance of S. Typhi against ciprofloxacin and azithromycin. Two relevant studies found a similar trend revealing excessive resistance of azithromycin and ciprofloxacin as 95.29 and 90.0%, respectively (Rahman, 2015; Vlieghe et al., 2012). Similarly, decreased ciprofloxacin susceptibility for S. Typhi has been witnessed by some studies in India recently (Chandel and Chaudhsry, 2001). In addition, a study in Pakistan reported the enhanced resistance of S. Typhi for ciprofloxacin, that is, consistent with our finding, but that same study found reduced sensitivity to ampicillin which is not consistent with our finding (Qamar et al., 2014). S. typhi was also highly resistant to antibiotics like gentamycin and amikacin. In sharp contrast to us, a community-based study in Indonesia showed almost no resistance against ceftriaxone or ciprofloxacin (Punjabi et al., 2013). The antibiotic resistance pattern may vary among the countries.
Furthermore, the current study revealed that S. paratyphi A was greatly sensitive to cefepime, ceftriaxone, imipenem, tetracycline, cefixime, ceftazidime, cephalexin, cotrimoxazole, piperacillin, aztreonam, amikacin, amoxiclav and cefuroxime. In agreement with this, S. Paratyphi A showed complete sensitivity to ceftriaxone (Bhatia et al., 2007). Interestingly, like S. Typhi strain, S. Paratyphi A also became resistant to ciprofloxacin. But, unlike S. Typhi which showed considerable insensitivity to azithromycin, S. Paratyphi A was sensitive against the same antibiotic. Earlier studies, contrarily to our outcomes, found azithromycin as highly sensitive to both Salmonella spp. (Chandey and Multani, 2012). We also observed a strikingly resemblance between S. Typhi and S. Paratyphi A as they both demonstrated similar enhanced insensitivity to two other antibiotics: gentamycin and amikacin. In contrast, Naheed et al. (2010) found that all S. Paratyphi A isolates were susceptible to all antimicrobial agents they tested. In Bangladesh, alarmingly, both S. Typhi and S. Paratyphi A lost the susceptibility to azithromycin.
Azithromycin’s insusceptibility to both S. Typhi and S. Paratyphi A poses an emerging public health concern as treatment failures have been reported (Molloy et al., 2010). Over-use of ciprofloxacin and azithromycin resulting from over-the-counter availability and easy oral administration, coupled with incomplete dose treatment by them might contribute to their high antibiotic resistance in Bangladesh. In the present study, not any single antibiotic had complete susceptibility to the total S. typhi isolates tested. Unless this increasing antibiotic resistance rate for Salmonella is checked, options for treating enteric fever cases would be lost shortly. Bangladesh Government should cryingly implement a national guideline on the proper usage of antibiotics.
CONCLUSION
The study unraveled the current antibiotic resistance patterns of S. Typhi and S. Paratyphi A to help medical practitioners so that they can make informed decisions and provide better treatment for enteric fever patients. This study revealed male and children were more susceptible to enteric fevers. Both S. Typhi and S. Paratyphi A were equally highly resistant to ciprofloxacin, gentamicin, and amikacin. Several antimicrobials presented significant variation in resistance against S. Typhi and S. Paratyphi A. Researchers and policymakers could find this study helpful in prioritizing their research scopes to tackle the upcoming challenges of antibiotic resistance among enteric fever patients.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Ahmed D, Nahid MA, Sami AB, Halim F, Akter N, Sadique T, Rana MS, Elahi MS, Rahman MM (2017). Bacterial etiology of bloodstream infections and antimicrobial resistance in Dhaka, Bangladesh, 2005-2014. Antimicrobial Resistance and Infection Control 6(1):2. |
|
|
Ahmed I, Rabbi MB, Sultana S (2019). Antibiotic resistance in Bangladesh: A systematic review. International Journal of Infectious Diseases 80:54-61. |
|
|
Bauer HW, Kirby WM, Sherris JC, Turck M (1966). Antibiotic susceptibility testing by standard single disc method. American Journal of Clinical Pathology 45(4):494-549. |
|
|
Bhatia JK, Mathur AD, Arora MM (2007). Re-emergence of chloramphenicol sensitivity in enteric fever. Medical Journal Armed Forces India 63(3):212-214. |
|
|
Britto CD, Wong VK, Dougan G, Pollard AJ (2018). A systematic review of antimicrobial resistance in Salmonella enterica serovar Typhi, the etiological agent of typhoid. PLoS Neglected Tropical Diseases 12(10). |
|
|
Brooks WA, Hossain A, Goswami D, Sharmeen AT, Nahar K, Alam K, Ahmed N, Naheed A, Nair GB, Luby S, Breiman RF (2005). Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerging Infectious diseases 11(2):326. |
|
|
Chandel DS, Chaudhry R (2001). Enteric Fever Treatment Failures: A Global Concern. Emerging Infectious Diseases 7(4):762-763. |
|
|
Chandey M, Multani AS (2012). A comparative study of efficacy and safety of azithromycin and ofloxacin in uncomplicated typhoid fever: A randomised, open labelled study. Journal of Clinical and Diagnostic Research 6(10):1736-1739. |
|
|
Chowta N, Chowta M (2005). Study of Clinical Profile and Antibiotic Response in Typhoid Fever. Indian Journal of Medical Microbiology 23(2):125. |
|
|
Clinical and Laboratory Standards Institute (CLSI) (2017). Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA 27th informational supplement. M100-S27. |
|
|
Connor BA, Schwartz E (2005). Typhoid and paratyphoid fever in travellers. Lancet Infect Diseases 5(10):623-628. |
|
|
Crump JA, Mintz ED (2010). Global trends in typhoid and paratyphoid fever. Clinical infectious diseases 50(2):241-246. |
|
|
Das S, Samajpati S, Ray U, Roy I, Dutta S (2007). Antimicrobial resistance and molecular subtypes of Salmonella enterica serovar Typhi isolates from Kolkata, India over a 15 years period 1998-2012. International Journal of Medical Microbiology 307(1):28-36. |
|
|
Ferreccio C, Levine MM, Manterola A, Rodriguez G, Rivara I, Prenzel I, Black R, Mancuso T, Bulas D (1984). Benign bacteremia caused by Salmonella typhi and paratyphi in children younger than 2 years. The Journal of Pediatrics 104(6):899-901. |
|
|
Global Burden of Disease Study (GBD) (2017). Data Resources | GHDx. (n.d.). Retrieved December 20, 2020, from |
|
|
Guha S, Jalan BY, Dey S, Easow JM, Wilson G, Shivananda PG (2005). Salmonella bacteraemia in Pokhara: emergence of antibiotic resistance. Nepal Medical College Journal 7(1):23-25. |
|
|
Lugito HPN, Cucunawangsih (2017). Antimicrobial resistance of salmonella enterica serovars Typhi and paratyphi isolates from a general Hospital in Karawaci, Tangerang, Indonesia: A five-year review. International Journal of Microbiology. Volume 2017 Article ID 6215136. |
|
|
Iyer RN, Jangam RR, Jacinth A, Venkatalakshmi A, Nahdi FB (2017). Prevalence and trends in the antimicrobial susceptibility pattern of Salmonella enterica serovars Typhi and Paratyphi A among children in a pediatric tertiary care hospital in South India over a period of ten years: a retrospective study. European Journal of Clinical Microbiology and Infectious Diseases 36(12):2399-404. |
|
|
Khanam C, Sayeed MA, Choudhury FK, Sheikh A, Ahmed D, Goswami D, Hossain L, Brooks A, Calderwood SB, Charles RC, Cravioto A, Ryan ET, Qadri F (2015). Typhoid Fever in Young Children in Bangladesh: Clinical Findings, Antibiotic Susceptibility Pattern and Immune Responses. PLoS Neglected Tropical Diseases 9(4):3619. |
|
|
Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ (2015). World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Medicine 12(12). |
|
|
Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong V, Dallman T, Nair S, Baker S, Shaheen G, Qureshi S, Yousafzai MT, Saleem MK, Hasan Z, Dougan G, Hasan R (2018). Emergence of an extensively drug-resistant Salmonella enterica serovar typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. MBio 9(1). |
|
|
Kumar S, Rizvi M, Berry N (2008). Rising prevalence of enteric fever due to multidrug-resistant Salmonella: An epidemiological study. Journal of Medical Microbiology 57(10):1247-1250. |
|
|
Molloy A, Nair S, Cooke FJ, Wain J, Farrington M, Lehner PJ, Torok ME (2010). First report of Salmonella enterica serotype paratyphi A azithromycin resistance leading to treatment failure. Journal of Clinical Microbiology 48(12):4655-4657. |
|
|
Naheed A, Ram PK, Brooks WA, Hossain MA, Parsons MB, Talukder KA, Mintz E, Luby S, Breiman RF (2010). Burden of typhoid and paratyphoid fever in a densely populated urban community, Dhaka, Bangladesh. International Journal of Infectious Diseases 14:93-99. |
|
|
Olarte J, Galindo E (1973). Salmonella typhi resistant to chloramphenicol, ampicillin, and other antimicrobial agents: strains isolated during an extensive typhoid fever epidemic in Mexico. Antimicrobial Agents and Chemotherapy 4(6):597-601. |
|
|
Punjabi NH, Agtini MD, Ochiai RL, Simanjuntak CH, Lesmana M, Subekti D, Oyofo BA, von Seidlein L, Deen J, Shin S, Acosta C, Wangsasaputra F, Pulungsih SP, Saroso S, Suyeti S, Suharno R, Sudarmono P, Syarurachman A, Suwandono A, Arjoso S, Beecham HJ, Corwin AL, Clemens JD (2013). Enteric fever burden in North Jakarta, Indonesia: A prospective, community-based study. Journal of Infection in Developing Countries 7(11):781-787. |
|
|
Qamar FN, Azmatullah A, Kazi AM, Khan E, Zaidi AKM (2014). A three-year review of antimicrobial resistance of Salmonella enterica serovars Typhi and Paratyphi A in Pakistan. Journal of Infection in Developing Countries 8(8):981-986. |
|
|
Raffatellu M, Wilson RP, Winter SE, Baumler AJ (2008). Clinical pathogenesis of typhoid fever. Journal of infection in Developing Countries 2(04):260-266. |
|
|
Rahman MA (2015). Antimicrobial Resistance Patterns of Salmonella Typhi Isolated from Stool Culture. Chattagram Maa-O-Shishu Hospital Medical College Journal 14(1):26-30. |
|
|
Raza S, Tamrakar R, Bhatt CP, Joshi SK (2012). Antimicrobial susceptibility patterns of Salmonella typhi and Salmonella paratyphi A in a tertiary care hospital. Journal of Nepal Health Research Council 10(22):214-217. |
|
|
Saha SK, Saha S, Ruhulamin M, Hanif M, Islam MA (1997). Decreasing trend of multiresistant Salmonella typhi in Bangladesh. Journal of Antimicrobial Chemotherapy 39(4):554-556. |
|
|
Saha S, Saha S, Das RC, Faruque ASG, Abdus Salam M, Islam M, Salam MA, Saha S (2018). Enteric fever and related contextual factors in Bangladesh. American Journal of Tropical Medicine and Hygiene 99(3):20-25. |
|
|
Sattar AA, Chowdhury MSJH, Yusuf MA, Jesmin S, Ara S, Islam MB (2017). Age and Gender Difference of Typhoid Fever among Paediatric Patients Attended at a Tertiary Care Hospital in Bangladesh. Bangladesh Journal of Infectious Diseases 3(2):36-39. |
|
|
Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, Bhan M (1999). Typhoid fever in children aged less than 5 years. Lancet 354(9180):734-737. |
|
|
Sur D, Ali M, Von Seidlein L, Manna B, Deen JL, Acosta CJ, Clemens JD, Bhattacharya SK (2007). Comparisons of predictors for typhoid and paratyphoid fever in Kolkata, India. BMC Public Health 7(1):289. |
|
|
Vlieghe ER, Phe T, De Smet B, Veng CH, Kham C, Bertrand S, Vanhoof R, Lynen L, Peetermans WE, Jacobs JA (2012). Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Neglected Tropical Diseases 6(12):e1933. |
|
|
Yaxian J, Hui Z, Hua N, Xiaoqin M, Fengliang L, Ning X, Jiajia L, Jie J, Rui Z (2015). Antimicrobial resistance surveillance of Salmonella isolates from the First People's Hospital of Yunnan Province, China. Journal of Infection in Developing Countries 9(4):333-337. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0