ABSTRACT
Cabbage is one of the most important vegetable crops under cultivation throughout the world, especially in Africa including Ethiopia. Cabbage aphid is a sap sucking insect pest that damage cabbage. Growers use synthetic chemicals to control cabbage aphids. The aim of this study was to evaluate the efficacy of Azadirachta indicae, Otostegia integrifolia and Crinum ornatum aqueous extract against cabbage aphids. The field experiment was carried out at Kobo agricultural research sub-center from December 2016 to April 2017. The experiment was conducted in a Randomized Complete Block Design (RCBD) with 21 treatments along with standard check and untreated control and three replications. Four applications of extracts were applied at the rate of 2.5,5 and 7.5% solely and in combinations. The study revealed that effects of botanicals on aphid mortality, infestation level, area of cabbage leaves, damage of leaves, cabbage head formation, estimation of the yield and economic values. All botanical treatments were toxic against cabbage aphids. Among botanicals, neem and crinum at 7.5% concentration provided maximum cabbage yields that were comparable with dimethoate 40% E.C. Further studies should be conducted on effectiveness of these botanicals in different seasons.
Key words: Cabbage aphid, plants aqueous extract, efficacy.
Cabbage (Brassicae oleracea var. capitata Linnaeus) is a versatile vegetable crop that belongs to the Brassicaceae family (Richardson, 2016). It is widely grown vegetables throughout the world. It also remains as a very vital crop for farmers and gardeners that enabling small scale farmer financially viable mainly in Africa and Asia. Therefore, it is also one of the major Ethiopian economically important vegetables, which have recently emerged as export item (Emana et al., 2015).
Cabbage is one of the most popular food crops and grows well in many parts of the country (Embaby and Lotfy, 2015). It is grown for domestic uses as well as, for the market as one source of business (Munthali and Tshegofatso, 2014). It is also important vegetable that reduces human health problems and used to make cabbage based conventional medicines: Heart disease, stroke, alleviate rheumatism and skin problems (Rokayya et al., 2013).
Leskovar (2014) stated that cabbage production during the fall and winter season mainly depends on supplemental irrigation. In the present study area, small scale farmers continuously use irrigation for cabbage and other crop cultivations. Wubie et al. (2014) reported that one of the constraints for the production and use of cabbage is cabbage aphids which damages cabbage from seedling to final growth (head formation) stage.
Cabbage aphid (Brevicoryne brassicae L.) is insect pest, which belongs to the family Aphididae of the order Hemiptera, grouped under serious plant sap sucking pest’s worldwide (Wubie et al., 2014). They exist in large numbers underside of the leaves and growing region of infested vegetables (Munthali and Tshegofatso, 2014). They also appear as grayish-white to powdery blue due to waxy covering (honeydew), but naturally, they are grayish green in color (Bodaiah et al., 2016). They can reduce cabbage yields and its quality for the marketable value and nutritional contents (Wubie et al., 2014). They are occupied and cause severe plant infestation that gives the reduction of plant growth, number of side branches and the oil content (Embaby and Lotfy, 2015).
Application of synthetic chemicals for, plant protection plays an important role in addition to other agronomic managements for the maximum crop production (Iqbal et al., 2011). Therefore, small-scale farmers are continuously using chemical insecticides to control aphids and associated with many undesirable and sometimes lethal consequences (Phoofolo et al., 2013). The continued dependence and use of insecticides over the years increased problems, such as: resistance, residues in the harvested product, toxicity to farmers due to improper use and loss of beneficial insects and loss of money (Abdulkadir, 1992). Those problems are associated with pesticide accumulation in animal tissues and plant materials.
Knowing such information’s on the effect of synthetic chemicals and pest damage, it can encourage a person who works a research and investigates for safer alternative control methods (botanicals) that can reduce synthetic chemical related problems (Abdulkadir, 1992). With having the above points in view, the current study was done to find out alternative methods for the control of cabbage aphid and other related problems.
Botanical pesticides are an important group of naturally occurring, often slow-acting crop protectants that are usually safer to humans and the environment than synthetic pesticides, and with minimal residual effects (Devi et al., 2016). Most of botanical products either solution or powder form are accepted to be less toxic to non-target organisms, easily degradable, highly effective and do not accumulate in the environment as dissimilar to synthetic chemicals which often end up being pollutants (Mwine et al., 2013).
Farmers have some skill and practice for the preparation and use of botanical pesticides against cabbage aphids. Due to high costs of synthetic pesticides, concern over environmental pollution associated with continuous use the persistence chemicals there is a rehabilitated interest in the use of botanicals for crop protection (Mwine et al., 2013). Botanical are easily prepared and sustainable controlling methods on cabbage aphids from local plants. In addition, this mechanism helps to reduce pest infestation and conventional insecticide related problems. By having all the above points in view, this study was carried out to evaluate the efficacy of Azadirachta indicae (A.Juss), Crinum ornatum (Ait) and Otostegia integrifolia (Benth) plant material aqueous extracts solely and in different mixture and concentration on cabbage aphids’ population under field condition.
This study was carried out in Sirinka Agricultural Research Center (SARC) Kobo sub-center in Kobo District, North Wollo Zone, Amhara region, Ethiopia during winter season from December 2016 to April 2017. Latitude of 11° 54’ 04”, 12° 20’ 56” N and longitude of 39° 25’ 56” and 39° 49’ 04” E with 1400 to 3100 m above sea level. The average annual rainfall was between 500 and 800 mm and annual temperature was 19.48 to 26.06°C (Magna Magazine, 2015). The experimental field has a clay loam type of soil.
Experimental plants were selected on the bases of their traditional practices and insecticidal properties, abundance and familiarity. However, Neem and Tinjut leaves were collected around Kobo district. Crinum bulb was collected from Abuhoy Mountain in Gidan district (Table 1).
The cabbage nursery bed was prepared on an area of 9 m2 during the first week of December/2016 and seeds were sown through line spacing at 0.5 inch depth. The seedlings with 6 to 7 true leaves were transplanted during the first week of January 2017. The recommended agronomic practices were followed.
The field experiment was laid out in Randomized Complete Block Design (RCBD) with three replications and 23 treatments including the control groups and standard check. The experiment contained 3 blocks and 69 plots, each with area of 2 m2. The space between blocks and plots was 1 and 0.5, m respectively. Each plot had 2 rows and 14 cabbage seedlings. Rows and seedlings were distant by 0.5 and 0.3 m, respectively.
The plant parts (leaves or bulb) were washed with tap water and dried in shade with sufficient air supply for 2 weeks (Sarwar, 2015). The dried materials were cut and grinded into very fine powder by using electrical grinder. Thirty percent stock solution was prepared for each plant material separately (Hailemichael and Raja, 2012). The extraction was made by 3 kg of powder mixed with 7 L of hot water for each sample plant separately. The mixtures were stirred thoroughly with a repeated agitation at 3 h interval for 24 h. After a day, the solution was filtered with the help of fine cotton cloth and thin wire mush and 10 litters of 30% stock solutions were made.
The solution was kept in refrigerator until sprayed.
Cabbage aphids were properly appeared two weeks after seedling transplantation on both lower and upper surface of the leaf. Identification of cabbage aphids was done based on the world’s aphid identification guide (Blackman and Eastog, 2000). The plant aqueous extract stock solutions were diluted and treated at a rate of 1 L per plot using a hand sprayer. Four superiors were done at weekly interval during morning hours.
Data collection was done 2 weeks after transplantation of the seedlings up to harvest from mid January to April, 2017. Five plants were selected randomly in each plot and four leaves per plant were marked. The total numbers of cabbage aphids were counted with the help of a hand lens a day before each treatment application. Mean number of cabbage aphids per plant (efficacy of treatments) were calculated (Shiberu and Mulugeta, 2016).
Where: Sci = initial score and Scf = final score.
The numbers of infested plants were counted and recorded before each treatment application interval and expressed as percentage (Baidoo and Adam, 2012):
Area of the leaf was measured by using a grid square paper (0.25 mm2) at the mid cabbage growing stages (Mwine et al., 2013) and three leaves (large, medium and small) per plant were selected from 5 marked plants in each plot purposively. Damaged leaves were selected with purposive sampling methods and damaged levels of cabbage leaves were calculated by subtracting the measured or windowed area of the leaves from the whole area of leaf. The process was done a week after the last treatment application. The mean percentages of damaged leaves were calculated as a proportion of the damaged area to total surface area of the leaf covered by the plant per plot using the following formula:
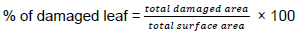
Cabbages with and without head in all experimental plots were identified and counted a day before harvesting. The total mean number of cabbages with and without head in each treatment, including control group were calculated. The total yields of both marketable and unmarketable cabbage head were measured by using an electronic sensitive beam balance to get mean weight in kilogram per hectare. The total yield was multiplied with current market price to calculate gross benefit with net benefit was calculated by subtracting total cost.
The data were subjected to one-way analysis of variance (ANOVA) using SAS (version 9.00) statistical software and means separation was calculated by using DMRT (Duncan’s multiple range tests) test (P < 0.05). All tables drown using the Excel software 2007.
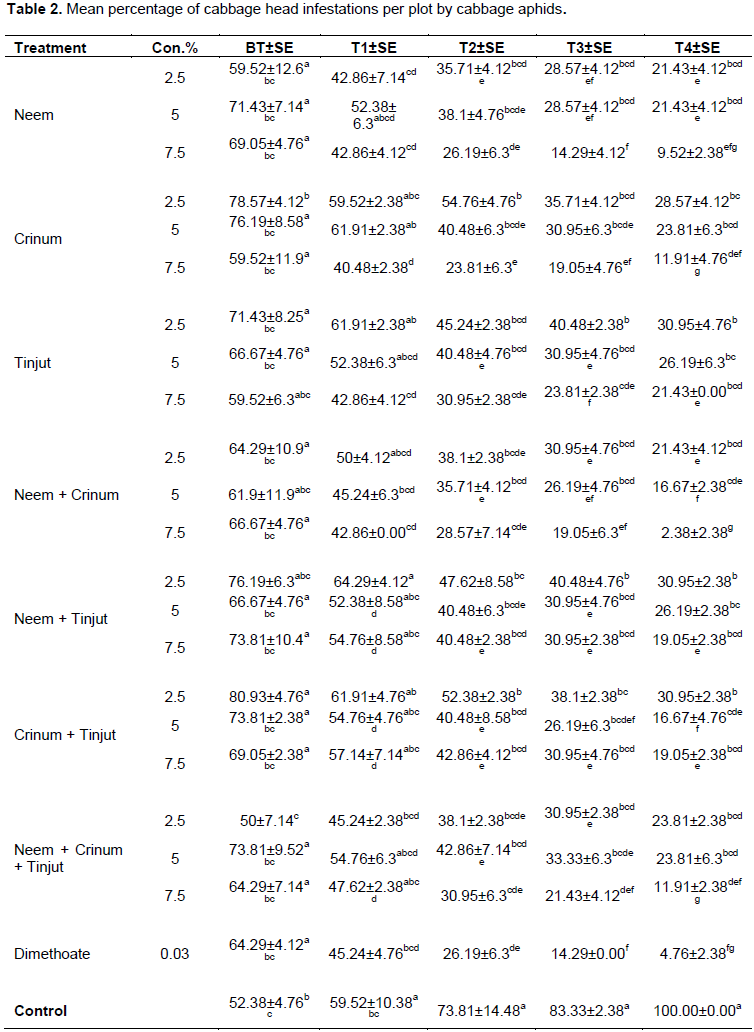
The roles of selected botanicals on aphid infestation were found significantly different among treatments (P<0.05) (Table 2). After the first treatment application (T1), infestations were reduced in all treated plots, while increased in untreated plots (52.38±4.76 to 59.52±10.38). In the second treatment application (T2), the maximum reduction was observed in Crinum 7.5% next to dimethoate (0.03%). Likewise second application, infestation increased in untreated plots by 24%. After the 3th treatment application (T3), mean percentage of infestation levels were lowered by 45.5% in plots treated with neem 7.5% and after the last treatment application (T4), infestation was remarkably decreased in all treated plots and the best one was neem + crinum with higher concentration (7.5%). On the contrary, infestation was reached its peak in untreated plots. Infestation was highly reduced in mixture (neem + crinum) at maximum concentration (7.5%) with increasing applications.
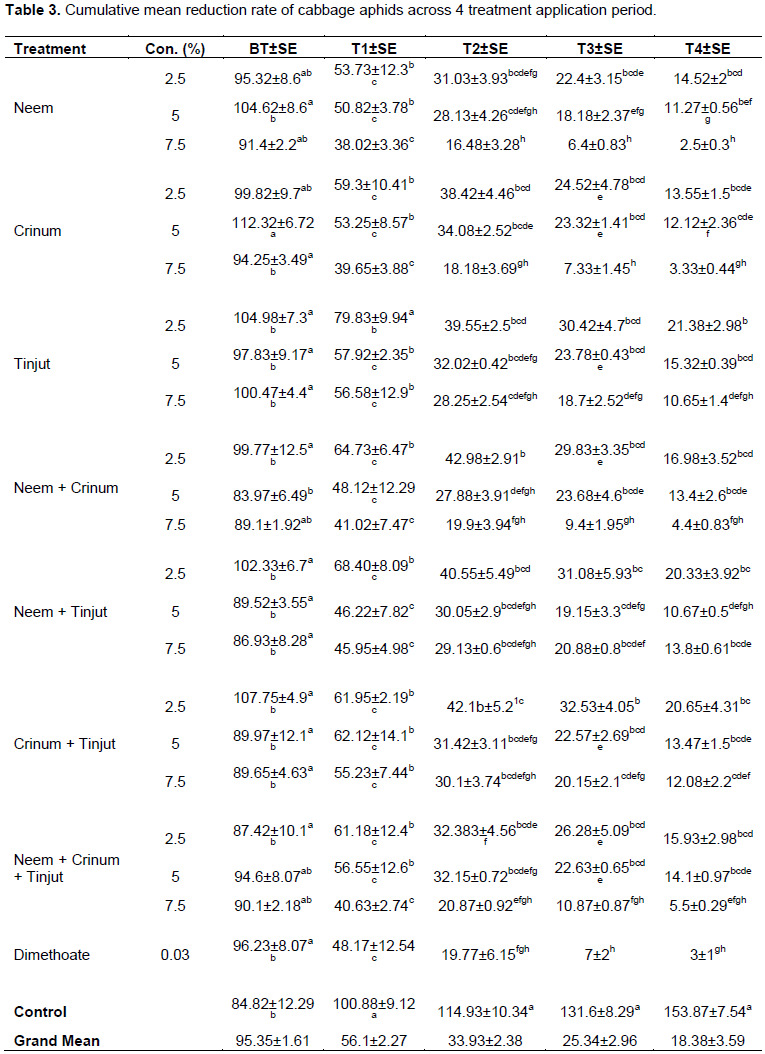
Across treatment application period, significant differences (P< 0.05) on the number of cabbage aphids per plot were observed (Table 3). The numbers of cabbage aphids were reduced in all treatments in each application interval, while increased in untreated plots. The highest reduction rate of cabbage aphids were recorded in plots treated with neem and crinum + neem with higher concentration (7.5%). Whereas the least numbers of reduction were recorded in plots treated with tinjut in lower concentration (2.5%). Generally, mean number of cabbage aphids were reduced in all treatments across treatment application interval. Likewise, in untreated plots mean number of cabbage aphids were extremely increased across treatment applications.
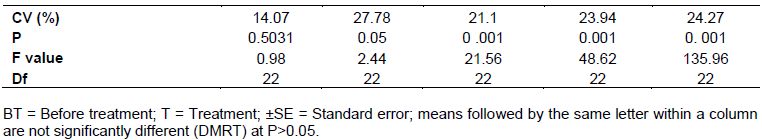
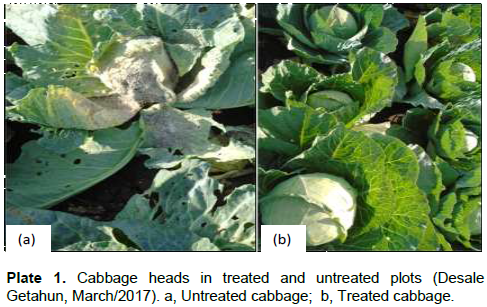
The extents of leaf damages caused by cabbage aphids were shown a significant difference (P <0.05) among treatments and control group (0 to 71.84%) (Table 4). Leaf damage was significantly lower in plots treated with botanicals than untreated plots. No damage was observed in plots treated with neem +crinum at 7.5%. But it reached its peak level in untreated plot (>71.84%) (Plate 1).
There was a significant different (P<0.05%) among treatments and control groups in affecting cabbage leaf area (Table 4). Cabbages with larger leaf area were recorded in plots treated with crinum next to neem + crinum at higher concentration (7.5%). However, the smallest leaf area was in untreated plot (control group) of cabbages. Medium sized leaf was measured from all the remaining treatments comparable with dimethoate. Finally, the statistical analysis of leaf area revealed that, application of treatments were completely increased the leaf area of head cabbage as compared to untreated cabbage leaves that ranges from 43.52 to 19.5 cm2.
The formation of cabbage heads were significantly different (P<0.05) among treatments and control groups (Table 4). The present study revealed that, highest percentage of cabbage heads (97.619%) were observed in plots treated with neem + crinum, neem and neem + crinum + tinjut at higher concentration (7.5%) which was better than dimethoate produced 92.86% per plot. In contrast, minimum percentage of cabbages with head (78.57%) was observed in plots treated with neem + crinum + tinjut at lower concentration (2.5%). Other treatments also provided enough head relative to the control group (33.19%).
The total yield (marketable and unmarketable) of head were shown significant difference (P<0.05) among treatments and control groups across treatment application period (Table 5). All botanical treatments were improved the yields than untreated plot of cabbages. The best yields per plots were recorded in plots treated with neem and crinum at higher concentration (7.5%). In contrast, the lowest yields were gained in botanicals in triple mixture with lower concentration (2.5%). Similarly, marketable yields per plot were significantly different (P<0.05) among treatments and control groups (Table 5). However, the highest marketable yield per plot was obtained from plots treated with crinum and neem in higher concentration (7.5%) than synthetic chemicals. Furthermore, cabbages treated with the remaining treatments gave comparable yields with dimethoate.
While, untreated plot (control group) of cabbages produced the lowest yields.
The final economic effectiveness of the yields were shown a significant difference (P<0.05%) among treatments and control groups (Table 6).The highest net benefit was gained from cabbages treated with crinum and neem at higher concentration (7.5%). The other botanical treatments were economically very effective than dimethoate 0.03% and untreated plots. Dimethoate showed maximum costs than the other treatments and supplied lowest net benefit. The untreated (control) plots resulted in the lowest net benefit.
Botanical insecticides are considered as plant protection methods, which are naturally safe and harmless to the health of users and consumers. Moreover, botanical insecticides are less expensive and easily prepared. During the present treatments like neem, crinum, neem + crinum and neem + crinum + tinjut with higher concentration (7.5%) were provided greatest efficiency against cabbage aphids. As a result, aphicidal activity of botanicals increased with increasing their concentration and exposure period. The reason might be bioactive compounds found in plant materials.
The present study has shown that, infestation and reduction rate of cabbage aphids showed significant different (P< 0.05) among tested botanical treatments and control groups (Table 2 and 3). Neem was highly effective than dimethoate (0.03%) and the control groups. Similarly, Djomaha et al. (2016) stated that aphid infestations in all treated plots (imidacloprid, neem and control) were significantly different. Pissinati and Ventura (2015) reported that, after the first treatment application, infestation was maximum in cabbage treated with neem [0.5%] than the mixture of neem + Pyroligneous [0.5%]. In contrast, the present study revealed that infestation was lower in cabbage treated with neem [2.5%] than neem + crinum [2.5%]. Therefore, the efficacy of neem was greater than neem + crinum and neem + tinjut at any of the three tested [2.5, 5 and 7.5%] concentrations. In studies made by Begna (2014), cabbage treated with botanicals: such as, garlic, chilli, neem and Phytolacca dodecandra L'Herit (endod in Amharic were recorded higher infestation level than conventional (diazinon) pesticides. In contrast, the present study revealed that botanicals, neem and crinum aqueous extracts with higher concentration [7.5%] scored lower percentage of cabbage aphid infestation than dimethoate (Table 2). Therefore, the efficacy of botanicals against cabbage aphid infestations depends upon their concentration. The maximum reduction rate of infestation was observed in neem followed by crinum and lastly tinjut.
The study also revealed that from the first to last treatment application period, tested botanical treatments were shown high percentage efficacy against cabbage aphids. Among them neem and crinum with higher concentration had higher efficacy than conventional insecticides, dimethoate and control groups. It was confirmed with the finding of Ezena et al. (2016), reported that botanicals had considerably reduced number of aphids than conventional insecticides, sunhalothrin and the tap water plots in the minor growing season.
Treatment concentration and application rate had a direct relationship with mortality/reduction rate of cabbage aphids. Phoofolo et al. (2013) reported that an increase in plant extract concentration resulted in an increase in the percentage of aphid mortality. Birhanu et al. (2011), who stated that mortality of cabbage aphids had related to the toxic odor of extracts entered into their spiracle and block the oxygen supply. Similarly, the present study was shown that neem with higher concentration also effectively reduced the number of cabbage aphids than the other botanical pesticides and standard check (Table 3). This might be due to the plants (botanicals) ability to attack aphids, as antifeedant, replant, and toxicant effects. In studies made by Nagappan (2012), reported that aqueous extract of Milia azadarach dry fruit was effective in reducing the cabbage aphids, cabbage aphid and important to get maximum benefit. Same way, the present study showed that, aqueous extract solutions of the neem, A. indica leaves were more effective than crinum bulb and tinjut leaf aqueous extracts against cabbage aphids. Sarwar (2015) reported that botanicals may not be killed insects for hours or days, but they were acting very quickly to stop its feeding. Similarly, it is evident from the present study that numbers of aphids were drastically reduced from first to last treatment application period. However, numbers of aphids were exceptionally increased in untreated plots. Treatment concentration was the other factor that determines the effectiveness of botanicals compared with conventional insecticides, dimethoate (0.03%). As a result, mortality (reduction) rate of cabbage aphids increased with increasing their concentration.
Mwine et al. (2013) believed that leaf damage levels continuously increased in all treatments and in some cases, cabbage leaf damage were as high as damage from control plots. Unlikely, in the present study in all treatments percentages of cabbage leaf damage were low. It was also observed in plots treated with neem + crinum 7.5% but higher in untreated plots, 71.84% (Table 4). The present results were confirmed with the finding of Begna and Damtew (2015) reported that highest leaf damage was recorded in control plots, whereas the least was in neem treated plots. In studies made by Bhat and Dhoj (2005), concentration of sample plant extract and treatment rate were the most effective which reduced damaged scale of cabbage leaves by controlling aphid population and their infestation level. Sharma and Gupta (2009) reported that the antifeedant effect of different concentration, irrespective of extracts, decreased with lower concentration from 5 to 1%. Likewise, in the present study, the scale of leaf damage was sharply increased from lower to higher botanical [7.5 %< 5 %< 2.5%] concentration. Therefore, percentages of damaged leaves were higher in untreated plots than treated plots.
A good botanical pesticide should protect a crop against target pests to levels below economic threshold (Mwine et al., 2013). In the current study, maximum percentage of cabbage heads per plot were observed in treated plots than untreated plots. In a repeated application of botanicals with higher concentration gave surplus amount of cabbage yields per plot. However, cabbage head development primarily depends upon the treatment efficacy that reduced impact of cabbage aphids. This may be due to toxic, antifeedant or deterrent effect of botanicals that against cabbage aphids. The application of different plant aqueous extracts increased the yield contributing characters; such as, number of leaves per plant, area of leaves, number of heads per plot and finally increasing the quality and quantity of the yield. Ezena et al. (2016) reported that no significant difference among treatments in cabbage yield with the exception of neem seed extract plots which had the highest yield. In contrast, in the current study there was significant difference (P<0.05) among treatments and control groups. Cabbages treated with neem and crinum in higher concentration (7.5%) produced highest yields (62,392 kg/ha). However, the lowest cabbage yield was harvested in untreated plots (Table 5).
Bhat and Dhoj (2005) reported that control plots have very low marketable yield compared with treated plots. Likewise, in the present findings highest number of marketable yield per hectare were gained from plots treated with crinum followed by neem in higher concentration (7.5%). This marketable yield variability was formed due to the treatment aphicidal action and concentration differences. The reason might be due to cabbage aphids affecting the yield by producing honeydew on the leaf surface that reduced photosynthesis, transmits viral disease and feeding growing parts that cause leaf damage and head deformation.
In the present study, cabbages treated with botanical aqueous extracts were provided more economic benefit than dimethoate and control groups. Neem and crinum in high concentration was produced peak net benefit per hectare, while no benefit (credit) in untreated plots (Table 6). Rokayya et al. (2013) also reported that the cost of plant protection using pesticides was higher than the use of botanicals. The final income (net benefit) of cabbage was depending upon the total cost and marketability of the yield. Crinum bulb, neem and tinjut leave aqueous extracts were effective in producing more net benefit as compared to dimethoate and untreated plots. The reason might be due to the less cost of botanicals used and produced more crop yield, while conventional dimethoate used more cost than the yield of the crop produced.
The present study revealed that all the treatments showed aphicidal activity against cabbage aphids but the leaf extract of neem followed by bulb extract of crinum plants with higher concentration have been proved the best treatment for the controlling cabbage aphids populations and achieving high yield. Therefore, gardeners especially small scale farmers protect their cabbages from cabbage aphid by using tested botanical aqueous extracts than conventional insecticides. Furthermore, studies should be conducted on the effectiveness of tested plants against cabbage aphid on different cabbage growing seasons.
The authors have not declared any conflict of interests.
The author appreciates the Amhara regional state for funding the field experimental study. Debre Markos University, Department of Biology is acknowledged for logistic support and fulfilled other necessary requirements for the study. The researcher benefited from Sirinka Agricultural Research center and kobo sub-center researchers for their suggestion, material and experimental land support 2016/17. They extend their thanks to Dr. Sudarsan Reddy Malkireddy and Ato Mulat Ayenew who provided many important suggestions and comments.
REFERENCES
|
Abdulkadir H (1992). Potential of Baculoviruses for control Diamondback moth and Crocidolomia binotalis on cabbage. Basic Research Division, MARDI, Serdang, Malaysia.
|
|
|
|
Amoabeng B, Gurr G, Gitau C, Stevenson P (2014). Cost: benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Protection, 57:71-76.
Crossref
|
|
|
|
|
Baidoo P, Adam J (2012). The effects of extracts of Lantana camara (l.) and Azadirachta indica (A. juss) on the population dynamics of Plutella xylostella, Brevicoryne brassicae and hellula undalis on cabbage. Sustainable Agriculture Research, 1(2):229-234.
Crossref
|
|
|
|
|
Begna F (2014). Evaluation of botanical insecticides for managing diamondback moth, plutella xylostella l. (lepidoptera: plutellidae) on head cabbage. M.sc.thesis. Haramaya University, Haramaya.
|
|
|
|
|
Begna F, Damtew T (2015). Evaluation of four botanical insecticides against Diamondback Moth, Plutella Xylostella L. (Lepidoptera: Plutellidae) on head cabbage in the central rift valley of Ethiopia. Sky Journal of Agricultural Research, 4(5):097-105.
|
|
|
|
|
Bhat B, Dhoj Y (2005). Effect of Melia Azedarach on Aphid (Brevicoryne brassicae) of organic cabbage farming. Team Leader, Ecological Service Centre, Bharatpur-2, Chitwan.
|
|
|
|
|
Birhanu M, Awoke Y, Tahgas A, Nagappan R (2011). Efficacy of Melia azadarach and Mentha piperita plant extracts against cabbage aphids, Brevicoryne brassicae (homoptera: Aphididae). World Applied Sciences Journal, 12(11):2150-2154.
|
|
|
|
|
Blackman R, Eastog V (2000). The identification and information guide second edition. In: Aphids on the Worlds crops. pp. 167-168. The natural history, Museum, Cross Well Road, Londen. (Wiky, J. and Sons, eds.). England.
|
|
|
|
|
Bodaiah B, Kumar A, Varma R, Anuhya G, Sudhakar P (2016). Pesticidal Activity of Plants on Selected insects. International Journal of Recent Scientific Research, 7(3):9298-9304,
|
|
|
|
|
Devi E, Devi P, Deepshikha (2016). A Review on Prospects of Entomopathogenic Fungi as Potent Biological Control Agents of Insect Pests. International Journal of Current Research Biosciences and Plant Biology, 3(9):74-82
Crossref
|
|
|
|
|
Djomaha ES, Ghogomu RT, Hanna R, Ngatat ES, Lontchi NF (2016). The effects of extract Azadirachta indica (Neem) oil and Imidacloprid (IRON 30SC) on the population dynamics of Brevicoryne brassicae, Lipaphispseudo brassicae and Plutella xylostella on white cabbage. International Journal of Agronomy and Agricultural Research, 8(4):135-142
|
|
|
|
|
Emana B, Afari-Sefa V, Dinssa F, Ayana A, Balemi T, Temesgen M (2015). Characterization and Assessment of Vegetable Production and Marketing Systems in the Humid Tropics of Ethiopia. Quarterly Journal of International Agriculture, 54(2):163-187.
|
|
|
|
|
Embaby S, Lotfy D (2015). Ecological studies on Cabbage pests. Journal of Agricultural Technology, 11(5): 1145-1160
|
|
|
|
|
Ezena GN, Akotsen HC, Fening KO (2016). Exploiting the insecticidal potential of the invasive siam weed, chromolaena odoratal (asteraceae) in the management of the major pests of cabbage and their natural enemies in southern Ghana. Advances in Crop Science and Technology, 4(4):2329-8863
|
|
|
|
|
Hailemichael A, Nagappan R (2012). Evaluation of Melia azedarach (Linn) Croton macrostachys (Hochst) and Schinus molle (Linn) Plant Extracts against Cabbage Aphid Brevicoryne brassicae Linn and their Natural Enemies. Asian Journal of Agricultural Sciences, 4(6):411-418.
|
|
|
|
|
Iqbal M, Kahloon M, Nawaz M, Javaid M (2011). Effectiveness of some botanical extracts on wheat aphids. The Journal of Animal & Plant Sciences, 21(1):114-115
|
|
|
|
|
Leskovar D (2014). A growth, physiology and yield response of cabbage is to deficit irrigation. Horticultural Science, 41: 138-146.
Crossref
|
|
|
|
|
Magn Magazin (2015). Kobo district governmental information and communication office. Kobo, Ethiopia. 8:1-40.
|
|
|
|
|
Munthali D, Tshegofatso A (2014). Factors Affecting Abundance and Damage Caused by Cabbage Aphid, Brevicoryne brassicae on. Four Brassica Leafy Vegetables: Brassica oleracea, var. Acephala, B. chinense, B. napusand B. carinata. The Open Entomology Journal, 8:1-9
Crossref
|
|
|
|
|
Mwine J, Ssekyewa C, Kalanzi K, Damme P (2013). Evaluation of selected pesticidal plant extracts against major cabbage insect pests in the field. Journal of Medicinal Plants Research, 7(22): 1580-1586.
|
|
|
|
|
Nagappan R (2012). Impact of Melia azedarach (Linn). (Meliaceae) dry fruit Extracts, farmyard Manure and nitrogenous fertilizer application against cabbage aphid Brevicornye brassicae Linn. (Homoptera: Aphididae) in home garden. Asian Journal of Agricultural Sciences, 4(3):2041-3890
|
|
|
|
|
Phoofolo M, Mabaleha S, Mekbib S (2013). Laboratory assessment of insecticidal properties of Tagetes minuta aqueous extracts against Brevicoryne brassicae on cabbage. Journal of Entomology and Nematology, 5(6): 70-76.
Crossref
|
|
|
|
|
Pissinati A, Ventura M (2015). Control Cabbage Aphid (Brevicoryne brassicae, L.) using Kaolin and Neem Oil. Journal of Entomology, 12(1):1812-5670.
Crossref
|
|
|
|
|
Richardson K (2016). Evaluation of the Broccoli (Brassica oleraceae L.) Variety imperial. Gladstone Road Agricultural Centre Crop Research Report.
|
|
|
|
|
Rokayya S, Li C, Zhao Y, Ying L, Sun C (2013). Cabbage (Brassica oleracea L.var. capitata) Phytochemicals with Antioxidant and Anti-inflammatory Potential. Asian Pac Journal Cancer Prevalence, 14(11):6657-666.
Crossref
|
|
|
|
|
Sarwar M (2015). The Killer Chemicals for Control of Agriculture Insect Pests: The Botanical Insecticides. International Journal of Chemical and Biomolecular Science, 1(3):123-128.
|
|
|
|
|
Sharma A, Gupta R (2009). Biological activity of some plant extracts against Pieris brassicae (Linn.). Journal of Biopesticides, 2(1):26-31.
|
|
|
|
|
Shiberu T, Mulugeta N (2016). Effects of synthetic insecticides and aqueous botanicals extracts on cabbage aphid, Brevicoryne brassicae (L.) on cabbage. Journal of Fertilizers and Pesticides, 7:162.
Crossref
|
|
|
|
|
Wubie M, Negash A, Guadie F, Molla G, Kassaye K, Nagappan R (2014). Repellent and Insecticidal Activity of Mentha piperita (L.) Plant Extracts against Cabbage Aphid [Brevicoryne brassicae Linn. (Homoptera:Aphididae)]. American-Eurasian Journal of Scientific Research, 9(6):150-156.
|
|