ABSTRACT
The green method of synthesizing nanoparticles is an eco-friendly, reliable and cost effective approach which has proven to be an alternative to chemical based methods over the years (Dipankar and Murugan, 2012). This research focuses on the synthesis and characterization of super-paramagnetic iron oxide nanoparticles (FeNPs), adopted a co-precipitation procedure by the interaction of ferric chloride solution (precursor) with aqueous extract of wonderful kola seed. The characterization phase was investigated using the Ultra-violet visible spectroscopy, Fourier Transform Infra- red, X-ray Diffraction and Scanning Electron Microscopy. The SEM analysis showed that the particles have irregular shapes with the average particle diameter measured between 10 to 40 nm. The crystalline nature of the nanoparticles was confirmed by the X-ray diffraction method with the crystallite size range between 10 and 47 nm. The absorbance peak of the synthesized wonderful kola FeNPs was investigated using the ultra-violet visible spectroscopy with wavelengths of 431, 488, 613 and 642 nm and corresponding band gap energies of 2.87, 2.54, 2.02 and 1.93eV respectively; while the absorbance peak of wonderful kola extract has a wavelength of 256 nm with band gap energy of 4.84 eV showing the tendency of a new compound formed. The FTIR peak at 3336.0cm-1 corresponds to the –OH bond stretching, with a strong and broad peak intensity (S, B) indicating the presence of alcohols and phenol. The peak value at 1636.3 cm-1 corresponds to C=O bond stretching vibration with strong intensity, indicating the presence of carbonyl and acids. The peak values also denote the change in fundamental vibrational levels of most molecules present in the sample. The synthesized magnetic nanoparticles could be applied as an enhancement tool in microwave tumour ablation.
Key words: Iron oxide nanoparticle, wonderful kola (Bulchholzia coriacea), super-paramagnetic, phytochemicals, FeNP characterization.
Microwaves occupy that portion of the electromagnetic spectrum between frequencies of 300 MHz and 300 GHz. Microwave ablation technique is similar to radiofrequency ablation in that it uses heats to destroy tissues. In the microwave frequency range, energy is deposited into tissues through waveguides or antennas (called applicators). Microwave ablation allows for flexible approaches to treatment of tumours through percutaneous, laparoscopic and open surgical access. With either computed tomographic or ultrasound guidance, the tumor is localized and the applicator is inserted. Because of the inherent properties of the electromagnetic wave, the device does not need to be grounded, thus alleviating the problem of grounding pad burns. The commonly used frequency bands for microwave ablation are 915 MHz and 2.45 GHz. Other frequencies explored for therapeutic applications include 433 MHz and broadband pulses with frequencies between 1 GHz and 10 GHz (Brace, 2011). Due to the fact that water molecules (H2O) are polar; when an oscillatory electric field interacts with water molecules, it causes the molecules to flip. As a result of the radiation interaction, the polar water molecules flip back and forth depending on the frequency of the microwave resulting in temperature increase. Therefore, microwave heats matter by agitating water and other polar molecules in the surrounding tissue, thus inducing cellular death via tissue coagulation and necrosis (Simon et al., 2005) at sufficiently high temperature. The temperature profile in tissue during ablation is obtained by solving a bio-heat equation according to Pennes (1948), for modeling thermal therapy procedures. The bio-heat equation is given by:

Where, 𜌠represents the density of nanoparticle.
The potential benefits of microwave technology include consistently higher intra-tumoral temperatures, larger
tumor ablation volumes, shorter ablation times, ability to use multiple applicators, improved convection profile, optimal heating of cystic masses and less procedural pain (Prakash, 2010). Biosynthesis, characterization and application of plant mediated metallic nanoparticles could reduce the exposure of patient to high dose of ionizing radiation. Microwave ablation has been reported to overcome most challenges associated with radiofrequency ablation (Ibitoye et al., 2016), hence the interest for this research work. The dynamic and unlimited applications of magnetic iron oxide nano-sized particles vary from industrial, medical, engineering to general applications. With a focus on the medical application of magnetic nanoparticles, its application varies from biosensors, magnetic resonance imaging, diagnostics, tissue engineering, theranostics, hyperthermia, magnetic separation to drug targeting and delivery (CD Creative Diagnostics and bio-particles products, 2009-2020). Numerous physical and chemical approaches have been formulated for the synthesis of nanoparticles of desired shape, wavelength, absorbance and size. Therefore, green synthesis has been considered as one of the promising methods for synthesis of nanoparticles because of their bio-compatibility, low toxicity and eco-friendliness in nature (Malik et al., 2014). The phytochemicals present in aqueous wonderful kola seeds function as a highly potent reducing and stabilizing agents for metals and as capping agents to create a sustainable coating on the metallic NP nanoparticles in a single step (https//uses.plantnetproject.org/en/Buchholzia_thollaniana_(PROTA)#other_botanical_information).
Purchase of experimental materials and identification
The seeds of the wonderful kola plant (Buchholzia coriacea) were purchased from the central market of Kaduna Metropolis and Oshodi market in Lagos State. The seeds were identified and validated at The University of Lagos Herbarium, Department of Botany, Akoka, Lagos with identification credentials of the seeds stated as (Scientific name: B. coriacea, family: Capparacea, LUH: 8248, Determinavit: Mr Nodza). The purity of ferric chloride hexahydrate, deionized water and other solvents used were strictly kept in check and ascertained to be of standard quality.
Processing and extraction of wonderful kola seeds
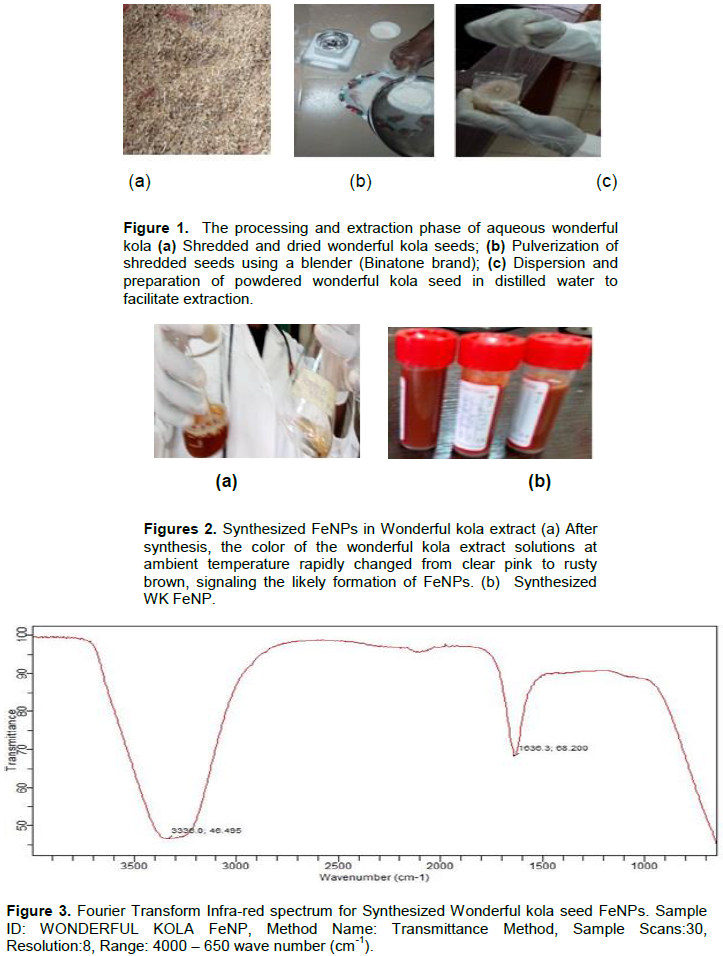
Ten wonderful kola seeds were washed meticulously with distilled water to remove dirts, epiphytes and decaying residues. The seeds were shredded and shade dried at ambient temperature for a period of 3 weeks. An electric food processor blender (Binatone brand) was used to pulverize the dried seeds. 20 grams of powdered wonderful kola seed was dissolved in 200 ml of distilled water in a beaker and mixed homogeneously. The mixture was covered with an Aluminum foil, placed inside a water bathe, covered and boiled at 60°C for 1 h to facilitate extraction. After boiling for an hour, the broth content was sieved into a clean beaker using a filter and Whitman filter paper to extract the filtrate and stored at 4° C for experimental use in accordance to the research study of Adelere et al. (2017). Figures 1 to 3 show the extraction phase of wonderful kola.
Synthesis of wonderful kola FeNPs
The aqueous extract of wonderful kola was used to synthesize iron nanoparticles where 0.2M of FeCl3 was reacted with 30 g/l of wonderful kola extract in a ratio of 2:3 at room temperature with constant stirring. An instant colour change from transparent pink to mild brown colour was observed. Furthermore, while stirring the mixture, 1.0 M of NaOH solution (prepared in 100 ml) was added to the mixture drop wise until a pH of 6.0 was achieved in accordance to Shahwan et al. (2011). A gradual colour change from mild brown to rusty brown colour was obtained. The introduction of the chloride of ferric solution to serve as a precursor agent to the wonderful kola seed extract (containing tannin, flavonoid, glycoside, saponins and alkaloids) possibly resulted in the reduction of iron salt component and nanoparticle stabilization. The activity of the hydroxyl group (OH) in the reduction of iron salt brought about a decline in the pH of the sample during the synthesis of iron oxide NPs. In the chemical reaction, the hydrolyzation of iron chloride (FeCl3) solution resulted in the formation of ferric hydroxide, which later releases H+ ions. The aqueous wonderful kola seed extract (R) in the reaction partially reduces the ferric hydroxide to form FeNPs with the oxidation of the aldehyde group to respective acids in relation to the study of Mahdavi et al. (2013) and Becerra et al. (2007). The presence of other organic compounds contained in the wonderful kola seed extract also aided the synthesis process to stabilize the value of the pH at 6, a prerequisite for enzymic reactions (Kanagasubbulakshmi and Kadirvelu, 2017). The biological reduction of iron oxide by the aqueous extract of wonderful kola seed for the synthesis of FeNP nano-sized particles was followed by centrifugation procedure for the isolation of Iron oxide NP from other compounds present in the sample. The prepared sample was transferred to new sample bottles and centrifuged at 1200rpm for 20 min. The surfactant was extracted from the supernatant into fresh sample bottles for drying at ambient temperature and also for characterization purpose.
Characterization of wonderful kola FeNPs
Different phases of characterization to ascertain the physical, chemical and morphological parameters of the synthesized wonderful kola seed nanoparticles were carried out which include: Fourier Transform Infra-red Spectroscopy (FTIR model, Cary 630, Agilent technologies, USA), X-Ray Diffraction (XRD model, EMPYREAN by Malvern Panalytical UK.), Ultraviolet Visible Spectroscopy (UV-Vis model, T90+ UV/VIS Spectrometer, PG Instruments Limited), Scanning Electron Microscopy (SEM model, Phenom ProX, by PHENOMWORLD ENDHOVEN the Netherlands).
Fourier transform infra-red spectroscopy
Fourier transform (a mathematical process) was adopted to transform the raw information into the infrared spectrum (Griffith et al., 2007) in this research study. FTIR Spectroscopy was adopted as a tool to identify the functional groups of the key elements with regards to determining the peak point (Full Width, Half Maximum FWHM) in the spectrum. Figure 3 depicts the FTIR spectrum of synthesized wonderful kola FeNPs obtained.
The FTIR analysis shows the stretching vibrations at 3336.0 and 1636.3 cm-1 with corresponding transmittance at a value of 46.495 and 68.200, respectively (Figure 3); it falls in the mid-infrared IR spectrum region of 400 to 4000 cm-1. The interpretation of the FTIR peak at 3336.0 cm-1 corresponds to the –OH bond stretch, with a strong and broad peak intensity (S, B). It indicates the presence of alcohols and phenols denoting the aqueous phase as well as aiding the reduction of the ferric chloride. The peak value at 1636.3 cm-1 corresponds to C=O bond stretch with strong intensity, indicating the presence of carbonyl and acids. This indicates the metabolites and phytochemicals available in the wonderful kola extract and amino acids, which aids the capping and stabilization process in the formation of FeNPs (https://www.compoundchem.com/). The peak values also depict the change in fundamental vibrational levels of most molecules present in the sample. The presence of traces of organic acid is denoted by unlabeled and visible peaks. These peaks reduce the pH of the samples which aids the synthesis of wonderful kola FeNPs (www.masterorganicchemistry.com).
X-Ray diffraction
The crystallinity in terms of the structure and the phase identification of the wonderful kola nano-sized particle was determined and analyzed by x-ray diffraction technique. Figure 4 depicts the x-ray diffraction of synthesized wonderful kola FeNPs. The presence of diffraction peaks with 2θ values of 23.0°, 29.4°, 31.6°, 34.4°, 36.0°, 39.4°, 43.2°, 47.5°, 48.5°, 56.6°, 57.4°, 60.6°, 64.6° corresponds to dspacing value of 0.39, 0.30,0.28, 0.26, 0.25, 0.23, 0.21, 0.19, ……. and 0.14 nm respectively of wonderful kola nano-sized crystals as obtained from Figure 4. The data obtained were compared with literatures and are in agreement with the x-ray diffraction standard for superparamagnetic nanoparticles (Table 1). The Debye Scherrer equation was adopted to calculate the average crystallite particle sizes which ranges approximately from 10 to 47 nm. The Debye Scherrer equation defines the relationship between the peak broadening and particle size in X-ray diffraction as denoted by the equation below (Patterson, 1939):

Equation 7: The Debye Scherrer Equation. Particle size of the crystal (nm) is d, Scherrer constant is k (dimensionless) (ranges from 0.9 to 1.0),
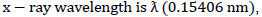
, width of the XRD peak at half-maximum is β (radian), bragg’s diffraction angle is θ (degrees). The value of the inter plane spacing
Ultra-violent visible spectroscopy
The Ultraviolet-visible spectroscopy is termed as the absorption spectroscopy in the UV-Visible spectral region. It makes use of light in the visible and adjacent (near – UV and near-infrared) ranges. In this region of the electromagnetic spectrum, molecules undergo electronic transitions (Skoog et al., 2007) (Figure 5).
In this research study, the absorbance peak of wonderful kola extract has a wavelength of 256nm absorbance 1.65340AU with band gap energy of 4.84eV. The absorbance peak of synthesized wonderful kola FeNPs displayed an onset of broad absorption maxima at wavelength of 431nm (absorbance 0.10047AU), 488nm (absorbance 0.15959AU), 613nm (absorbance 3.7274 E-3) and 642nm (absorbance 7.5321 E-2), accordingly in visible range between 200 to 800 nm wavelength. This indicates the formation of FeNPs having rusty brown color, which was investigated using the Ultra-violet visible spectroscopy. Band gap energy is calculated with respect to the Equation.

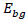
is the band-gap energy and Æ› is the wavelength of the nanoparticle. Corresponding band gap energies with reference to the wavelengths are 2.87, 2.54, 2.02 and 1.93eV. The presence of more than one broad absorption peaks at different wavelengths indicates the formation of iron nanoparticles which is believed to be due to the residue of collective oscillation of surface plasmons, with exposure to air new absorption bands appear as a result of oxidation with several maxima depicting the presence of numerous iron oxide nanoparticles in relation to the study of Klacanova et al. (2012).
Scanning electron microscopy
The Scanning Electron Micrograph (SEM) and Image-J software were adopted in this research to study the size distribution and measurement parameters of wonderful kola FeNPs. The average size of the Wonderful kola FeNPs was in the range of 10 to 40 nm. The nanoparticles in Figures 6 to 8 were observed to have inhomogeneous and irregular shapes (octahedral, spherical and unquantifiable shapes as shown above). The physical and structural features of the synthesized nano-sized particle are largely affected by the irregular shape of the NP induced by the constituent of the synthesized sample (Table 2).
The use of wonderful kola seed in the synthesis of zero-valent super-paramagnetic nano-sized particles was achieved. The co-precipitation method adopted proved effective and easily replicated. A colour change from clear pink to rusty brown was observed from the synthesis of wonderful kola iron oxide nanoparticle. In the XRD peak analysis, the Debye Scherer equation was adopted to calculate the crystallite particle sizes which ranges approximately from 10 to 47 nm. The crystallite particle sizes as depicted by the SEM analysis ranges from 10 to 40 nm, with the presence of irregular shapes (octahedral, spherical, cubic and other unspecified shapes). The FTIR peak at 3336.0 cm-1 corresponds to the –OH bond stretch, with a strong and broad peak intensity (S, B) indicating the presence of alcohols and phenols denoting the aqueous phase as well as aiding the reduction of the ferric chloride. The peak value also shows traces of N-H stretch with a medium intensity, indicating the presence of amine and amides. The peak value at 1636.3 cm-1 corresponds to C=O bond stretch with strong (S) intensity, indicating the presence of carbonyl and acids. This denotes the phytochemicals available in the wonderful kola extract and amino acids, which aids the capping and stabilization process in the formation of FeNPs. The peak values also depict the change in fundamental vibrational levels of most molecules present in the sample. In the UV-Vis spectrum analysis, the absorbance peak of wonderful kola extract has a wavelength of 265 nm with band gap energy of 4.68 eV. The absorbance peak of synthesized WK FeNPs has a wavelength of 431, 488, 613 and 642 nm accordingly which was investigated using the ultra-violet visible spectroscopy; corresponding band gap energies with reference to the wavelengths are 2.87, 2.54, 2.02 and 1.93 eV.
The authors are grateful to the Nigerian Defence Academy, Department of Physics, Kaduna, The College of Medicine, Department of Radiation Biology, Radiotherapy and Radio diagnostics, Department of Pharmaceutical Chemistry, Central Research Laboratory (The College of Medicine, University of Lagos, Nigeria). This research study was supported by the Nigerian Defence Academy, Ribadu Campus, Kaduna State, Nigeria.
The authors declare no conflict of interest.
REFERENCES
|
Adelere IA, Agbaje L, Aboyeji DO, Abdulsalam R, Adabara NU, Bala JD (2017). Biosynthesis of Silver Nanoparticles Using Aqueous Extract of Buchholzia Coriacea (Wonderful kola) Seeds and Their Antimicrobial Activities. Annals Food Science and Technology 18(4):1-4.
|
|
|
|
Becerra RH, Zorrilla C, Ascencio JA (2007). Production of iron oxide nanoparticles by a biosynthesis method: An environmentally friendly route. Journal of Physical Chemistry 111(44):16147-16153.
Crossref
|
|
|
|
|
Brace CL (2011). Microwave Tissue Ablation biophysics, Technology and Applications. Critical Reviews in Biomedical Engineering 38(1):65-78.
Crossref
|
|
|
|
|
CD Creative Diagnostics and bio-particles products, (2009 - 2020) Properties and applications off magnetic nanoparticles.
View
|
|
|
|
|
Dipankar C, Murugan S (2012). The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from iresine herbstii leaf aqueous extract, Colloids and Surfaces, Sciencedirect: Bio interface 98:112-119.
Crossref
|
|
|
|
|
Griffith PR, De Hasseth JA, Winefordner JD (2007). Fourier Transform Spectroscopy, 2nd Edition, Wiley-Blackwell. pp. 1-560.
|
|
|
|
|
Ibitoye AZ, Nwoye EO, Aweda AM, Oremosu AA, Anunobi CC, Akanmu NO (2016). Microwave ablation of ex vivo bovine tissues using a dual slot antenna with a floating metallic sleeve. International Journal of Hyperthermia 32(8):923-930.
Crossref
|
|
|
|
|
Kanagasubbulakshmi S, Kadirvelu K (2017). Green Synthesis of Iron Oxide Nanoparticle using Lagenaria Siceria and Evaluation of its Antimicrobial Activity. Defence Life Science Journal 2(4):422-425.
Crossref
|
|
|
|
|
Klacanova K, Fodran P, Simon P, Rapta P, Boca R, Jori V, Miglierini M, Kolek E, Caplovic L (2012). Formation of Fe(O) - Nanoparticles via reduction of Fe (II) compounds by amino acids and their subsequent oxidation to iron oxides. Hindawi Publishing Corporation. Journal of Chemistry 2013:1-10.
Crossref
|
|
|
|
|
Mahdavi M, Farideh N, Mansor BA, Rosfarizan M (2013). Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18(5):5954-5964.
Crossref
|
|
|
|
|
Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK (2014). Green chemistry based benign routes for nanoparticle synthesis. Journal of Nanoparticles 2014:1-14.
Crossref
|
|
|
|
|
MasterOrganicChemistry.
|
|
|
|
|
Patterson A (1939). 'The Scherrer formula for x-ray particle size determination. American Physical Society 56(10):978-982.
Crossref
|
|
|
|
|
Pennes HH (1948). Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. Journal of Applied Physiology 1(1):93-122.
Crossref
|
|
|
|
|
Prakash P (2010). Theoretical modeling for hepatic microwave ablation. The Open Biomedical Engineering Journal 4:27-38.
Crossref
|
|
|
|
|
Rachel L, Kashif A, Minh L, Nancy LO (2003). Fluorescence Resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Research 63(17):5194-5197.
|
|
|
|
|
Shahwan T, Abu SS, Nairat M, Scott TB (2011). Green synthesis of iron nanoparticles and their applications as a fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Elsevier, Chemical Engineering Journal 172(1):258-266.
Crossref
|
|
|
|
|
Simon CJ, Dupuy DE, Mayo-Smith WW (2005). Microwave ablation: principles and applications. RadioGraphics 25:S69-S83.
Crossref
|
|
|
|
|
Skoog DA, Holler FJ, Crouch SR (2007). Principles of Instrumental Analysis, 6th edition, Belmont, CA. Thomson books/cole pp.169-173.
|
|
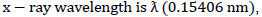 , width of the XRD peak at half-maximum is β (radian), bragg’s diffraction angle is θ (degrees). The value of the inter plane spacing
, width of the XRD peak at half-maximum is β (radian), bragg’s diffraction angle is θ (degrees). The value of the inter plane spacing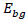 is the band-gap energy and Æ› is the wavelength of the nanoparticle. Corresponding band gap energies with reference to the wavelengths are 2.87, 2.54, 2.02 and 1.93eV. The presence of more than one broad absorption peaks at different wavelengths indicates the formation of iron nanoparticles which is believed to be due to the residue of collective oscillation of surface plasmons, with exposure to air new absorption bands appear as a result of oxidation with several maxima depicting the presence of numerous iron oxide nanoparticles in relation to the study of Klacanova et al. (2012).
is the band-gap energy and Æ› is the wavelength of the nanoparticle. Corresponding band gap energies with reference to the wavelengths are 2.87, 2.54, 2.02 and 1.93eV. The presence of more than one broad absorption peaks at different wavelengths indicates the formation of iron nanoparticles which is believed to be due to the residue of collective oscillation of surface plasmons, with exposure to air new absorption bands appear as a result of oxidation with several maxima depicting the presence of numerous iron oxide nanoparticles in relation to the study of Klacanova et al. (2012).