ABSTRACT
Scutellaria or skullcap is a genus containing approximately 350 species of flowering plants, many of which are sold and marketed for their medicinal value. Flavonoids found in Scutellaria spp. have demonstrated anti-inflammatory, antiviral, sedative, antithrombotic and antioxidant affects. Baicalin, a flavonoid produced by Scutellaria spp., is an important compound used to treat anxiety. Scutellaria spp. have potential as commercially valuable specialty crops based on their visual and medicinal properties; however, a lack of commercial production techniques for successful cultivation of this genus precludes adoption by most growers. The influence of plant production techniques on flowering and baicalin synthesis is undocumented; thus, empirical research is needed for development of commercial production protocol. Objectives of this research were to investigate the effect of nutrient application rate and plant available water on growth and baicalin synthesis in Scutellaria arenicola and Scutellaria integrifolia, two common species of Scutellaria found in eastern United States. To accomplish these objectives, S. arenicola and S. integrifolia were cultivated in a greenhouse and subjected to one out of four nutrient application rates and one out of two volumetric water content rates. Results demonstrated that synthesis of baicalin, the main flavonoid of the Scutellaria genus that contributes to its reported medicinal benefits, occurred in both species. Fertilization rate and volumetric water content were found to influence both plant growth and baicalin concentration in S. integrifolia. In contrast to results observed for S. integrifolia, volumetric soil water and nutrient application rate did not influence plant growth in S. arenicola. Scutellaria spp. cultivated in the greenhouse had similar concentrations of baicalin to those harvested from the wild, undisturbed natural habitats. Results from this investigation will assist in development of commercial production protocol for these species and provides the first foundational research that has reported the presence of baicalin, a high value medicinal compound in S. arenicola.
Key words: Skullcap, medicinal, flavonoid, cultivation, production.
Plants of the Scutellaria genus are found around the globe and are prized for their medicinal qualities. In many Asian countries, S. baicalensis or Baikal skullcap, is prescribed to patients as an anti-inflammatory, antiviral and antithrombotic medicine (Shang et al., 2010). In the United States, S. lateriflora or American skullcap, is sold for its reported anxiolytic properties. Commercial sales and marketing for medicinal applications of S. lateriflora exist despite the lack of research documenting the presence of medicinally active compounds within the plant (Shang et al., 2010). Medicinal benefits of Scutellaria spp. are attributed to flavonoids present in vegetative and root tissue of most species. Flavonoids found in many species of Scutellaria include baicalin, baicalein and wogonin (Similien et al., 2016). Baicalin, one of the main flavonoids found in many species of Scutellaria, is valued for its anxiolytic effects without any sedative or myorelaxant effects (Liao et al., 2003; Xu et al., 2006). In the United States, skullcap has not been evaluated by the Food and Drug Administration as a medicine and thus has been designated as a herb of “undefined safety” (Awad et al., 2003). Nonetheless, dried skullcap shoots are commonly found in the market as an ingredient in tea, as a vitamin, and as liquid extract. Two species of Scutellaria, native to United States, Florida, S. integrifolia and S. arenicola, possess potential for commercial production; however, neither of them have been evaluated for growth and performance or baicalin content when cultivated within a greenhouse production environment.
Scutellaria spp. containing high concentrations of baicalin had high market value (Similien et al., 2016). Production of plant secondary metabolic compounds, such as baicalin, are believed to be produced by plants to protect plant cellular tissue, especially when plant stress is experienced (Kumar and Pandey, 2013). Plant stress experienced in natural, undisturbed environments are believed to result in greater secondary compound synthesis (Shippmann et al., 2002). When cultivated in protected commercial environments, medicinal plants are thought to produce decreased concentrations of secondary compounds. Relationships between nutrient availability and synthesis of baicalin may be explained by the Carbon-Nutrient Balance Hypothesis (CNBH) (Hamilton et al., 2001). The hypothesis predicts production of secondary metabolic compounds when deficiencies in carbon and nitrogen exist (Hamilton et al., 2001). Flavonoid development within Scutellaria spp. has been reported to occur in response to water and nutrient availability. Cao et al. (2012) found that baicalin concentrations in S. baicalensis were negatively correlated with nitrogen fertilizer application. Similarly, Similien et al. (2016) observed negative correlations between baicalin concentration and nutrient availability in S. lateriflora. Yuan et al. (2012) observed increased flavonoid concentrations in root and leaf tissue of S. baicalensis when subject to mild water stress (12% soil water content) as compared to a control that received sufficient water (16% soil water content). These results are in contrast to findings by Similien et al. (2016), who observed increased baicalin concentrations in S. lateriflora when irrigation was applied to plants cultivated on open field. When cultivated under 40% shaded conditions, however, field-grown S. lateriflora flavonoid concentrations were not significantly influenced by irrigation applications.
Rising popularity and interest in herbal medicines, coupled with the unknown influence of greenhouse production practices on plant growth and synthesis of the flavonoid baicalin within two Scutellaria spp., supports the need for research on this specialty crop. The objective of this study was to investigate the influence of nutrient application and plant available water on growth and baicalin synthesis of two native North American skullcap species, S. integrifolia and S. arenicola, within a protected greenhouse production environment. Results from this experiment can be used to develop cultivation practices for these potentially valuable medicinal plant species.
Cultivation and preparation
On 6th June 2017, 48 cuttings of S. integrifolia were taken from a single stock plant, dipped in indole-3-butyric acid rooting hormone (Hormidin 1; OHP Inc., Mainland, PA, United States) and transplanted into 4 cm3 rockwool cubes (Grodan, Milton, ON, Canada). Cuttings were placed in propagation chambers under continuous fluorescent lighting (T5; Sunblaster, Langley, BC, Canada), and kept at 25.5 °C and above 50% humidity for three weeks until rooted. On 27 June 2017, individual cuttings were transplanted into 2 L containers filled with a soilless substrate with a composition of 30% pine bark, 48% peat, 10% perlite and 12% vermiculite (Fafard 4P, Sun Gro Horticulture Canada, Ltd., Agawam, MA, United States) and placed on a bench in a 30.5 x 14.6 m gutter-connected greenhouse with 30% light reducing polycarbonate paneling located in Apopka, Florida, United States (latitude 28°38’ N, longitude 81°33’ W). Environmental conditions within the greenhouse were measured and recorded every 15 min by a data logger (WatchDog 2475; Spectrum Technologies, Inc., Aurora, IL, United States). Forty-eight cuttings of S. arenicola were taken on 5 July 2017 and subject to the same propagation methods previously described for S. integrifolia. Due to poor rooting of this species, only 32 cuttings of S. arenicola were transplanted on 28th July 2017.
To quantify baicalin concentrations from plants found growing in a natural, undisturbed habitat (Lake County, FL, United States), field-harvested samples of S. integrifolia and S. arenicola were collected in August 2017. Given the commercial popularity of S. latiflora as an anxiolytic herbal medicine, samples of S. latiflora leaves were purchased from a local herbal medicine market (Leaves & Roots; Orlando, FL, United States).
Fertilization treatment
One week after transplant (WAT) on 5th July 2017, Osmocote 15-9-12 slow release fertilizer (Everris NA Inc., Dublin, OH, United States) was applied to S. integrifolia replicates (n=12) at one of the four rates: 0 (none), 3.8 (low), 9.2 (medium) or 13.8 g (high). Fertilizer formula includes 8.4% ammoniacal and 6.6% nitrate nitrogen, 9% phosphate and 12% soluble potash. Fertilizer application rate was selected based on manufacturer’s recommendations. Upon transplant into 2 L containers, each plant received 112.5 mL of water daily using a pressure-compensating drip irrigation emitter (Woodpecker Pressure Compensating drippers; Netafim, Fresno, CA, United States). This treatment was repeated for S. arenicola on 3rd August 2017.
Volumetric water content treatment
Volumetric water content (VWC) was measured and recorded every 60 min throughout the experiment utilizing soil moisture sensors connected to a data logger (Em50; Decagon Devices, Pullman, WA, United States). Throughout WAT 1 to 3, substrate moisture was maintained among all plants at 56.2% VWC. Three weeks after transplant, half of the plants for each fertilizer treatment (n=6) for S. integrifolia were selected at random and subjected to reduced VWC of 9.9% to induce water stress. For S. arenicola, half of the plants for each fertilizer treatment (n=4) were selected at random and subjected to reduced VWC of 9.9% 4 WAT on 21st August 2017. An additional week of growth prior to implementation of reduced VWC for S. arenicola was provided to allow plants to reach sufficient size.
Measurements and data collection
Beginning from 1 WAT, plant growth indices (PGI = height × width1 × width2) were recorded weekly and continued until termination of the experiment 5 WAT. S. integrifolia were terminated 2nd August 2017 (5 WAT) when plants began flowering. Plants were destructively harvested by cutting the plant 2.5 cm above the crown and mass was recorded using a laboratory balance (Adventurer Analytical; Ohaus, Parsippany, NJ, United States). Plants were dried at room temperature until ≥ 70% moisture was lost and dry mass yield (DMY) was recorded. Dried plant material from the same treatment group were combined and vacuum-sealed for storage until ready for baicalin analysis using high performance liquid chromatography (HPLC). S. arenicola were also cultivated for five weeks and destructively harvested on 1 September 2017. Plant DMY was recorded and all replicates of each treatment were combined and vacuum-sealed for storage until HPLC analysis could be completed.
Preparation of leaf and stem extracts
Aliquots were prepared by randomly selecting a 0.1 g sample from the previously vacuum-sealed containers. Samples were placed in a test tube (13 × 100 mm Beaded Rim, Pyrex, Corning, NY, United States) and chemical extraction was performed using a prepared 0.005% acetic acid in a methanol : water extraction solution (80:20). 2 mL of extraction solution was added to each test tube and homogenized (Homogenizer 850; Thermo Fisher Scientific Inc., Waltham, MA, United States) at 21,000 rpm for a duration of 1 min. Extracts were placed into a 1.5 mL Eppendorf tube (1.5 mL Graduated Natural; Thermo Fisher Scientific Inc., Waltham, MA, United States) and centrifuged (Eppendorf Centrifuge 5418; Hauppauge, NY, United States) at 10,000 rpm for 10 min. Samples were then filtered through a 0.2 µm filter (25 mm Nylon Syringe Filter; Thermo Fisher Scientific Inc., Waltham, MA, United States) and placed in 2 mL HPLC ready vials (Clear Glass Sure Stop Vial; Thermo Fisher Scientific Inc., Waltham, MA, United States). Four extracts of each treatment combination for S. integrifolia were prepared and analyzed. Due to poor growth and lack of plant material, only two samples of each treatment combination for S. arenicola were prepared and analyzed. Four samples of field-harvested S. integrifolia and S. arenicola, and four samples of locally purchased S. lateriflora were extracted using the previously described extraction technique.
HPLC analysis
Baicalin concentration analysis was performed using a Dionex UltiMate 3000 HPLC (Thermo Fisher Scientific Inc., Waltham, MA, United States) with a C18 3 µm 3 × 150 mm column (Thermo Fisher Scientific Inc., Waltham, MA, United States). Analysis was performed utilizing the method described by Li et al. (2015). Mobile phase solutions were compromised of 0.01% phosphoric acid aqueous solution (A) and acetonitrile (B). The gradient elution was as follows: 0 to 3.5 min, 27% v/v B; 3.5-6 min, 27-60% B; 6-7 min, 60-40% B; 7-10 min, 40-27%; and then returned to initial conditions for 2 min for a total run time of 12 min. Column temperature was maintained at 30°C, scan wavelength ranged from 220-400 nm, flow rate was maintained at 0.8 mL min-1, and sample injection volume was 1 µL. The wavelength for baicalin absorption, as determined by Li et al. (2015), was 278 nm. Peak detection occurred 1.89 min after sample injection (Figure 1).
Standard calibration
Baicalin standard was purchased from Indofine Chemical Company (Hillsborough, NJ, United States). Solid baicalin standard was dissolved in 100% methanol to achieve a standard concentration of 250 µg mL-1. Standard calibration was achieved by doing serial injection at rates of 1, 5, 10, and 15 µL and resulted in a calibration correlation of r2=0.99.
Statistical analysis
The experiment was arranged as a complete randomized design with irrigation and fertilizer application rate assigned as independent variables. Six replicates were cultivated for each treatment combination for a total of 48 experimental units for S. integrifolia. Four replicates were cultivated for each treatment combination for S. arenicola. Statistical analysis of PGI, DMY and baicalin was analyzed using mixed model analysis in JMP® Pro 13 (SAS; Cary, NC) with post-hoc mean separation tests performed using Tukey’s honest significant difference test by WAT with variance within treatment combination replicates defined as the random error term. Statistical tests were considered significant if P ≤ 0.05.
Greenhouse environmental conditions
Mean daily greenhouse temperature ranged from 24.0 to 33.6°C throughout the duration of the experiment. Mean relative humidity was 79.8% and photosynthetic active radiation varied from 59.6 to 311.6 µmol m‑2 s-1.
Plant growth index
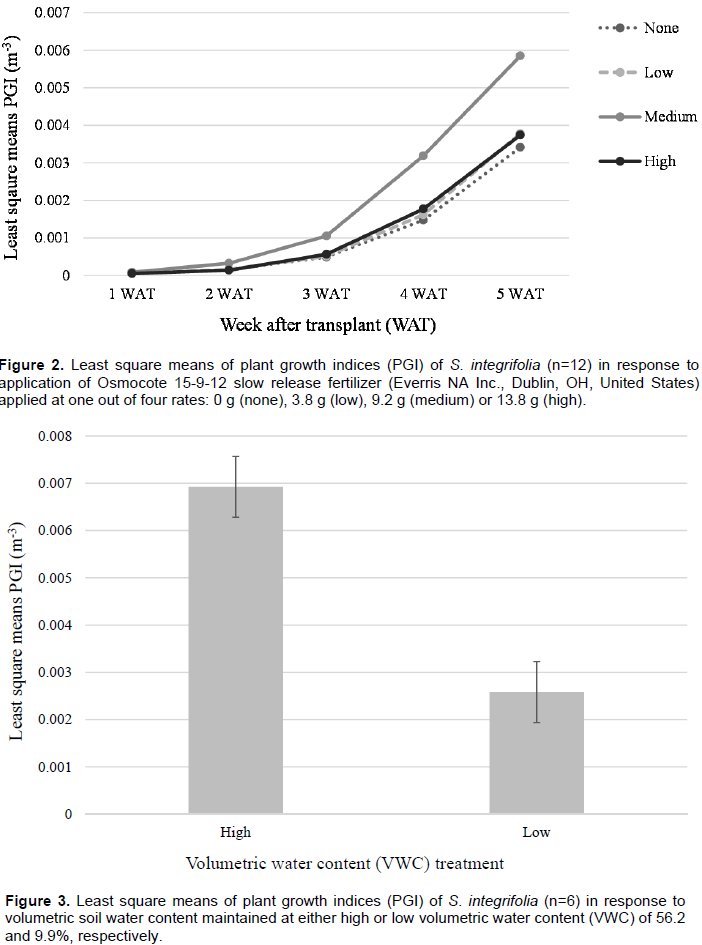
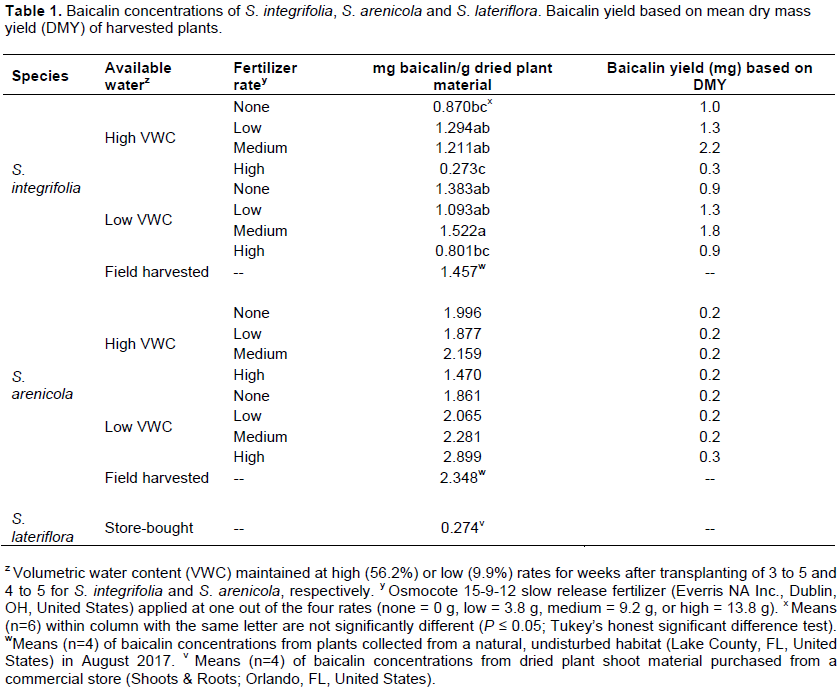
No significant effects were observed for PGI among fertilizer or water stress treatments for S. integrifolia between 1 and 4 WAT. Plant growth was minimal between 1-2 WAT; however, beginning from 3 WAT, growth was quadratic (Figure 2). Plants subjected to the medium fertilizer application rate had the highest PGI, between 2 and 5 WAT. At termination of the experiment, 5 WAT, a water stress effect was observed (Figure 3). Least square means of PGI for plants subjected to high VWC were significantly greater (5.8 e-3 m-3) for plants that were maintained at a high VWC than those subjected to low VWC (2.6 e-3 m-3).
No significant effects were observed for PGI among fertilizer or water stress treatments for S. arenicola throughout the duration of the experiment. Moreover, PGI did not increase between between 1 and 5 WAT (data not shown). Plants that received the high fertilizer rates, regardless of water stress treatment, had the highest PGI at termination of the experiment with a least square mean of 4.1 e-5 m3. Plants that received medium rate applications of fertilizer possessed the lowest overall PGI 5 WAT with a least square mean of 2.8 e-5 m3.
Plant dry mass
No significant differences among treatment combinations were observed for DMY of S. integrifolia (Figure 4). Plants that received medium fertilizer application rates and subjected to high VWC had highest dry mass (1.8 g). Lowest DMY (0.7 g) was recorded for S. integrifolia that received no fertilizer and subjected to low VWC. Similar to results observed for S. integrifolia, no significant differences among treatment combinations were observed for S. arenicola (Figure 4). S. arenicola that received low fertilizer application rates and subjected to high VWC had the highest DMY of 0.11 g. Contrary to observations for S. integrifolia, S. arenicola that received medium fertilizer application rates and subjected to high VWC had the lowest DMY at 0.08 g.
Baicalin concentration
Baicalin concentrations ranged from 0.801 to 1.383 mg g plant shoot tissue-1 (Table 1). A fertilizer and water stress interaction was observed for baicalin concentrations of S. integrifolia. High concentrations of baicalin were observed in plants that received low and medium application rates of fertilizer, regardless of water stress treatment. S. integrifolia that received no fertilizer and subjected to low VWC possessed a high mean baicalin concentration (1.383 mg baicalin g plant shoot tissue-1); however, plants that received the same fertilizer treatment (none) but were maintained at high VWC, possessed significantly less mean baicalin (0.870 mg g plant shoot tissue-1). Based on DMY, greatest baicalin yield was observed when S. integrifolia received medium rate applications of fertilizer. When high rates of fertilizer were used during cultivation of S. integrifolia, low concentrations of baicalin were observed within the plant shoot tissue regardless of water stress treatment.
Baicalin concentrations in S. arenicola ranged from 1.861 to 2.899 mg g plant shoot tissue-1 (Table 1). Baicalin concentrations resulting from each treatment combination were approximately 2-fold greater than those for similar treatment combinations for S. integrifolia; however, limited plant mass resulted in insufficient replication to perform statistical mean separation tests among imposed treatments. Greatest baicalin concentrations were observed for S. arenicola that received high fertilizer application rates and subjected to low VWC. Given poor growth, baicalin yield of S. arenicola was low (0.2 to 0.3 mg).
Field harvested S. integrifolia and S. arenicola had mean baicalin concentrations of 1.457 and 2.348 mg g plant shoot tissue-1, respectively (Table 1). Baicalin concentrations were similar to those cultivated within the greenhouse and subjected to experimental treatments. S. lateriflora, purchased at a local medicinal herbal store, had low concentrations of baicalin 0.274 mg g plant shoot tissue-1 and were similar to concentrations observed for S. integrifolia that received high rates of fertilizer and high VWC (0.273 mg g plant shoot tissue-1).
S. arenicola responded poorly to clonal propagation. Unlike S. integrifolia, which displayed logarithmic growth during the five-week study, S. arenicola did not increase in plant mass. Poor growth and performance of S. arenicola suggests that commercial cultivation techniques imposed in this study were not ideal for this species. Despite poor growth, however, baicalin concentrations in this species were approximately 2-fold greater than those observed in S. integrifolia. Detection of baicalin in S. arenicola has not been reported before. Relatively high concentrations of baicalin observed in S. arenicola warrant additional investigations to better understand relationships between plant growth and baicalin synthesis.
Increased fertilizer application rate, from none (0 g) to low (3.8 g) and medium (9.2 g), resulted in increased concentration of baicalin in both S. integrifolia and S. arenicola, regardless of VWC treatment. In contradiction to this trend, high fertilizer application rates (13.8 g) resulted in low baicalin concentrations in both S. integrifolia and S. arenicola, with exception of S. arenicola subjected to the low VWC treatment. Decreased baicalin concentration in response to increased application of fertilizer rates has been observed in both S. baicalensis and S. lateriflora (Cao et al., 2012; Shiwakoti et al., 2016; Similien et al., 2016). Results observed in the current investigation largely contradict these findings, thus suggest relationships between nutrient and water availability and baicalin concentration are likely species dependent, and cannot be predicted simply by application of the CNBH. Empirical studies are therefore necessary to establish these relationships and assist in development of recommended commercial production practices.
Both S. integrifolia and S. arenicola cultivated in the current study were found to possess similar concentrations of baicalin to plants found growing in local, undisturbed natural environments. Results, therefore, do not support the assertion that medicinal plants produced within protected commercial environments will unremittingly possess lower concentrations of secondary metabolic compounds, such as baicalin, due to decreased exposure to environmental stressors. Baicalin concentrations within all cultivated plants in this study were found to be greater than concentrations observed within the limited, medicinal commercial samples of S. lateriflora analyzed in this investigation. Although, not an objective of this investigation, a more diverse sampling of commercially available Scutellaria spp. medicinal products would help define variability that exists in the marketplace. Given its relatively rapid growth rate coupled with high baicalin concentration, S. integrifolia likely possess qualities that would allow it to be successfully produced commercially for medicinal application. More specifically, production practices implemented in this investigation (medium application rates of fertilizer coupled with sufficient volumetric water content) provide a foundation for successful production of high yielding plant material that possess relatively high concentrations of baicalin.
Production of secondary metabolic compounds such as baicalin are believed to decrease in response to applications of fertilizer and when plants are cultivated within protected commercial greenhouse environments. Results of this investigation, however, showed that greenhouse cultivation of S. integrifolia and S. arenicola did not result in decreased production of baicalin as compared to plants obtained from local, undisturbed natural environments. Moreover, applications of fertilizer increased synthesis of baicalin in majority of the imposed treatments. S. integrifolia exhibited fast growth, reaching reproductive stage by five weeks, and responded well to commercial clonal propagation techniques. Medium fertilizer application rates and high VWC produced S. integrifolia with high baicalin yield. Although, S. arenicola exhibited poor growth trends throughout this study, high concentrations of baicalin present within this species support future studies. Empirical studies that examine the relationships between horticultural production techniques and plant growth and response, such as those presented here, are necessary for the establishment of commercial production practices for medicinally important plant species.
The authors have not declared any conflict of interests.
The authors thank Wendy Poag of Lake County Parks and Trails, for her assistance in obtaining plant materials for this experiment and her guidance in experiment concepts.
REFERENCES
|
Awad R, Arnason J, Trudeau V, Bergeron C, Budzinski J, Foster B, Merali Z (2003). Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine 10(8):640-649.
Crossref
|
|
|
|
Cao X, Xu F, Wang W, Wang J, Huang S, Zhang X (2012). Responses of Scutellaria baicalensis 'Georgi' yield and root baicalin content to the fertilization rates of nitrogen, phosphorus, and potassium. The Journal of Applied Ecology 23(8):2171-2177.
|
|
|
|
|
Hamilton J, Zangerl A, DeLucia E, Berenbaum M (2001). The carbon–nutrient balance hypothesis: its rise and fall. Ecology Letters 4(1):86-95.
Crossref
|
|
|
|
|
Kumar S, Pandey A (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal. doi: 10.1155/2013/162750.
Crossref
|
|
|
|
|
Li B, Chen J, Li J, Wang X, Zhai H, Zhang X (2015). Highâ€performance liquid chromatography with photodiode array detection and chemometrics method for the analysis of multiple components in the traditional Chinese medicine Shuanghuanglian oral liquid. Journal of Separation Science 38(24):4187-4195.
Crossref
|
|
|
|
|
Liao JF, Hung WY, Chen CF (2003). Anxiolytic-life effects of baicalein and baicalin in the Vogel conflict test in mice. European Journal of Pharmacology 464(2-3):141-146.
Crossref
|
|
|
|
|
Shang X, He X, He X, Li M, Zhang R, Fan P, Zhang Q, Jia Z (2010). The genus Scutellaria and ethnopharmacological and phytochemical review. Journal of Enthopharmacology 128(2):279-313.
Crossref
|
|
|
|
|
Shippmann U, Leaman DJ, Cunningham AB (2002). Impact of cultivation and gathering of medicinal plants on biodiversity: Global trends and issues. Biodiversity and the ecosystem approach in agriculture, forestry, and fisheries. Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture, Rome, Italy.
|
|
|
|
|
Shiwakoti S, Shannon D, Wood C, Joshee N, Rimando A, Lawrence K, Kemppainen B (2016). Nitrogen, phosphorus, and potassium effects on biomass yield and flavonoid content of American skullcap (Scutellaria lateriflora). Journal of Plant Nutrition 39(9):1240-1249.
Crossref
|
|
|
|
|
Similien A, Shannon D, Wesley Wood C, Rimando A, Kemppainen B, van Santen E, Joshee N (2016). Shade, Irrigation, and Nutrients Affect Flavonoid Concentration and Yield in American Skullcap. Crop Science 56(3):1213-1224.
Crossref
|
|
|
|
|
Xu Z, Wang F, Tsang, SY, Ho KH, Zheng H, Yuen CT, Chow CY, Xue H (2006). Anxiolytic-like effect of baicalin and its additivity with other anxiolytics. Planta Medica 72(2):189-192.
Crossref
|
|
|
|
|
Yuan Y, Liu Y, Wu C, Chen S, Wang Z, Yang Z, Huang L (2012). Water deficit affected flavonoid accumulation by regulating hormone metabolism in Scutellaria baicalensis 'Georgi' roots. PLoS One 7(10):e42946.
Crossref
|
|