ABSTRACT
Amaranth is one of the most commonly produced and consumed indigenous vegetables on the African continent. In Tanzania amaranth constitutes about 5.3% of total vegetable hectarage planted annually. Most cultivated varieties of amaranth are landraces with relatively poor leaf and grain yield. This study was conducted to identify genotypes with potential for dual purpose (leaf and grain) use for promotion or further cultivar development. An experiment was carried out in two seasons at AVRDC - The World Vegetable Center in Arusha, Tanzania from Feb to May and June to Sep 2012. Fourteen genotypes were used in a randomized complete block design. Results indicated that leaf yield differed significantly among the genotypes in both trial 1 (p ≤ 0.01) and 2 (p ≤ 0.05). The highest leaf yields were obtained in genotypes RVI00117 (32.8 t/ha) and RVI00002 (14 t/ha) in trial 1 and 2, respectively. The lowest leaf yields were obtained from genotypes RVI00121 and RV00090 (4 and 6.3 t/ha) in trials 1 and 2, respectively. There were significant differences (p ≤ 0.001) among genotypes for grain yield obtained after leaf harvesting. Genotype RVI00022 had the highest seed yield (1971.3 kg/ha) over the two seasons. Where leaf was not harvested, genotype RVI00121 had the highest seed yield (2920 kg/ha) over the two seasons. From this study, we recommend genotypes RVI00121 and RVI00001 for grain production. For dual purpose use, we recommend RVI00007 during warm and wet conditions and RVI00022 during cool and dry condition.
Key words: Amaranth, leaf yield, seed yield, genotype performance,
Amaranth (Amaranthus spp.), a C4 plant, is extensively grown as a green leafy vegetable and for its grain in many tropical countries in Africa, Central and Southern America, Mexico and parts of Asia (DAFF, 2010). It is one of the oldest food crops in the world; evidence of its cultivation is dating back 6700 BC (Itúrbide and Gispert, 1994; DAFF, 2010). The crop is one of few plant species whose leaves are eaten as a vegetable and can also be grown for their seeds. This is the case of some introduced varieties of American origin (Wu et al., 2000). Grain amaranth is not commonly cultivated in Africa (Grubben and Denton, 2004). However recently, a few farmers have taken the growing of grain amaranth more seriously and are supplying millers and supermarkets in Zimbabwe, Kenya, Uganda and Ethiopia (Achigan-Dako et al., 2014). The genus Amaranthus consists over 60 species, several of which are cultivated as leaf vege-tables, grains, or ornamental plants, while others are con-sidered weeds (Maboko, 1999; DAFF, 2010). However, the majority of the species grown for vegetables are represented by Amaranthus dubius, A. lividus, and A. hybridus (Mlakar et al., 2010). Three principal species most considered for grain include, A. hypochondriacus, A. cruentus and A. caudatus (Teutonico and Knorr, 1985; Muyonga et al., 2008; Mlakar et al., 2010).
Amaranth is one of the most commonly produced and consumed indigenous vegetables on the African continent (Grubben and Denton, 2004). It is extensively grown as a green leaf vegetable in many tropical countries in Africa like Tanzania, Benin, Togo, Sierra Leone, DR Congo and Kenya. It is also common in tropical areas outside Africa like in India, Bangladesh, Sri Lanka and Caribbean (Grubben and Denton, 2004). Of the more than 78,000 ha of vegetables planted annually in Tanzania, amaranth constitutes about 5.3% (National Bureau of Statistics, 2012). A study by Keller (2004) indicates that amaranth is an important traditional leafy vegetable in northeast Tanzania, listed first in the top five vegetables grown in the region.
The combination of its anatomical features and its C4 metabolism might have contributed to its wide geographical adaptation under diverse environmental conditions (Stallknecht and Schulz-Schaeffer, 1993; Kaul et al., 1996). Amaranth is an annual crop that grows rapidly and is harvested within 3 to 4 weeks after sowing for leaves, while the grain can be harvested 60 to 90 days. The crop is tolerant to common vegetable insect pest and less labour-demanding (Maundu et al., 2009). There is no distinct separation between the vegetable and grain types, except black grains are not preferred by most farmers and consumers. Leaves of young plants grown for grain are used not only for human consumption but also used as animal feed, in South America, Africa, Asia and Eastern Europe (Kaul et al., 1996; Muyonga et al., 2008). Amaranth leaf can be used as greens in salads, boiled or fried in oil and mixed with meat or fish. Cooked greens can be used as side dish in soups or as an ingredient in sauce and baby food (Mlakar et al., 2010). The grain of amaranth can also be used in numerous recipes ranging from popped amaranth snack, porridge, stiff porridge, chapatti (flat bread), bread, creamy soup, pancakes, cakes, scones, pizza, etc.
Amaranth leaves are rich in vitamins A (2917 IU) and vitamin C (43.5 mg), while both leaves and grains contain, iron (2.32 mg; 2.1 mg), calcium (215 mg; 47 mg), potassium (611 mg; 135 mg), phosphorus (148 mg; 50 mg) and protein (2.46 g; 3.8 g), respectively. All of these are essential nutrients lacking in most people’s diets.
Despite its positive agronomic and nutritional characteristics, the majority of cultivated genotypes of amaranth in Africa including Tanzania are low yielding relative to their potential of up to 40 tons and 600 kg per ha for leaf and grain, respectively (Svirskis, 2003; Moinester, 2007). Only a few improved varieties are available as a result of which the majority of farmers grow their local cultivars. Studies for both leaf and grain yield and its contributing quantitative and qualitative traits are scarce (Shukla et al., 2006). However, there are a number of germplasm collections available in AVRDC genebank for evaluation and direct release and/or use in breeding programs. Harvest of leaves and grain from the same plant (dual-purpose) allows smallholder farmers to exploit the full nutritional benefits of amaranth. Therefore, the current study was conducted to identify dual purpose (leaf and grain) amaranth genotype for possible release as new varieties or further cultivar enhancement.
Genetic materials and experimental design
A total of 14 amaranth lines were evaluated on-station at AVRDC - The World Vegetable Center, Regional Center for Africa (AVRDC-RCA), Arusha, Tanzania (Table 1). Materials selected were based on suitability for using in grain and leaf such as grain colour (brown or cream). The materials were evaluated for leaf and grain yields in two trials. In trial 1, plants were evaluated for both leaf and grain yields. Side leaves were continuously harvested/picked weekly allowing the plant to flower and give grain. In trial 2, the genotypes were grown for grain yield evaluation without leaf harvesting. The experiments were conducted in 2012 in two seasons, first season (Feb - May) and second season (May - Sep). The trials were laid out in randomized complete block design (RCBD) with three replications in a plot size of two rows at 60 cm spacing between rows and 25 cm between plants; there were 24 plants per row.
Experimental location
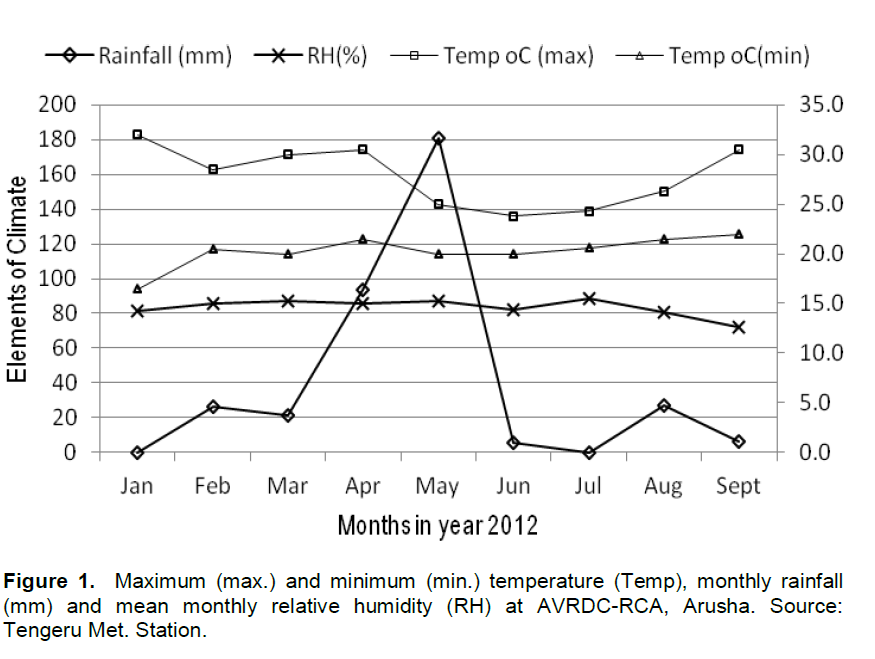
The trial site, AVRDC-RCA’s research station, is located in Arusha, Tanzania at 1290 m a.sl, and 4.8° N latitude and 37° E longitude. The site has clay loam soil with a pH ranging 6.0 to 6.7. The average temperature during the first season (Feb - May) was 25.1°C with a mean daily maximum of 28.5°C and daily minimum of 20.5°C, while the average during the second season (May - Sep) was 24.3°C with 26.1 and 21°C mean daily maximum and minimum, respectively. The location has bimodal rainfall with the main rainfall occurring from Feb to Jun and the short rain from Sep to Dec. The total amount of rainfall received during the first and the second season was 322.1 and 32.7 mm, respectively. Average relative humidity in the first and second season was 86.3 and 80.8%, respectively.
General agronomic practices
Land was ploughed and harrowed by tractor, and ridges were made manually by hand hoe. Seeds were sown directly at the rate of 1 kg per ha by drilling after mixing with sand in 1:4 seed to sand ratio. Seed was sown on the 7th Feb in 1st season for both trial 1 and 2, and 29th May 2012 in the 2nd season for trial 1 and 2. Thinning was carried out twice at 14 and 22 days after sowing (DAS) leaving a spacing of 25 cm between plants and a total of 24 plants per row. Fertilizer was applied at the rate of 200 kg/ha Diammonium phosphate (DAP) 18:46:0 as a basal application at sowing, and at 120 kg/ha urea (46:0:0) as side-dressing in two split applications, 60 kg/ha each, at two and six weeks after sowing. Selecron® (a.i. profenofos 720 g/l EC) was used to control cutworm and whiteflies at the rate of 1 ml/l of water while Actellic® (a.i. pirimiphos-methyl, 1.5 ml/l) was used to control, aphids and caterpillars twice at 14 and 42 DAS. Folicur (a.i. Tebuconazole 430 g/l) at the rate of 1 ml/l and Ridomil (a.i. Metalaxyl-M) at 3 g/l of water were used to control dumping off once at 7 DAS. Weed was controlled by hand-hoeing at 2-weeks interval starting 14 days after germination, but the frequency reduced as the plants grew forming canopy. Furrow irrigation was used to supplement rainfall.
Data collection
Data collected in trial-1 (experiment with leaf harvesting) included leaf yield, number of leaf harvested per plant, leaf length and width, number of branches per plant, days to 50% flowering, plant height and grain yield. Grain yield was measured in trial-2 (the experiment without leaf harvesting) to see the potential of the genotypes in grain yield when grown without leaf harvested. The first leaf harvesting per plot was started 6 weeks after sowing and continued at bi-weekly interval until a total of 4 harvests in the first season and 3 harvests in the second season. The leaf harvesting was done by plucking off tender leaves without topping. Fresh leaf weight was measured immediately using a kitchen balance (model Globe Brand; Globe Food Equipment Company Dayton, Ohio, USA). At each harvest, number of leaves harvested per plot was counted. Leaf length and width (cm), number of branches per plant, and plant height (cm) at flowering stage were measured on 10 plants randomly selected per plot. In the experiment without leaf harvesting, the materials were allowed to flower and give grain without any disturbance. Grain yield harvesting in both experiments was conducted when inflorescence colour had turned yellow. Plants were cut and threshed and clean grains were put in net bags and dried on seed drier (locally made with air blowing by fan under neath) to 6.5% moisture content before weighing using an electronic balance.
Data analysis
Data collected were subjected to both individual and combined analyses of variances (ANOVA) using CoStat version 6.204 (CoHort Software, CA, USA). Correlation analysis was performed to see the association among the various parameters.
Genotype (G) by Season (S) interactions were significant for leaf yield per plant, leaf yield per ha, number of leaves per plant, number of branches per plant, days to 50% flowering and plant height.
Leaf yield
The best leaf yielding genotypes in season-1 were not the best in season-2 and vice versa (Table 2). The highest
fresh leaf yield in season-1 was obtained in genotype RVI00117 (32.8 t/ha) followed by genotypes RVI00002 (20.9 t/ha) and RVI00021 (20.6 t/ha). The lowest leaf yield was obtained in genotype RVI00121 (14.1 t/ha). The highest mean leaf yield in season-2 was obtained in genotypes RVI00002 (14 t/ha) and RVI00001 (13.7 t/ha), while the lowest yield was in genotype RVI00090 (6.3 t/ha).
Number of leaf harvested per plant
The differences among the genotypes were significant at p≤0.01 in season-1 and at p≤0.001 in season-2. Genotype RVI00117 had the highest mean leaf number harvested per plant in season-1, while RVI00002 gave the highest in season-2 (Table 2). The lowest mean leaf number harvested per plant in both seasons was in genotype RVI00090.
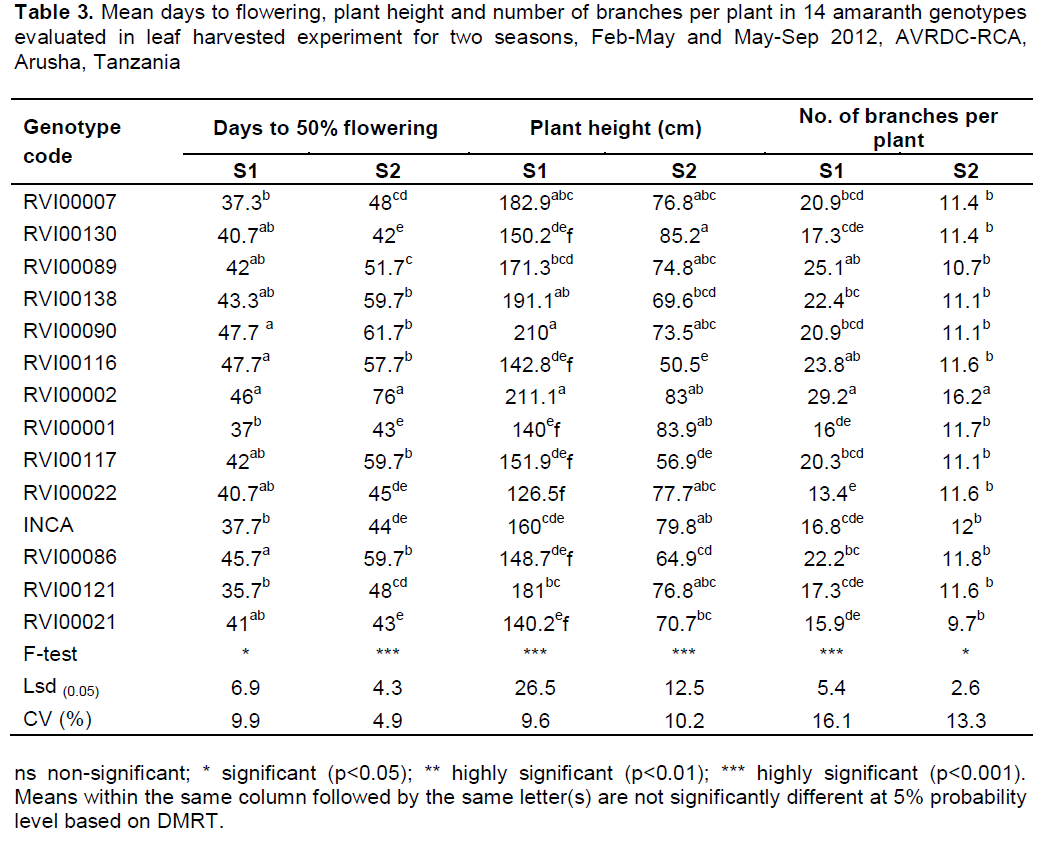
Number of branches per plant and plant height
The genotypes significantly differed in number of branches per plant at p≤0.001 in season-1 and at p≤0.05 in season-2. Genotype RVI00002 had many number of branches per plant in both seasons (Table 3). On the other hand a few numbers of branches per plant were observed in genotypes RVI00022 in season-1 and in genotype RVI00021 in season-2. Some of the tallest genotypes in season-1 were not the tallest in season-2. RVI00002 and RVI00090 were the tallest genotypes in season-1 while RVI00130 was the tallest in season-2 followed by RVI00001 and RVI00002 (Table 3).
Days to 50% flowering
RVI00007 and RVI00001 were the earliest genotypes in season-1, whereas genotype RVI00130 was the earliest in season-2 (Table 3). The longest number of days to attain 50% flowering in season-1 was recorded in genotypes RVI00090, RVI00116 and RVI00002, while in season-2 the longest number of days was observed in RVI00002.
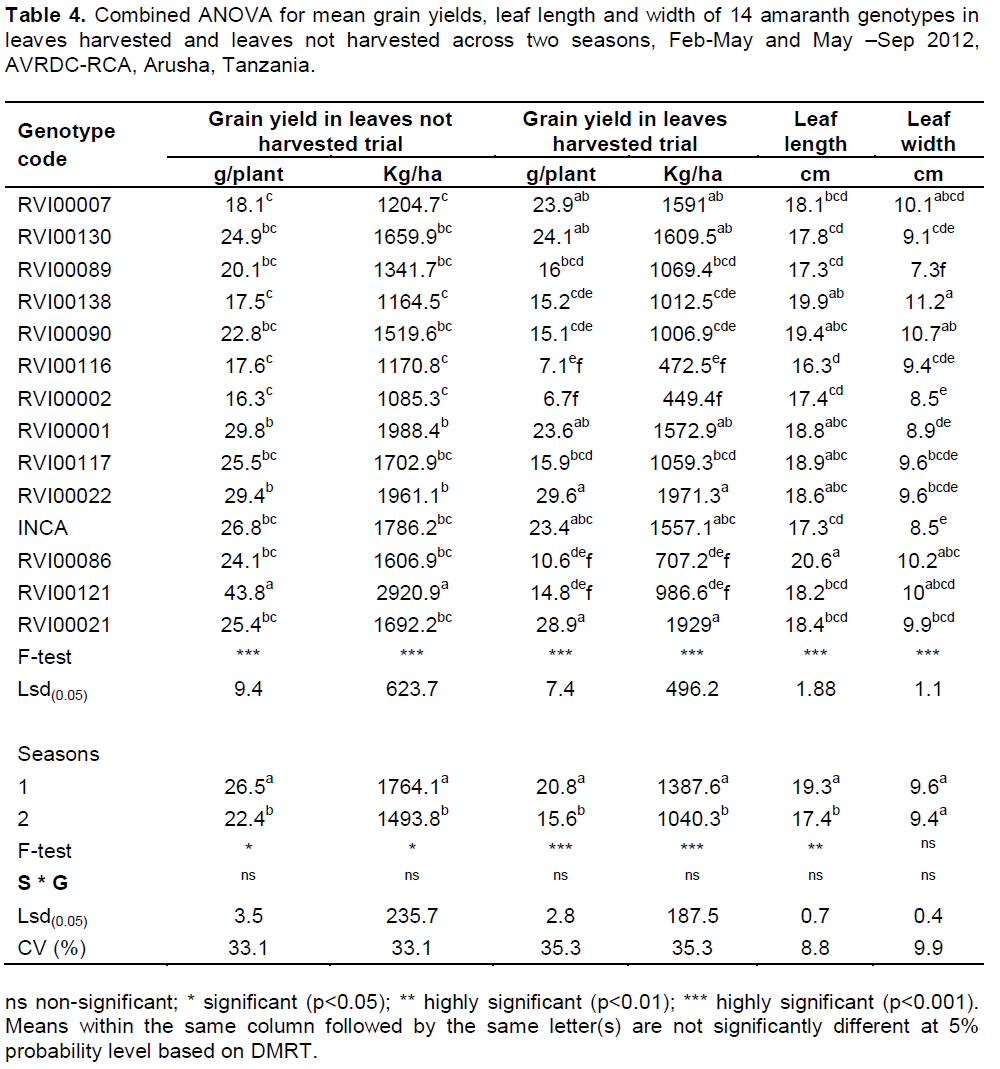
Grain yield, leaf length and leaf width
Grain yield in both harvested and non-harvested experiments, leaf length and leaf width were three traits for which G×S interactions were non-significant in this study. Combined analysis of variance indicated that there was a significant difference (p≤0.001) among genotypes in grain yield (Table 4). The highest mean grain yield in non-leaf-harvested experiment was observed in genotype RVI00121 (2921 kg/ha) followed by RVI00022 (1961 kg/ha), whereas the lowest yield was observed in genotype RVI00002 (1085 kg/ha). On the other hand in trial-1, where leaves were harvested, the highest grain yield was recorded in genotype RVI00022 (1971 kg/ha) and RVI00021 (1929 kg/ha). The differences among genotypes in leaf length and width were significant in both seasons. Genotype RVI00086 had the longest leaf, while the shortest leaf was recorded in genotype RVI00116 (Table 4). The broadest leaf was recorded in genotype RVI00138 and the narrowest in genotype RVI00089.
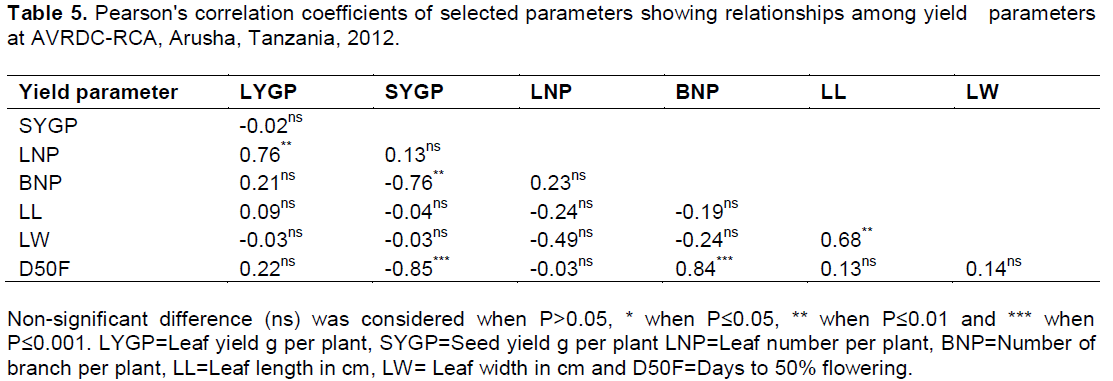
Correlation of yield parameters
Correlation analysis conducted among traits on the average of data of the two seasons indicated that leaf yield per plant had strong positive correlation with number of leaf per plant, while it was not correlated with other traits (Table 5). Grain yield per plant indicated negative correlation with days to 50% flowering and branch number per plant, implying that genotypes with late flowering and few number of branches per plant had low seed yield and vice versa. There was no correlation between grain yield and leaf yield.
Leaf and grain yield
The variations in leaf and seed yield between the two seasons might be due to the influence of the growing environment condition. The first season was characterized by warm (mean temp 25.1°C) and wet (322 mm rainfall), while the second season was cool (mean temp 24°C) and dry (32.7mm rainfall) (Figure 1). Warm and wet conditions seems to be optimum for amaranth production since it affects other traits like plant height and number of branches which might affect directly or indirectly leaf and grain yield.
It has been reported that fresh leaf yield of amaranth may vary from 10 to 70 t ha-1, while seed yield ranges from 1 to 6 t ha-1 (Svirskis, 2003). Grain yield could go below 1 t/ha. Gupta et al. (1994) reported grain yields of 0.3 t and 0.7 t ha-1 under unfavorable and optimum growing conditions in Kenya, respectively. Leaf yield reported in the present study were generally lower, but comparable to those reported earlier for Amaranthu species, A. cruentus, A. hypochondriacus and A. dubius (Oluoch et al., 2009) that varied between 17.8 t and 32 t/ha with different harvesting techniques. The higher values reported in the earlier study may be explained by differences in harvesting methods and genotypes evaluated. In the present study, differences among the genotypes in leaf and grain yields indicate their differences for dual purpose or grain amaranths.
In general, the grain yield reported in this study was within the yield ranges reported earlier (Svirskis, 2003). Variations among genotypes in grain yield in leaf harvested experiment and in leaf not harvested indicate that in many cases leaf defoliation reduces grain yield. Removal of specific green tissues inhibits photosynthesis and alters sink-source relationships. Leaf harvesting/defoliation limit the production of exportable sugars which are required as a resource for meristematic activity and for the growth of sink organs, mainly the grain in this case. Saidi et al. (2007) reported the highest grain loss in cowpea when leaf harvesting frequency was as per appearance. In the present study, however, some genotypes (RVI00007 and RVI00021) gave higher grain yields in leaf harvested experiment than under leaf not harvested experiment. We observed in these genotypes, where leaves were harvested there were few branches and light inflorescence that were not breaking/loading. However, in plots where leaves were not harvested both branching and inflorescences became heavy resulting in lodging, and breakage of inflorescences during windy and/or rainy days. This resulted in significant grain yield loss before harvesting.
Plant height and days to 50% flowering
Both plant height and days to 50% flowering were affected by season. Plant height ranged from 127 to 211 cm and 51 to 85 cm in the first and second season, respectively. The same trend was observed in days to 50% flowering where the entries took more days in the second season. These variations can be attributed to differences in genotypes response to the different seasons. In the first season the weather condition was warm and wet while the second season was cool and dry. Vegetable amaranth has been reported to achieve optimum growth at temperature ranges 25 to 30°C (Whitehead et al., 2002). The result of the current study is in agreement with the finding of Kauffmann and Weber (1990) who reported that some traits of amaranths such as plant height, days to maturity and plant architecture are affected by environmental conditions.
Number of leaves and branches per plant
Differences observed in number of leaves harvested and number of branches per plant in each season might be due to genotype and seasons differences. Highest leaf yield was harvested in Season-2.The genotype RVI00002 took longer time to flower in both seasons as compared to other genotypes, and therefore its vegetative phase extended, which resulted in higher number of branches as well as leaves harvested. This observation is in line with findings by Okokoh and Bisong (2011) who observed the sharp decline of leaf productivity in A. cruentus after on-set of flowering.
Relationship among yield parameters
Weak negative correlations between leaf yield with seed yield and leaf width, suggest that high leaf yielding genotype had relatively low grain yield as well as thinner leaves. This was shown by the genotype RVI00002, which had relatively higher leaf yield in both seasons, but low in grain yield.
Amaranth is one of the vegetables that have potential for nutrition and food security, and income diversification. There is, therefore, a need of improving its productivity. It was indicated from this study that genotypes RVI00121 and RVI00001 were the best for grain production while RVI00007 and RVI00022 were recommended for dual purpose (leaf and grain) during warm wet and cool dry conditions, respectively; further study might be required to understand the effects of environment on yield and quality of both leafy and grain, and genotype by environment interaction. Generally, genotypic differences appear to strongly affect the choice of amaranth for leaf, grain or dual purpose production.
The authors have not declared any conflict of interest.
REFERENCES
|
Achigan-Dako GE, Sogbohossou OED, Maundu P (2014). Current knowledge on Amaranthus spp: Research avenues for improved nutritional value and yield in leaf amaranth in Sub-Sahara Africa. Euphytica 196(3) |
|
|
|
DAFF (2010). Amaranthus: Production guideline. Department of Agriculture, Forestry and Fisheries, Directorate of Agricultural Information Services Pretoria. Republic of South Africa. |
|
|
|
Grubben GJH, Denton OA (2004). Plant Genetic Resources of Tropical Africa 2: Vegetable. Wageningen, Netherlands: PROTA Foundation. |
|
|
|
Gupta C, Dobos G, Gretzmacher R (1994). Comparison of the grain amaranth species A. cruentus and A. hypochondriacus. Symposium on Breeding of Oil and Crops in Albena, Bulgarien. |
|
|
|
Itúrbide GA, Gispert M (1994). Grain amaranths (Amaranthus spp.). In: Hernándo Bermejo JE, León J (Eds.), Neglected crops: 1492 from a different perspective. Plant production and Protection series No. 26. FAO, Rome, Italy. |
|
|
|
Kauffman CS, Weber EL (1990). Grain amaranth. p. 127-139. In: Janick J, Simon JE (Eds.), Advances in new crops. Timber Press, Portland, OR. |
|
|
|
Kaul HP, Aufhammer W, Laible B, Nalborczyk E, Pirog S, Wasiak K (1996). The suitability of amaranth genotypes for grain and fodder use in Central Europe. Die Bodenkultur 47(3):173-181 |
|
|
|
Keller G (2004). African Nightshade, Eggplant, Spiderflower et al – Production and Consumption of Traditional Vegetables in Tanzania from the Farmers' Point of View. MSc thesis, Georg-August Universität, Göttingen, Germany |
|
|
|
Maboko SM (1999). Vegetable amaranth improvement for South Africa [Ongenotype]. Available from htt:/www.newcrops.uq.edu.au/newslett/ncn11169.htm. |
|
|
|
|
Maundu P, Achigan-Dako E, Morimoto Y (2009). Biodiversity of African vegetables. In: Lichtfouse E, Hamelin M, Nararrete M, Debaeke P (Eds.), Sustainable Agriculture volume 2. London. EDP Sciences. Chapter III.
Crossref |
|
|
|
Mlakar SG, Turinek M, Jakop M, Bavec M, Bavec F (2010). Grain Amaranth as an alternative and Perspective crop in Temperate climate. J. Geogr. 5(1):135-145. |
|
|
|
Moinester AJ (2007). Determinants of Adoption for Improved Vegetable Amaranth Seed: The case of small-scale farmers in Northeastern and Central Tanzania. Msc. Thesis University of California, Davis. |
|
|
|
Muyonga HJ, Nabakabya D, Nakimbugwe DN, Masinde D (2008). Efforts to promote amaranth production and consumption in Uganda to fight malnutrition. In: Robertson GL, Lupien RJ (Eds.), Using Food science and Technology to improve nutrition and promote national development. Ontario. IUoFST. Ch 8, P 2. |
|
|
|
National Bureau of Statistics (2012). National sample census of Agriculture 2007/2008. Small holder Agriculture volume II: Crop sector- National Report. |
|
|
|
Okokoh SJ, Bisong WB (2011). Effect of Poultry manure and Urea-N of flowering occurrence and leaf productivity of Amaranthus cruentus. J. Appl. Sci. Environ. Manage 15(1):13 - 15. |
|
|
|
Oluoch MO, Pichop GN, Silue` D, Abukutsa-Onyango MO, Diouf M, Shackleton CM (2009). Production and Harvesting Systems for African Indigenous Vegetable. In: Lichtfouse E, Hamelin M, Nararrete M, Debaeke P (Eds.), Sustainable Agriculture Volume 2. London: EDP Sciences. Chapter 5. |
|
|
Saidi M, Ngouajio M, Itulya FM, Ehlers J (2007). Leaf harvesting initiation time and frequency affect biomass partitioning and yield of cowpea. Crop Science 47:1159-1166.
Crossref |
|
|
Shukla S, Bhargava A, Chatterjee A, Srivastava A, Singh SP (2006). Genotypic variability in vegetable amaranth (Amaranthus tricolor L.) for foliage yield and its contributing traits over successive cuttings and years. Euphytica 151:103-110.
Crossref |
|
|
|
Stallknecht GF, Schulz-Schaeffer JR (1993). Amaranth rediscovered. In: Janick J, Simon JE (Eds). New crops. Wiley, New York. pp. 211-218. |
|
|
|
Svirskis A (2003). Investigation of amaranth cultivation and utilization in Lithuania. Agron. Res. 1(2):253-264 |
|
|
|
Whitehead WF, Carter J, Sigh BP (2002). Effect of planting date on vegetable Amaranth leaf yield, plant height and gas exchange. HortScience 37(5):773-777. |
|
|
Wu H, Sun M, Yue S, Sun H, Cai Y, Huang R, Brenner D, Corke H (2000). Field evaluation of an Amaranthus genetic resource collection in China. Genet. Resour. Crop 47(1):43–53.
Crossref |