ABSTRACT
The use of accurate cutting age in vegetative propagation is a key element in improving plant performance. To investigate the effect of physiological age on Cordia africana, branch cuttings from 1-3-, 8-10- and ≥15-year-old trees were grown under open-field conditions for two months. The cuttings were monitored for flushing and growth. The number of days to flushing was lowest for the 8-10-year-old and highest for the ≥15-year-old age class. Rooted cuttings from the 8-10-year-old trees had higher values of height, stem volume, number of leaves, and leaf width than counterparts from the other two age classes, where there were no significant differences between each other for these parameters. Moreover, the differences in stem volume and number of leaves between the youngest and oldest age classes were not statistically significant. Relative growth rates of height, diameter, and stem volume were unresponsive to physiological age. The findings of this study suggest that 8-10-year-old trees could be the most suitable donors of vegetative propagation material for C. africana. It is, however, important that rooting of the cuttings be evaluated to determine if the age-related trends in shoot growth can be sustained.
Key words: Cordia africana, cutting, growth, ortet, physiological age, vegetative propagation.
Although both stand- and tree-level factors may contribute to age-related declines in forest productivity (Day et al., 2001), those operating at the scale of individual trees are likely to play a major role (Martínez-Vilalta et al., 2007). The alterations associated with the adult habit are due to stable changes in the apical meristem called maturation (Thomas, 2013), while superimposed on these are the effects of tree size and complexity (Matsushita et al., 2015). Maturation in woody plants is an ongoing process that results in developmental changes involving lower growth potential (Sweet, 1973), change in type of foliage, changed branching patterns (Poethig, 2013), diminished apical dominance, and reduced regenerative competence (Greenwood, 2000). Trees generally increase in size as they mature and while changes due to size are reversible, those due to maturation are normally not. The effects of age can be decoupled from those of size by using rooted cuttings or grafted scions taken from different aged ortets, to produce trees of uniform size, but with shoots of different physiological ages (Browne et al., 1997; Krakowsky et al., 2005). The basis of these approaches is that maturation-related properties of trees are associated with changes in gene expression (You et al., 2014), and can, therefore, be inherited by vegetative propagules (Greenwood et al., 1989; Hutchinson et al., 1990). Most of the work on maturation has been descriptive, and comprehensive experimental studies on woody species are rare. Nonetheless, maturation is not only an interesting developmental phenomenon, but a major concern to those aiming to use vegetative propagation for forest renewal. In some studies, however, the maturation hypothesis has been reported not to explain the age-related decline in growth (Bond, 2000; Mencuccini et al., 2005; Martínez-Vilalta et al., 2007), suggesting that the phenomenon may be influenced by other factors.
Cordia africana (Lam.) is an early colonizer in forest re-growth (Hundera et al., 2015). This montane tree species is an important component of the Bamenda Highlands Forest Biome in North West Cameroon (Ndenecho, 2011). Among other economic and ecological benefits, C. africana is an important source of food, firewood, bee forage, and timber for construction and making household tools and farm implements (Alemayehu et al., 2016). The high market value of its moderately hard and durable wood has placed this tree species under pressure from over-exploitation (Robi and Edris, 2017). Moreover, the productivity and structure of forest landscapes in Cameroon are likely to be under threat from changing global climatic conditions (Sonwa et al., 2012). Thus, it is crucial to enhance the supply of C. africana via selecting reforestation material with high growth potential and to ensure its sustainability by putting in place adaptive management strategies.
C. africana possesses two silvical characteristics that make it well suited to natural regeneration. First, it is a prolific seed producer and due to the hard nature of its seed coat (Dedefo et al., 2017) the seeds cannot be easily digested by wildlife. The ability of hard-coated seed to pass through the gut of herbivores and out in faeces unharmed has also been observed in Acacia tortilis (Shorrocks and Bates, 2015). Second, it has the ability to be reproduced vegetatively by rooting of plant parts and by coppicing (Fern, 2014). Reforestation of C. africana by vegetative means is quite popular in the Bamenda Highlands region. The reasons for this vary, but commonly include the ease and speed with which new plants are produced. For many forest nursery workers, raising plants from branch cuttings is the method of choice. In the face of an ongoing debate on mechanisms underpinning the developmental declines in tree growth, the possibility has been raised by Ambebe and Dang (2009) that maturation- and size-mediated controls of growth may be species specific. This study was aimed at investigating the effect of physiological age on the growth of C. africana cuttings.
Experimental design
The study used a randomized complete block design with three ortet age classes (1-3, 8-10 and ≥15 years) in two blocks. Separated by a distance of 2 km, each block represented a collection site for cuttings in Big Babanki (1177 m a.s.l; 6.117° N, 10.250° E). Big Babanki is a small village located 30 km north of Bamenda, the capital of the North West Region of Cameroon. The village is characterized by forest patches that constitute part of the expansive Bamenda highlands forest.
On the 24th of September 2016, ten trees were randomly chosen from each block and ortet age class, and two hardwood cuttings were taken from vigorous terminal long shoots of lateral branches in the lower quadrant of the live crown. The cuttings were bulked in a plastic bag, and transported to the Faculty of Science Laboratory of The University of Bamenda in Bambili (1444 m asl; 5.983° N, 10.250° E). They were decapitated and trimmed to a length of 25 cm, so that all were about the same diameter. The distal end of the cutting was set to a depth of 10 cm in moisture-saturated media (1 soil:1 sand) in a polythene bag. No pre-planting treatment of the cuttings with a plant hormone or chemical decontaminant was performed. The polybags were then moved to an out-door nursery where each ortet age class × block combination was randomly located. The nursery is situated at the Faculty of Science Laboratory. The mean annual temperature in Bambili is 20.8°C and the annual rainfall is 2140 mm (Climate-Data.org, 2017). With an average 386 mm, the most precipitation falls in September. January is the driest month. The average rainfall of September and October 2016 were 418.08 mm and 190.37 mm, respectively, and that of November 2016 when the experiment ended was 7.4 mm (Worldweatheronline.com, 2017). Since the experiment started in the month of September, irrigation was mainly natural. The natural precipitation was, however, supplemented with watering using normal tap water when necessary. The frequency of the artificial irrigation increased as the experiment progressed due to the transition from the rainy to dry season. No fertilizer application was made during the course of the experiment that lasted two months.
Data collection
The cuttings were monitored daily for flushing. This was considered to occur when 50% of the cuttings in a particular block had done so. The number of days to flushing for an ortet age class was then determined by taking the average for the two blocks and rounding the value to the nearest whole number.
Five cuttings were randomly chosen from each ortet age class and block for data collection. Height (H) and stem diameter (D) were measured with a ruler and caliper, respectively, after flushing and at the end of the experiment. Initial and end stem volumes (SV) were calculated from the following equation of Aphalo and Rikala (2003):
SV = D2H
Relative growth rates of H, D, and SV were determined by dividing increments of these parameters by the corresponding initial measurements.
The number of leaves per plant, leaf length and width of the most widely expanded leaf per plant were also determined at the end of the experiment. Leaf length was measured from the upper edge of the leaf to the lowest point, whereas leaf width was measured as the widest region across the lamina perpendicular to the length.
Statistical analysis
All growth and leaf traits were analyzed by the following linear model:
Yijk = µ + Bi + Aj + BAij + E(ij)k
Where B is the effect of block, A is the fixed effect of ortet age, and E(ij)k is the experimental error. Before conducting the Analysis of Variance (ANOVA), the data were checked to determine whether the model assumptions (normally distributed errors and homogeneity of variances) were fulfilled. The ANOVA was performed on untransformed data. When the effect of ortet age on a given parameter was suggested to be significant, means separation was conducted with Scheffé’s F-test. Pearson product-moment correlation was performed to determine the relationship between leaf length and width whereas the correlation coefficient (r) was tested for statistical significance using t-test. All the analyses were performed in Data Desk 6.01 at α ≤ 0.05.
Days to bud burst
The number of days to flushing was lowest for the intermediate ortet age class. Cuttings from 8-10-year-old trees flushed in 4 days, those from 1-3- and ≥15-year ortets did so in 7 and 9 days, respectively.
Growth
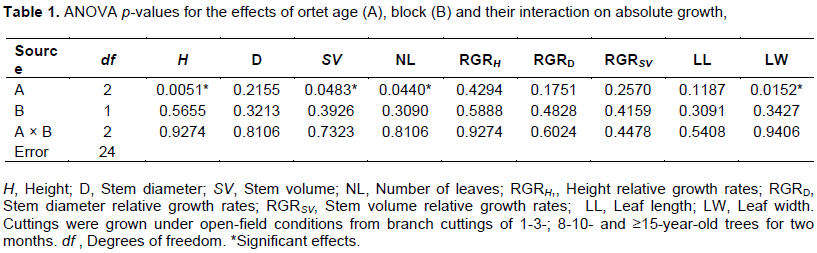
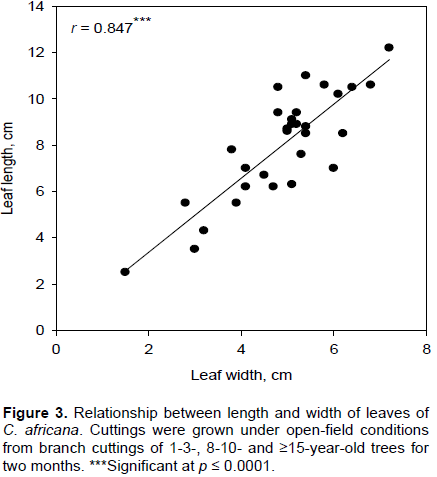
According to the ANOVA, height, stem volume, and number of leaves were affected by ortet age (Table 1). In contrast, neither stem diameter nor the relative growth rates of height, diameter, and stem volume showed any significant responses to age under the ANOVA (Table 1 and Figure 1B). Height was significantly greater for rooted cuttings grown from 8-10-year-old trees than from the other two ortet age classes (Figure 1A). The post-hoc test did not detect any significant differences in height between the 1-3- and ≥15-year-old ortets (Table 1 and Figure 1A).
For stem volume and number of leaves, there was a similar pattern of response of the rooted cuttings to ortet age (Figure 1C and D). Values of these parameters were highest for the 8-10- and lowest for 1-3-year-old ortets (Figure 1C and D). However, neither of these ortet ages showed a statistically significant difference with the ≥15-year-old for either stem volume or number of leaves (Table 1 and Figure 1C and D).
Leaf dimensions
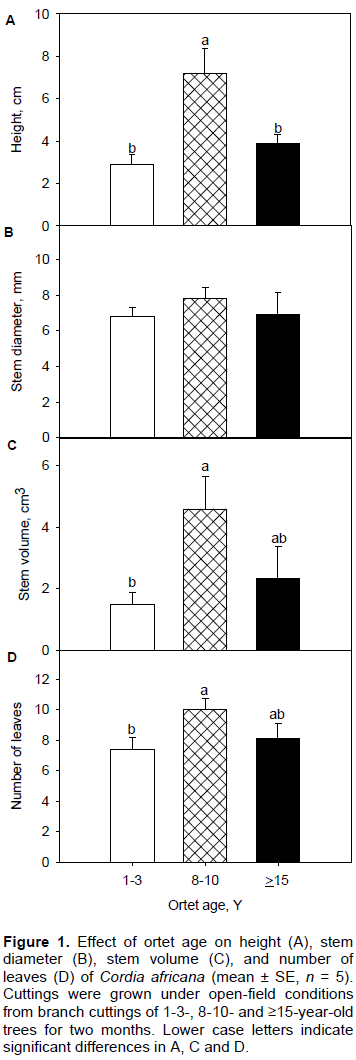
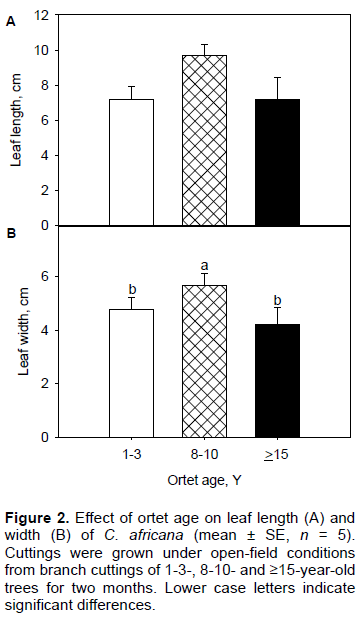
While leaf width was influenced by ortet age, leaf length was not (Table 1 and Figure 2A). Rooted cuttings from 8-10-year ortets had significantly higher responses of leaf width than those from the other two age classes which displayed similar responses of this trait (Table 1 and Figure 2B). However, regression analysis showed a highly significant positive relationship between leaf length and leaf width (Figure 3).
In general, the was no significant main effect of block or its interaction for any of the parameters examined (Table 1).
In the present study, growth of rooted cuttings increased from 1-3- to 8-10-year-old and then declined for the ≥15-year-old ortet age class, although the differences between the youngest and oldest age treatments were not statistically significant for some traits. This trend is consistent with the sigmoidal pattern of tree growth, which stipulates that growth generally increases to a peak after initiation and declines with further increase in tree age (Weiner, 2001; Bond et al., 2007). The results also emphasize the fact that a new independent plant produced by vegetative propagation is a clone, which can express morphological phenotypes of the donor tree (Hutchinson et al., 1990; Ritchie and Keeley, 1994; Hartmann et al., 2002).
The findings on C. africana reported here are in accordance with those of several other studies. For instance, in an investigation of the effect of physiological age on growth of Jatropha curcas L., it was found that cuttings from secondary branches outperformed their counterparts from primary and tertiary branches (Santoso and Parwata, 2014). Furthermore, height and diameter of grafted Larix laricina (Du Roi) K. Koch (Greenwood et al., 1989), leaf length of grafted Pseudotsuga menziesii (Mirb.) Franco (Ritchie and Keeley, 1994), number of leaves of Jatropha branch cuttings (Santoso and Parwata, 2014), and leaf area of Eucalyptus regnans F. Muell. (England and Attiwil, 2006) have all shown responses to ortet age in a manner similar to those of the present study. Although leaf area measurement was not performed, the response of leaf width to age and its relationship to leaf length could be useful indicators of the response of leaf area. Results from modelling studies such as Ugese et al. (2008) and Ogoke et al. (2015) have shown that leaf area is strongly positively correlated to these leaf dimensions. Perhaps the most interesting outcome of this study is the observation that cuttings from the intermediate ortet age were superior in height to those from the youngest and oldest tree age classes. In consistence with Udensi and Ikpeme (2012), the longer plant axis resulted in the formation of more leaves. In other studies (Greenwood et al., 1989; Mencuccini et al., 2007), the greater number of leaves is the result of a greater number of branches in taller than shorter plants. Number of leaves and leaf size are indicative of the surface area available for photosynthesis to drive further growth (Lambers et al., 2008).
Going by the maturation hypothesis, the age-related changes in morphology reported here are genetically controlled. This means that physiological processes that control growth are likely genetically regulated and should show direct age-mediated changes in trees. Hutchinson et al. (1990) explored the role of gene expression in the photosynthesis of grafted scions from L. laricina trees of different ages. The cab genes that encode elements of the photosynthetic apparatus were found to be expressed at lower levels in mature than young plants. The associated decrease in photosynthetic activity implies that older trees have lower growth rates. Another physiological process that is impacted by maturation is flushing behaviour. Rooted cuttings from younger trees flush considerably more and faster than counterparts of older origin (Zaczek et al., 1993; O’Reilly and Harper, 1999). In the present study, the earlier flushing seems to have allowed for the growth advantage exhibited by rooted cuttings from the intermediate ortet age over those from the other two age classes. The finding that relative growth rates were unresponsive to ortet age is in favour of this assertion. The absence of an effect of ortet age on shoot growth rates has been reported for other tree species such as Fraxinus excelsior L., Acer pseudoplatanus L., Pinus sylvestris L., Populus balsamifera L. (Mencuccini et al., 2005) and Pseudotsuga menziesii (Mirb.) Franco (Bond et al., 2007).
The results of this study suggest that cuttings for reforestation of C. africana should be obtained from trees that are neither very young nor old. The profound age-related adjustments in growth of the rooted cuttings can have important consequences on productivity of future forest stands since height and diameter of reforestation material are, evidently, good predictors of subsequent field growth (Ambebe et al., 2013). However, an important consideration on reforestation sites is the potential for survival of planting material. In general, larger diameter planting stock tend to have higher survival rates than those having smaller diameter (Mexal and Landis, 1990). It could not be determined here if the plants derived from the most suitable ortet age were superior in survival potential. The uncertainty is as a result of the lack of an effect of ortet age on stem diameter. However, some root morphological and physiological traits have been proven to have strong relationships with survival (Burdett, 1983; Jacobs et al., 2005; Ambebe et al., 2013), evoking the need for further studies in these areas for C. africana.
The authors have not declared any conflict of interests.
REFERENCES
|
Alemayehu G, Asfaw Z, Kelbessa E (2016). Cordia africana (Boraginaceae) in Ethiopia: A review of its taxonomy, distribution, ethnobotany and conservation status. Int. J. Bot. Stud. 1:38-46.
|
|
|
|
Ambebe TF, Dang QL (2009). Limits to tree growth and the "age-related decline" in aboveground forest productivity. In: W.P. Karam (ed), Tree growth: Influences, Layers and Types. Nova Science Publishers, New York. pp. 109-120.
|
|
|
|
|
Ambebe TF, Lum AF, Balgah RA, Njoya MTM (2013). Evaluation of regeneration stock alternatives for optimization of growth and survival of field-grown forest trees. J. Life Sci. 7:507-516.
|
|
|
|
|
Aphalo P, Rikala R (2003). Field performance of silver-birch planting-stock grown at different spacing and in containers of different volume. New For. 25:93-108.
Crossref
|
|
|
|
|
Bond BJ (2000). Age-related changes in photosynthesis of woody plants. Trends Plant Sci. 5:349-353.
Crossref
|
|
|
|
|
Bond BJ, Czarnomski NM, Cooper C, Day ME, Michael SG (2007). Developmental decline in height growth in Douglas-fir. Tree Physiol. 27:441-453.
Crossref
|
|
|
|
|
Browne RB, Davidson CG, Steeves TA, Dunstan DI (1997). Effects of ortet age on adventitious rooting of jack pine (Pinus banksiana) long-shoot cuttings. Can. J. For. Res. 27:91-96.
Crossref
|
|
|
|
|
Burdett AN (1983). Quality control in the production of forest planting stock. For. Chron. 59:133-138.
Crossref
|
|
|
|
|
Climate-Data.org (2017). Climate: Bambili. Available at
View. Accessed May 9, 2017.
|
|
|
|
|
Day ME, Greenwood MS, White AS (2001). Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates with tree age. Tree Physiol. 21:1195-1204.
Crossref
|
|
|
|
|
Dedefo K, Derero A, Tesfaye Y, Muriuki J (2017). Tree nursery and seed procurement characteristics influence on seedling quality in Oromia, Ethiopia. For. Trees Livelihoods 26:96-110.
Crossref
|
|
|
|
|
England JR, Attiwill PM (2006). Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees 20:79-90.
Crossref
|
|
|
|
|
Fern K (2014). Cordia africana. Useful Tropical Plants Database 2014. http:// tropical.theferns.info. Accessed 8 August 2017.
|
|
|
|
|
Greenwood MS (2000). Primary productivity of Norway spruce with increasing stand age. In: What does cause age-related decline in forest productivity? Age Decline Symposium Notes. ESA Annual Meeting, Snowbird, Utah.
|
|
|
|
|
Greenwood MS, Hopper CA, Hutchinson KW (1989). Maturation in larch : I. Effect of age on shoot growth, foliar characteristics, and DNA methylationPlant Physiol. 90:406-412.
|
|
|
|
|
Hartmann HT, Kester DE, Davies FT, Geneve RL (2002). Plant propagation: Principles and practices, 7th edn. Printice-Hall, New Jersey.
|
|
|
|
|
Hundera K, Honnay O, Aerts R, Muys B (2015). The potential of small exclosures in assisting regeneration of coffee shade trees in South-Western Ethiopian coffee forests. Afr. J. Ecol. 53:389-397.
Crossref
|
|
|
|
|
Hutchinson KW, Sherman CD, Weber J, Smith SS, Singer PB, Greenwood MS (1990). Maturation in larch. II. Effects of age on photosynthesis and gene expression in developing foliage. Plant Physiol. 94:1308-1315.
Crossref
|
|
|
|
|
Jacobs DF, Salifu KF, Seifert JR (2005). Relative contribution of initial root and shoot morphology in predicting field performance of hardwood seedlings. New For. 30:235-251.
Crossref
|
|
|
|
|
Krakowsky J, Benowicz A, Russell JH, El-Kassaby YA (2005). Effects of serial propagation on Chamaecyparis nootkatensis physiology and growth traits. Can. J. For. Res. 35:623-632.
Crossref
|
|
|
|
|
Lambers H, Chapin FS III, Pons TL (2008). Plant physiological ecology. Springer, New York.
Crossref
|
|
|
|
|
Martínez-Vilalta J, Vanderklein D, Mencuccini M (2007). Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 150:529-544.
Crossref
|
|
|
|
|
Matsushita M, Takata K, Hitsuma G, Yagihashi T, Noguchi M, Shibata M, Masaki T (2015). A novel growth model evaluating age-size effect on long-term trends in tree growth. Func. Ecol. 29:1250-1259.
Crossref
|
|
|
|
|
Mencuccini M, Martínez-Vilalta J, Hamid HA, Korakaki E, Vanderklein D (2007). Evidence for age- and size-mediated controls of tree growth from grafting studies. Tree Physiol. 27:463-473.
Crossref
|
|
|
|
|
Mencuccini M, Martínez-Vilalta J, Vanderklein D, Hamid HA, Korakaki E, Lee S, Michiels B (2005). Size-mediated ageing reduces vigour in trees. Ecol. Lett. 8:1183-1190.
Crossref
|
|
|
|
|
Mexal JG, Landis TD (1990). Target seedling concepts: height and diameter. In: R. Rose, S.J. Campbell and T.D. Landis (eds), Target Seedling Symposium. USDA Forest Service General Technical Report RM-200. pp. 17-35.
|
|
|
|
|
Ndenecho EN (2011). Ethnobotanic resources of tropical montane forests: Indigenous uses of plants in the Cameroon Highland Ecoregion. Langaa Research and Publishing CIG, Mankon, Bamenda.
|
|
|
|
|
Ogoke IJ, Ike GA, Echereobia CO, Ngwuta AA (2015). Non-destructive leaf area determination in African eggplant (Solanum macrocarpon). Agrosearch 15:13-20.
Crossref
|
|
|
|
|
O'Reilly C, Harper C (1999). The morphological characteristics, root growth potential and flushing response of rooted cuttings compared with transplants of Sitka spruce. Ann. For. Sci. 56:201-210.
Crossref
|
|
|
|
|
Poethig RS (2013). Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 105:125-152.
Crossref
|
|
|
|
|
Ritchie GA, Keeley JW (1994). Maturation in Douglas-fir: I. Changes in stem, branch and foliage characteristics associated with ontogenetic aging. Tree Physiol. 14:1245-1259.
Crossref
|
|
|
|
|
Robi MK, Edris EM (2017). Distribution, abundance, and population status of four indigenous threatened tree species in the Arba Minch Natural Forest, Southern Ethiopia. Int. J. Nat. Resource Ecol. Manag. 2:1-8.
|
|
|
|
|
Santoso BB, Parwata IGMA (2014). Seedling growth from stem cutting with different physiological ages of Jatropha curcas L. of West Nusa Tenggara genotypes. Int. J. Appl. Sci. Technol. 4:5-10.
|
|
|
|
|
Shorrocks B, Bates W (2015). The Biology of African Savannahs, 2nd edn. Oxford University Press.
|
|
|
|
|
Sonwa DJ, Somorin OA, Jum C, Bele MY, Nkem J (2012). Vulnerability, forest related sectors and climate change adaptation: The Case of Cameroon. For. Policy Econ. 23:1-9.
Crossref
|
|
|
|
|
Sweet GB (1973). The effect of maturation on the growth and form of vegetative propagules of radiata pine. New Zeal. J. For. Sci. 3:191-210.
|
|
|
|
|
Thomas H (2013). Senescence, ageing and death of the whole plant. New Phytol. 197:696-711.
Crossref
|
|
|
|
|
Udensi O, Ikpeme EV (2012). Correlation and path coefficient analyses of seed yield and its contributing traits in Cajanus cajan (L.) Mill sp. Am. J. Exp. Agric. 2:351-358.
Crossref
|
|
|
|
|
Ugese FD, Baiyeri KP, Mbah BN (2008). Leaf area determination of shea butter tree (Vitellaria paradoxa C.F. Gaertn.) Int. Agrophys. 22:167-170.
|
|
|
|
|
Weiner J (2001). The nature of tree growth and the age-related decline in forest productivity. Oikos 94:2.
Crossref
|
|
|
|
|
Worldweatheronline.com (2017). Bambili, Nord-Ouèst, Cameroon Weather Averages | Rainfall and Rain Days | World Weather Online.
|
|
|
|
|
You C-Y, Zhao Q, Wang X-F, Xie X-B, Feng X-M, Zhao L-L, Shu H-R, Hao Y-J (2014). A dsRNA-binding protein MdDRB1 associated with miRNA biogenesis modifies adventitious rooting and tree architecture in apple. Plant Biotech. J. 12:183-192.
Crossref
|
|
|
|
|
Zaczek JJ, Steiner KC, Heuser Jr CW (1993). Vegetative propagation of mature and juvenile northern red oak. In: Gillespie AR, Parker GR, Pope PE, Rink G (eds), Proceedings of the 9th Central Hardwood Forest Conference. General Technical Report NC-161. St Paul, MN: USDA Forest Service, North Central Forest Experiment Station. pp. 210-221.
|
|