ABSTRACT
The effect of rooting media and number of nodes per cutting on nursery performance of vanilla fragrance were evaluated in South-western Ethiopia in 2011/ 2012. Six rooting media (forest soil, decomposed animal manure, fine sand, 1:1 mixture of forest soil: fine sand, 1:1 mixture of decomposed animal manure: fine sand and 1:1:1 mixture of forest soil: decomposed animal manure: fine sand) and four levels of node number (two, three, four, and five node cutting) were used in this experiment. Treatments were arranged in randomized complete block design with three replications. The result revealed that interaction between the two factors was significant (P£0.05) for all parameters studied except sprouting percentage and root number. The highest shoot length, shoot girth, shoot fresh weight, shoot dry weigh, leaf number, leaf area, leaf fresh weight, leaf dry weight and root to shoot ratio were obtained from four node cuttings grown on a 1:1:1 mixture of forest soil: decomposed animal manure: fine sand rooting media, with the exception of the highest root length and rooting percentage of cuttings obtained from the rooting media containing pure sand. In addition highest root fresh weight, root volume and root dry weight were obtained from five nodal cuttings. Two nodal cutting grown on decomposed animal manure and pure fine sand media showed lower root initiation and shoot growth.
Key words: Vanilla fragrance, propagation, rooting media, node number.
Vanilla (
Vanilla planifolia Andr
.) belongs to the family Orchidaceae, of which some 110 species have been reported so far (Talubnak and Soytong, 2010). Etymologically, vanilla is derived from the
Spanish word "vainilla" meaning
little pod (James and Ackerman, 2003). Vanilla grows best under hot humid climate from sea level to an elevation of 1500 m. Most of its production is carried out between 10°- 20° above and below the equator. The ideal growing conditions include moderate rainfall of 1500 to 3000 mm evenly distributed through 10 months of the year; temperatures of 15 to 30°C during the day and 15 to 20°C during the night and relative humidity of around 80% (Bianchessi, 2004).
Ethiopia has favorable environment for vanilla production and the crop has a huge local and international market (Zerihun et al., 2009). Currently, three accessions of vanilla introduced at different times are under adaptation trial in South-western Ethiopia. The first accession (Van.1/93) was introduced from Bali Island, Indonesia in 2001; the second accession (Van.3/04) was introduced from Uganda in 2004 and the third accession (Van.2/05) was introduced from Mauritius in 2005 (Girma et al., 2008). Currently, all three accessions are very well adapted at Teppi and their seedlings are being multiplied at Teppi National Spices Research Center (TNSRC) for further study.
Predominantly, vanilla is propagated through the vegetative means mainly from stem cuttings collected from a healthy and vigorously growing mother plant (Carlos and Balakrishnan, 1991; KAU, 2002; Purseglove, 1973; Sengupta, 2003). Different experiences are available from various locations about the number of nodes per stem cutting and ideal rooting media for propagation of vanilla. The type of rooting medium used can have a major influence on the rooting capacity of cuttings (Hartman et al., 1990). Vanilla stem cuttings are adversely affected by the availability of water and nutrients in the medium used during propagation. The length of cuttings used for vegetative propagation of vanilla is commonly determined by the availability of mother plants (Palama et al., 2010). Preliminary study in TNSRC indicated that vanilla cuttings with a minimum of a single node, cuttings with two, three and more numbers or more can be used for propagation (Girma et al., 2011). There was also an increasing trend in mean values of most planting materials growth parameters such as numbers of leaves, length of vine and root dry weight as the number of nodes per original stem cutting was increased (Girma, 2012). In addition, the use of short cuttings (30 cm long with 3 to 4 nodes) is becoming widespread due to shortage and unavailability of the preferred planting materials (Namirembe-Ssonkko et al., 2005). Therefore, the objective of this research is to determine influence of different rooting media and number of nodes per stem cutting on nursery performance of vanilla.Vanilla (
Vanilla planifolia Andr
.) belongs to the family Orchidaceae, of which some 110 species have been reported so far (Talubnak and Soytong, 2010). Etymologically, vanilla is derived from the
Spanish word "vainilla" meaning
little pod (James and Ackerman, 2003). Vanilla grows best under hot humid climate from sea level to an elevation of 1500 m. Most of its production is carried out between 10°- 20° above and below the equator. The ideal growing conditions include moderate rainfall of 1500 to 3000 mm evenly distributed through 10 months of the year; temperatures of 15 to 30°C during the day and 15 to 20°C during the night and relative humidity of around 80% (Bianchessi, 2004).
Ethiopia has favorable environment for vanilla production and the crop has a huge local and international market (Zerihun et al., 2009). Currently, three accessions of vanilla introduced at different times are under adaptation trial in South-western Ethiopia. The first accession (Van.1/93) was introduced from Bali Island, Indonesia in 2001; the second accession (Van.3/04) was introduced from Uganda in 2004 and the third accession (Van.2/05) was introduced from Mauritius in 2005 (Girma et al., 2008). Currently, all three accessions are very well adapted at Teppi and their seedlings are being multiplied at Teppi National Spices Research Center (TNSRC) for further study.
Predominantly, vanilla is propagated through the vegetative means mainly from stem cuttings collected from a healthy and vigorously growing mother plant (Carlos and Balakrishnan, 1991; KAU, 2002; Purseglove, 1973; Sengupta, 2003). Different experiences are available from various locations about the number of nodes per stem cutting and ideal rooting media for propagation of vanilla. The type of rooting medium used can have a major influence on the rooting capacity of cuttings (Hartman et al., 1990). Vanilla stem cuttings are adversely affected by the availability of water and nutrients in the medium used during propagation. The length of cuttings used for vegetative propagation of vanilla is commonly determined by the availability of mother plants (Palama et al., 2010). Preliminary study in TNSRC indicated that vanilla cuttings with a minimum of a single node, cuttings with two, three and more numbers or more can be used for propagation (Girma et al., 2011). There was also an increasing trend in mean values of most planting materials growth parameters such as numbers of leaves, length of vine and root dry weight as the number of nodes per original stem cutting was increased (Girma, 2012). In addition, the use of short cuttings (30 cm long with 3 to 4 nodes) is becoming widespread due to shortage and unavailability of the preferred planting materials (Namirembe-Ssonkko et al., 2005). Therefore, the objective of this research is to determine influence of different rooting media and number of nodes per stem cutting on nursery performance of vanilla.
Description of the study area
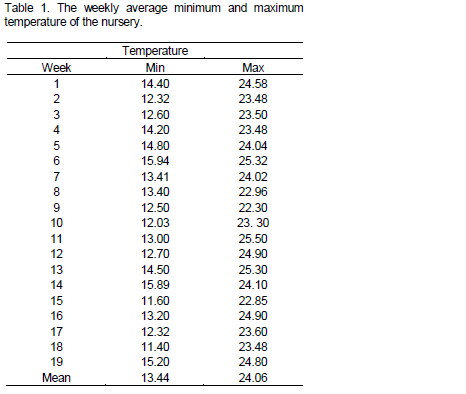
The experiment was conducted in South-western Ethiopia, Teppi National Spices Research Center (TNSRC) at nursery condition in 2011/2012. The site is located within latitude 7°3' N and longitude 35°0' E in an altitude of 1200 m a.s.l (metres above sea level) and receives a mean annual rainfall of 1688 mm. The mean maximum and minimum temperature of the area is 29.5 and 15.3°C, respectively. The soil type of the study area is dystric nitisols, eutric vertisols and vertic gleysols dominated by forest soil (Abayneh and Ashenafi, 2005). Minimum and maximum temperature of the experimental periods is presented in Table 1. In general, the area is characterized by high rainfall, temperature and humidity and representative hot humid low lands (EIAR, 2010).
Experimental materials
This experiment comprises two factors (rooting media composition and vanilla cuttings length). Semi hard wood cuttings of introduced vanilla accession (Van1. /93) with different node number (2, 3, 4 and 5) and six types of rooting media (fine sand, forest soil, decomposed animal manure, 1:1 mixture of forest soil and fine sand, fine sand: decomposed animal manure and 1:1:1 mixture of forest soil, decomposed animal manure and fine sand) were used for this experiment.
Preparation of rooting media and cuttings
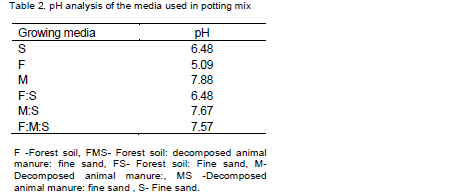
The materials used for the preparation of the potting mixture were fine sand (2.5 mm), forest soil and decomposed animal manure. Forest soil was collected from the upper 15 cm layer of the soil under a forest canopy at the TNSRC. Decomposed animal manure was also collected from the Centre and fine sand was purchased from the local market. All rooting media were sieved through a 2.5 mm mesh and fine sand thoroughly washed to remove soil particles and other undesirable materials (Plate 2). The pH of each potting mix was determined using JENWAY digital electrode pH meter on a 1:2.5 basis of soil and water (Table 2), respectively at the soil laboratory of the Jimma University College of Agriculture and Veterinary Medicine.
Semi hard wood slant cuttings of vanilla vine with 2, 3, 4 and 5 nodes per stem cutting were taken from healthy mother plants using a pruning shear disinfected with alcohol. All leaves were removed from the vine before planting (Plate 1) and the cuttings were planted in the respective growth media followed by watering. After planting, the necessary nursery management practices such as watering and weeding were applied as per the recommendation of Girma et al. (2011) (Plate 3).
Experimental design and treatments
The study was conducted as a factorial experiment (6 media types × 4 different node numbers) in a randomized complete block design (RCBD) with three replications. Each treatment consisted of ten cuttings and a total of 720 cuttings were used in the study.
Data collection and analyses
The parameters evaluated in this experiment were shoot length (cm), shoot girth (mm), shoot fresh and dry mass (g), leaf number, total leaf area (cm2·plant-1), leaf fresh and dry mass (g), root number, root fresh and dry mass (g), root volume (ml), root length (cm) per rooted cutting, root to shoot ratio in terms of dry mass (g) as well as percentages of rooting and sprouting (out of ten cuttings within a plot). In all cases, roots were gently washed with tap water before measuring root growth parameters. Total leaf area was measured using leaf area meter (ADC Bio scientific Ltd Area Meter AM 200, England) in cm2. Root volume was determined by the displacement method using a graduated cylinder half filled with water. For dry mass determination, both roots and shoots were oven dried at 80°C until the samples attained a constant mass (72 h). Except for percentage of rooted and sprouted cuttings the results are presented for discussion per plant basis.
The collected data on different growth parameters were analysed by Analysis of Variance (ANOVA). The ANOVA was done by using SAS version 9.2 Computer software (SAS Institute Inc., 2008).
When ANOVA showed significant differences, mean separation was carried out using Least Significant Difference (LSD) test at 5% level of significance (Gomez and Gomez, 1984). All the figures and tables were generated by Excel computer program.
All planting materials root and shoot growth parameters were significantly influenced by the type of rooting media and number of node per stem cuttings used (p≤0.05). However, root numbers were not significant. The highest root length (17.5 cm) (Plate 7) and percentage of rooting (99.3%) were obtained from four nodal cuttings which are grown in fine sand rooting media followed by five nodal cuttings (16.99 cm and 93.33% respectively) which were grown in 1:1:1 mixture of forest soil, decomposed animal manure and fine sand media. This could be due to the fact that low bulk density of fine sand medium invariably allows for greater root penetration leading to formation of longer roots. In accordance with this, Fagge et al. (2011) reported that influence of the medium is felt even before rooting occurs due to water retention and aeration properties and that the percentage and quality of roots in terms of number, length and weight can in many ways be directly linked to the rooting medium itself. Higher rooting percentage observed from longer cuttings could be linked to better initial carbohydrate reserves stored of longer cuttings (Ky-Dembele et al., 2011). In addition fine sand medium provides adequate aeration and drainage leading to increased porosity to promote better root initiation. This is supported by the work of Hans and Gisler (1983) where increasing air content of the rooting media improved rooting in Poinsettia and Hydrangea. While the lowest root length (6.05 cm) (Plate 8) and percentage of rooting (44.33%) were obtained from two nodal cuttings which were grown in decomposed animal manure (Table 3).
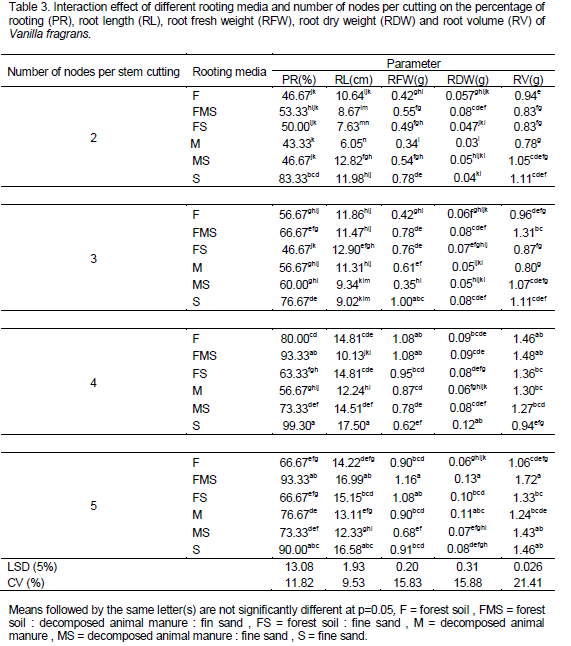
Maximum root fresh weight (1.16 g), root dry weight (0.13) and root volume (1.72 mm) was observed from five node cuttings grown on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand. This may reveal that longer cuttings denote longer roots. Longer roots can have an ability of deeper penetration and therefore greater capacity in water and nutrients absorption leading to heavier root fresh weights in this media (1:1:1 mixture of forest soil: decomposed animal manure: fine sand) as compared to the decomposed animal manure medium. The result of the present investigation is in agreement with the work of Girma et al. (2012). Whereas the lowest root fresh weight (0.34 g), root dry weight (0.03) and root volume (0.78 mm) was observed from two nodal cutting grown on decomposed animal manure (Table 3). Similar trends in root fresh and dry weight were reported by Umesha et al. (2011).
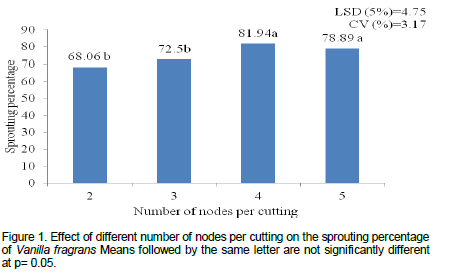
The result of this experiment on the percentage of cuttings that develop new shoot (sprouting percentage) showed that there was no significant difference between interaction of factors (rooting media and node number). However, there was significant (p<0.05) difference between node number treatments. The early sprouting and highest sprouting percentage of vanilla cuttings was recorded from four nodal cuttings (81.94%) which are statistically at par with five nodal cuttings (78.89%), whereas the lowest sprouting percentage of vanilla was recorded from cutting with two and three nodes (68.06 and 72.5%, respectively) (Figure 1). The current result supported by Umesha et al. (2011) who observed early sprouting and better shoots growth and development from longer vanilla cuttings than single and three nodal cuttings.
The highest leaf number (9.2), leaf fresh weight (7.16 g), leaf dry weight (2.127 g), shoot length (57.60 cm) and shoot fresh weight (33.38 g) was observed from four nodal cutting grown on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand rooting media. Consequently, the highest mean values from four nodal cutting were supportive to a report by Girma et al. (2012). Whereas the lowest leaf number (5.40) was observed from two nodal cuttings which are grown on sole fine sand but statistically on par with three nodal cuttings. In addition the lowest leaf fresh weight (6.34 g), leaf dry weight (2.127 g), shoot length (25.04 cm) and shoot fresh weight (14.53 g) was observed from two nodal cutting grown on sole fine sand (Table 4). Maximum shoot girth (0.43 mm) and shoot dry weight (4.16 g) was recorded from four and five node cutting grown on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand; whereas the lowest shoot girth (0.307 mm) observed from two nodal cutting grown on sole fine sand medium and the lowest shoot dry weight (2.56 g) observed from two nodal cutting grown on sole fine sand medium but statistically on par with three node cutting grown on fine sand medium (Table 4). Generally, there was an increasing trend in shoot growth and development as the node number per cutting increased.
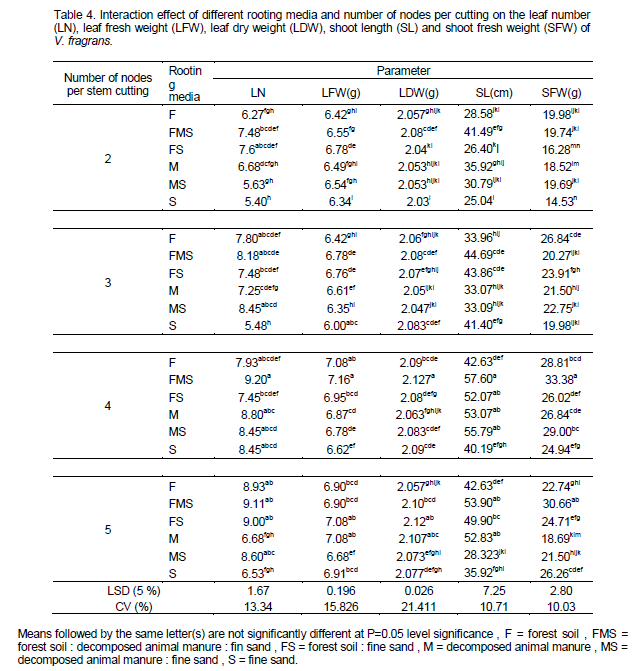
Maximum leaf area (78.85 and 78.51 cm2) was observed from four and five node cuttings grown on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand and 1:1 mixture of forest soil: fine sand rooting media, respectively; whereas the lowest leaf area (46.98 cm2) was observed from two node cuttings raised on pure fine sand. However, root number showed no significant difference (p > 0.05) between interaction of rooting media and number of nodes per stem cutting. Highest root to shoot dry weight ratio (0.133 g) was recorded from four node cutting grown on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand followed by five nodal cutting (0.103) grown on 1:1:1 mixture of forest soil: fine sand: decomposed animal manure and 1:1 mixture of forest soil: fine sand whereas the lowest root to shoot dry weight (0.04 g) ratio is recorded from two and three node cutting grown on sole fine sand rooting media (Table 5). On the other hand, an increasing pattern of root to shoot ratio was obtained from two nodes to four nodes cutting while a decreasing order was recorded from four nodes to five node cutting.
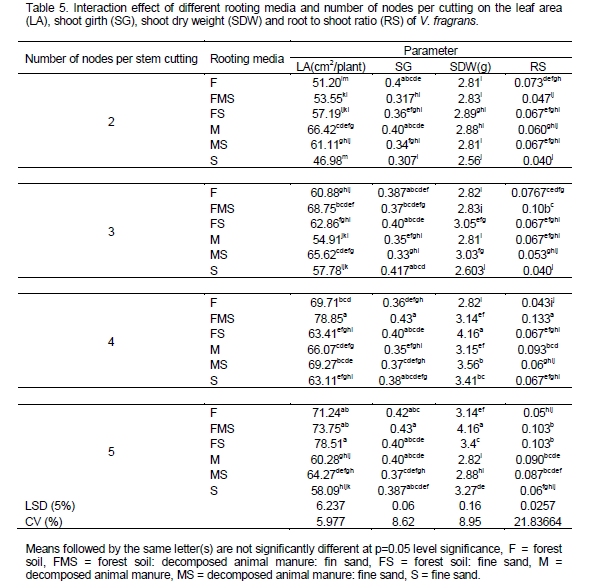
In general, longer cuttings grown on 1:1:1 mixture of forest soil: fine sand: decomposed animal manure resulted in highest root and shoot growth. However, cuttings from the medium composed of pure sand showed poor shoot growth and development and cuttings grown in pure decomposed animal manure showed lowest root growth (Plates 4, 5 and 6). The current findings in vanilla fragrance are also supported by the reports of Digafie (2006) who observed higher shoot growth response in black pepper cuttings raised on a multi-component media than those grown on single component. The results of using four node numbers for vanilla planting material preparation was also supporting to a recommendation by Girma et al. (2012). The authors stated that size and uniformity of planting materials from four node cutting were very promising. They also recommended using four node vanilla (V. fragrans) cutting is economical as well as environmental to raise nursery rooted planting materials in South western Ethiopia. According to Namirembe -Ssonkko et al. (2005) Vanilla fragrans can be easily and successfully propagated in the nursery using short stem cuttings (30cm, approximately 4 nodes) inserted in a medium with a high OM content and a good water holding capacity. Umesha et al. (2011) reported that root initiation is faster and vigorous in three to five node cuttings.


CONCLUSION AND RECOMMENDATION
Rooting media type showed considerable effect on all root and shoot development and growth parameters (except sprouting percentage, shoot girth and root number). In most of the case leaf number (9.2), leaf fresh (7.157 g) and dry weight (2.127 g), shoot length (57.60 cm) , shoot fresh weight (33.383 g) and root to shoot ratio (0.133 g) characters evaluated, better results were obtained from four node vanilla cuttings grown on a 1:1:1 mixture of forest soil: decomposed animal manure : fine sand, with the exception of the biggest root length (17.5 cm) and rooting percentage (99.3%) of cuttings obtained from the medium containing pure sand. cuttings obtained from the medium containing pure sand. 1:1:1 mixture of forest soil: decomposed animal manure: fine sand rooting media give rise to branched roots having better chance of adaptation after transplanting, but those grown from the medium composed of pure sand resulted in long, coarse and brittle roots that were not preferable. The highest value for some root parameters (root volume, root fresh and dry weight) was obtained from five node cuttings grown on a 1:1:1 mixture of forest soil: decomposed animal manure: fine sand. In addition early and highest sprouting percentage were obtained from longer (four and five node) cuttings. Higher mean values of leaf area, shoot girth and shoot dry weight were obtained from four and five node cuttings grown on a 1:1:1 mixture of forest soil: decomposed animal manure: fine sand. Therefore, the study reveals that vegetative propagation of vanilla could be successfully attained by raising four node cuttings on 1:1:1 mixture of forest soil: decomposed animal manure: fine sand rooting media.
The authors have not declared any conflict of interest.
REFERENCES
|
Abayneh E, Ashenafi A (2005). Soils of Tepi and Haru Agricultural Research Sub centers. National Soil Research Center, Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia. pp. 1-34. |
|
|
|
Bianchessi P (2004). Vanilla agriculture and curing techniques, a photographic handbook for vanilla farmers. Venue vanilla Co Ltd. Vanuatu. pp. 20-32. |
|
|
|
Carlos T, Juan, Balakrishnan S (1991). South pacific perennial spice production developments and prospects. CTA, ADB and IRETA. |
|
|
|
Digafie T (2006). Influence of Growth Media and Physiological Conditions of The Stock Plant on Root and Sprout Development of Black Pepper (Piper nigrum L.). A Master of Science Thesis Submitted to the Department of Plant Sciences, School of Graduate Studies, Haramaya. P 70. |
|
|
|
EIAR (Ethiopian Institute of Agricultural Research), 2009. One Step ahead in Spices Research. EIAR, Addis-Ababa. |
|
|
|
Fagge AA, Manga AA (2011). Effect of Sowing Media and Gibbrellic Acid on the Growth and Seedling Establishment of Bougainvillea glabra, Ixora coccinea and Rosa chinensis Characters. 2: Root Characterization. Bayero J. Pure Appl. Sci. 4(2):155 – 159 |
|
|
|
Girma H, Digafie T, Edossa E, Belay YB, Weyessa G (2008). Spices research achievements, revised edition. Ethiopian Institute of Agricultural Research, Addis Ababa Ethiopia. pp. 43-45. |
|
|
|
Girma H, Digafie T, Habtewold K, Haimanot M (2011). Effect of Node Number on the Nursery performance of vanilla (Vanilla fragrans) Cutting in South Western Ethiopia. Proceeding of the third biennial Ethiopian Horticultural Science Society (EHSS) Conference on Improving Quality Production Horticultural Crops for Sustainable Development, Jimma, Ethiopia. Jimma University College of Agriculture and Veterinary Medicine. |
|
|
|
Girma H, Digafie T, Habtewold K, Haimanot M (2012). The Effect of Different Node Number cuttings on Nursery performance of vanilla (Vanilla planifolia syn. fragrans) Cuttings in South Western Ethiopia. Int. Res. J. Agric. Sci. Soil Sci. 2(9):408-412. |
|
|
|
Gomez K, Gomez AA (1984). Statistical procedure for agricultural research, 2nd Edn. John Wiley and Sons, New York, USA. P 704. |
|
|
Hans R, Gisler D (1983). Physical conditions of propagation media and their influence on the rooting of cuttings. III. The effect of air content and temperature in different propagation media on the rooting of cuttings. Plant Soil 75:1-14.
Crossref |
|
|
Hartman HT, Kester DE, Davies FT (1990). Plant propagation: Principles and Practices, 5th ed. Prentice-Hall Publishers, Englewood Cliff, NJ, USA. P 647.
PMid:2111751 |
|
|
|
James D, Ackerman (2003). "Vanilla". Flora South America 26(4):507-516 |
|
|
|
KAU (Kerala Agricultural University) (2002). Package of practices and recommendations: Crops. 12th Edition Kerala Agricultural University. pp. 278-290. |
|
|
|
Ky-Dembele CK, Tigabu M, Bayala J, Savadogo P, Boussim IJ, Od’en PC (2011). Clonal Propagation of Khaya senegalenss. The Effects of Stem Length, Leaf Area, Auxins, Smoke Solution, and Stock plant Age. Int. J. Forest. Res. 20(11): 269-281 |
|
|
|
Namirembe-Ssonkko R, Lule A, Kyakimwa F (2005). Evaluation of Rooting Media for Commercial Nursery Propagation of Vanilla fragrans in Uganda. Proceedings of the Third Horticulture Workshop on Sustainable Horticultural Production in the Tropics. Maseno, Kenya, Maseno University, MSU. |
|
|
Palama T, Menard P, Fock I, Choi Y, Bourdon E, Govinden-Soulange J, Bahut M, Payet B, Verpoorte R, Kodja H (2010). Shoot differentiation from protocorm callus cultures of Vanilla planifolia (Orchidaceae): Proteomic and Metabolic responses at early stage. BMC Plant Biol. 10:82-94.
Crossref |
|
|
|
Purseglove JW (1973). General agronomic aspects of spice crops. In: Purseglove JW (eds.), Tropical Products Institute Proceedings of the Conference on Spices, London School of Pharmacy. pp. 85-90. |
|
|
|
SAS (Statistical Analysis System) (2008). SAS Institute. Cary, North Carolina, U.S.A. |
|
|
|
Sengupta R (2003). Vanilla. The spice of life for Indian farmers. pp. 5-10. |
|
|
|
Talubnak C, Soytong K (2010). Biological control of vanilla anthracnose using Emericella nidulans. J. Agric. Technol. 6(1):47-55. |
|
|
|
Umesha K, Murthy G, Smitha GR (2011). Environmental conditions and type of cuttings on rooting and growth of vanilla (Vanilla planifolia Andrews). J. Trop. Agric. 49(1-2):121-123. |
|
|
|
Zerihun A, Ayelign M, Alemayehu T, Wondyfraw T (2009). Efficient in vitro multiplication protocol for Vanilla planifolia using nodal explants in Ethiopia. Afr. J. Biotechnol. 8(24):6817-6821. |