ABSTRACT
This study focused on Vitex payos (Lour) Merr. which is a high valued indigenous fruit tree known for its social (nutritional and medicinal use) and economic potential in Kenya. However, domestication of this species is hampered by limited information on its vegetative propagation. The objective of the present study was to determine the effect of seasonality and grafting methods on graft take-off and survival. Scions were collected and evaluated using wedge, saddle and splice grafting methods. The experiment was conducted in a glasshouse under a complete randomized block design. Development, growth and survival of terminal shoots and lateral branch were assessed. Analysis of variance (ANOVA), post-hoc and regression analyses were performed using GenStat software 16th edition. There was significant difference in the number of terminal shoots developed across grafting methods (F2,159 = 3.60; p = 0.029). Similarly, there were significant differences in the mean growth of the terminal shoots across seasons and grafting types. Wedge grafts registered the highest number of terminal shoots followed by splice grafts. Wedge grafts that were done during the month of October recorded the tallest shoots on average. Growth of lateral shoots across the seasons and grafting methods were significantly different (p < 0.05). However, grafting method and season had no effect on survival of the grafts even though survival declined significantly over time. The findings of this study show that wedge grafts registered the best performance. Grafting in October or when the tree is almost breaking dormancy is recommended for better growth performance.
Key words: Vitex payos, vegetative propagation, grafting method, seasons, graft-take.
Vitex payos (Lour) Merr. is a small deciduous tree species in the Verbenaceae family. It is mainly found in Eastern and Southern African countries like Kenya, Democratic Republic of Congo (DR Congo), Tanzania, Zambia and Mozambique. In Kenya, V. payos grows in semi-arid parts namely: Makueni, Kitui, Mwingi, Mbeere, Tharaka, Machakos, Kilifi and Kwale counties (Mbora et al., 2008). The tree grows up to a height of 10 m and is characterized by low, sparsely branched and rounded crown with square branchlets (Beentje, 1994). V. payos grows naturally between 0 and 1600 m above sea level with mean annual rainfall of 650 to 850 mm. In arid environments, it thrives well near rock out-crops and utilizes the run-off for its sustenance. It often grows in sandy soil and less often, clay red soil (Mbora et al., 2008). Vitex payos is highly valued for its fruits for domestic and income (Muok et al., 2001). The fruit pulp can be processed into jam and juice. The pulp is rich in carbohydrates, macronutrients (K, Na, P and Ca), in vitamin C as well as micronutrients (Fe, Mn, and Zn) in quantities that can contribute to human diet. Its potassium content is 1600±40 mg100 g-1 dry matter and more abundant than that of Adansonia digitata 240 ± 30 mg 100 g-1 dry matter (Kimondo et al., 2012) and bananas 318.28 mg 100 g-1 dry weight (Mohapatra et al., 2010).
Ecologically, V. payos is commonly found in dry combretum wooded and bush savannah. The natural woodlands in which the fruit tree grows are increasingly being cleared for agriculture, and settlement (Muok et al., 2001). Even under agroforestry systems where farmers leave behind such fruit trees for various uses, regeneration and recruitment is affected by livestock that destroy the saplings. Propagation through seed is hampered by seed dormancy and anthropogenic factors (Mbora et al., 2008). Despite its high value, there is little or no effort in domestication of V. payos to ensure its ex situ conservation. This is attributed to lack of appropriate information on its propagation, and long period before fruiting. Therefore, the on farm and natural range populations of V. payos are declining causing a lot of concern on its future survival. Domestication of V. payos is further complicated by high variation in fruit qualities such as taste, size and productivity across its natural range (Muok et al., 2001). To solve this problem, appropriate vegetative propagation technique is recommended to shorten the duration to fruiting while maintaining high qualities of the mother tree. This technology enables farmers to cultivate improved varieties of V. payos in orchards (Pina and Errea, 2005; Mudge et al., 2009).
Grafting is widely practiced in breeding and fruit production programs. However, information on grafting of V. payos is scanty. Preliminary studies indicate feasibility of grafting V. payos (Kimondo, 2010). However, very low success in graft take-off of 15% was recorded during April to October 2008 with no significant differences in graft take-off across seasons. It is argued that the period after dry season, just before the rains, when the plant cells undergo rapid cell division is the best period for scion collection to achieve greatest graft take-off (Akinnifesi et al., 2007). In Kitui, this season lies between August and October. The past studies on V.payos species were of limited sample size and had significant variability across months that made it difficult to draw informed decision. Additionally, previous success of grafting methods is reported to vary with species (Anjarwalla et al., 2016). In the preliminary studies of grafting V. payos, grafting methods tested included wedge, splice; and whip and tongue method. The latter was found to be less practical for an ordinary farmer to perform easily because V. payos wood is hard. Therefore, there is need to develop a simple, effective and practical methods of grafting for the farmer.
Relatively few studies have been undertaken on grafting of indigenous fruit trees in East Africa (Munjuga et al., 2014; Anjarwalla et al., 2016). However, different methods revealed different levels of success in different species. For instance, studies of different grafting methods on Allanblackia floribunda indicated that grafts take-off in side tongue (80%) and side veneer (52%) was higher than Wedge/top cleft (50%) (Asaah et al., 2011). Grafting studies on baobab (Adansonia digitata) by Anjarwalla et al. (2016), showed that top cleft/wedge grafting recorded higher survival success than side veneer (71 vs. 55%). These studies suggest that appropriate grafting methods for a target fruit tree needs to be determined prior to the rolling out of massive grafting. Furthermore, tests on the effect of age of rootstock on grafting success in A. digitata showed that a 2-year old rootstock was more successful than a year old rootstocks (75 vs. 53%) , hence the age of rootstock is important in grafting operation (Anjarwalla et al., 2016).
This study was designed to determine the effect of seasonality (scion’s physiological state) on graft take-off and survival and evaluate the effect of different grafting methods on V. payos graft take-off and survival. Specifically, saddle (SA), splice or side (SI) and wedge (W) grafting methods were tested across three seasons: a period after long dry season just before the rains begin (the month of October) during the rains (the month of January) and the period just after the rains (early March).
Description of study sites, scion collection area and experimental sites
Scions were collected from Ikanga village, 50 km from Kitui town, Kitui county, Eastern Kenya. The experiment was conducted at the Drylands Eco-Region Research Programme (DERP) – Kitui, glasshouse. Both Ikanga and Kitui receive bimodal rainfall pattern with two peaks between March and May; and October and December. The rest of the months are dry with daily temperatures variation between 24 to 38°C. The annual rainfall ranges between 120 and 300 mm per annum (Bhollai, 2013). Ikanga area is characterized by sandy loam soils. The scions were collected on agricultural farms and open woodlands. The Ikanga population was chosen due to its relative proximity to Kitui and its uniform V. payos populations (Kimondo, 2010). V. payos rootstocks were raised from wildings (seedlings that naturally regenerated in the woodland) collected from central Kitui. Growth of the rootstocks was boosted by spraying them with foliar fertilizer for a period of eight months.
The scions were collected during three seasons: Before the onset of the long rains (October, 2012) when the tree is dormant and is about to burst its buds, in (January, 2013) when the leaves are fully developed and active growth is taking place, and lastly in the middle of dry season with high temperature (early March, 2013) before short rains. Scions were randomly harvested from the mother trees that exhibited the desired physiological characteristics. Scion harvesting was done early in the morning and the scions sparingly sprayed with water to avoid dehydration.
The scions were then wrapped with newspapers, packed in clear polythene tube and kept in cooler boxes. Grafting was performed a day after scions collection under shade condition to minimize dehydration. Secateurs and grafting knives were sterilized with sodium hypochlorite before scions’ harvesting and during grafting to reduce contamination and infection at the point of union.
Standard grafting procedures were adopted. The root stock was severed with surgical blades at 10 cm high and scion of about 5 cm length was attached. Three grafting methods that have shown some levels of success in V. payos (Kimondo, 2010) and have been reported to be simple for ordinary farmer to perform were evaluated. They include: 1. Splice or side graft where a diagonal or slanting cut is made on both the rootstock and scion; 2. Saddle where a deep cleft was made in the end of the scions by doing two sloping cuts and the end of the stock to make a wedge-shape to fit into the scions which is placed upon it saddlewise, and 3. Wedge grafting where two smooth cuts are made towards the base of the scions creating a blunt wedge. The wedge side of the scions is then inserted into a cut created on the rootstock to ensure cambial contact between the rootstock and the scions. In all the three methods, the rootstocks and scions were then brought together and tied with a para film or grafting tape to ensure strong union as well as good cambium contact. Splice was chosen because it is the simplest grafting method for any ordinary farmer and takes less time to perform (Garner, 2013). Though in the preliminary studies, it scored the lowest percentage of graft take-off, it acted here as the control since it does not have notched cuts to enhance cambial contacts as in the case of wedge and saddle grafts.
All grafts were then covered with a loose transparent plastic to ensure moisture buildup around the scion to prevent desiccation. Grafts were then placed in a glasshouse throughout the experimental period and watered daily in the morning.
Experimental design
A complete randomized block design (CRBD) was adopted for the 3 grafting methods and replicated four times. In each replicate, there were 10 individual grafts per method. The experiment was conducted in a glasshouse in Kitui with mean monthly temperature of 23.2°c in October, 22.7°c in November and 22°C in December months of 2011. Whereas in 2012, the mean monthly temperatures were 22.6°C in January, 24.4°c in February and 24.7°C in March.
Assessments, measurements and maintenance
The variables included: count of fully open terminal buds; number of lateral buds beginning to open; height of terminal shoot, length of longest side branches and graft survival. The procedure was repeated for the experiments conducted in January and March, 2013. The assessments for the terminal shoots and lateral shoots were done on surviving grafts but the percentage number of the developing terminal buds was calculated out of the total populations of grafts done inlcuding the dead.
The first assessments were done after the emergence of the scion sprouts with subsequent assessments performed forty nightly for two and a half months. Monitoring was done on weekly basis to remove suckers from the rootstocks to avoid unnecessary competition. After the development of terminal buds, the polythene tubes were removed and replaced with bigger translucent polythene tubes. This was done to avoid an abrupt exposure of the young sprouts to harsh weather condition. Weather data at Kitui was monitored and recorded.
Data analysis
Survival percentage in each category was done and calculated based on the total population, graft method and seasonality. The data was analyzed using general statistics (Genstat 16th edition) software. Significant differences were declared at 5% (p<0.05). Data was analyzed for descriptive statistics. Logistic regression model was used to determine the most appropriate grafting method and the best season for grafting. In this model, each number of grafts per replicate was assumed to be independent of a given outcome such as death or alive; opening of terminal buds or not. For each graft method, the counts of dead and alive (survival count); and counts of grafts with terminal buds opening were scored. The survival percentages of grafts and the percentage of grafts developing terminal buds were then calculated. Survival and number of terminal buds were analyzed as a function of different grafting methods (independent variable) across seasons and results interpreted as appropriate. Analysis of variance (ANOVA) was used to determine differences on mean height of terminal shoot and mean length of longest side branches among different grafting methods across all seasons. The season in entire dataset was also handled as covariate besides running data analysis per season.
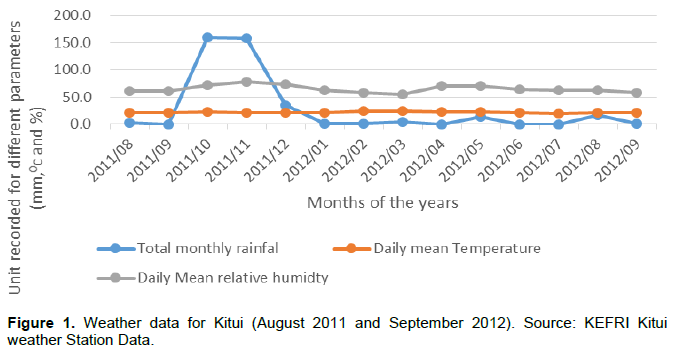
The weather data for DERP-Kitui experimental site include rainfall, temperature and humidity (Figure 1). The rainfall peaks were in November and May; however, data for 2012 was unavailable due to a hitch experienced in the data logger. The mean monthly temperature of the glasshouse was 23.2°c in October 22.7°c in November, 22°C in December, 2011, whereas in 2012, the mean monthly temperatures were 22.6°C in January, 24.4°c in February and 24.7°c in March. There was no much variation in the temperatures during the grafting periods.
Effect of season and grafting method on the survival of grafts
There were no significant differences on survival among different grafting techniques at the closure of the experiment at day 65 (F2, 156 = 2.15; p=0.119) nor was there sufficient evidence to show that seasons affected the survival of grafts (F2, 156 = 2.78; p=0.065). Both season and method of grafting accounted for less than 3% of the total variability. In all seasons, 58% of wedge grafts survived, whereas 50% of each of saddle and splice grafts survived (Figure 2a, b and c). Instead, findings show that graft survival declined significantly over days (F 4,156 =48.51; p<0.01) across all seasons (Figure 2a, b and c). The effect of days during experiment on survival accounted for 54% of the total variability.
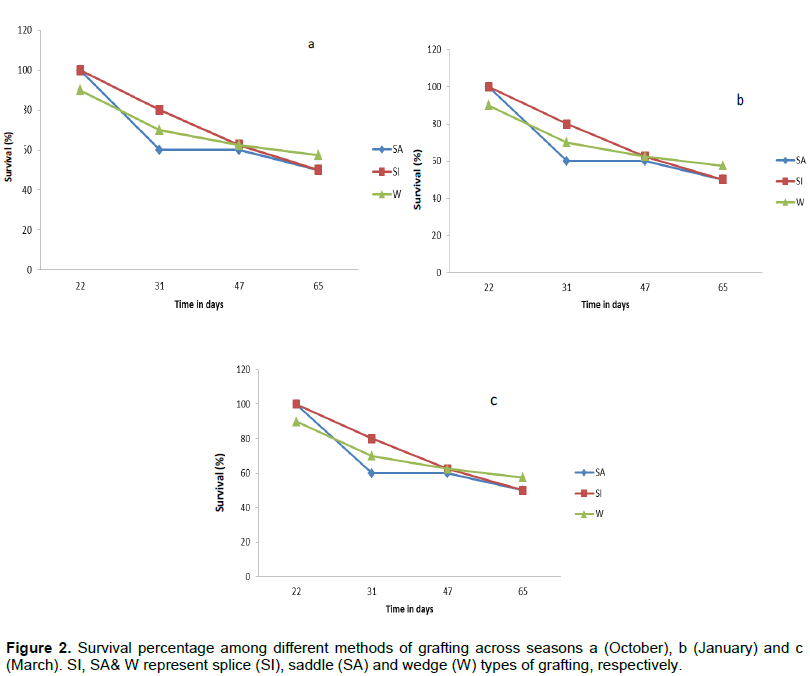
The present findings are in line with those reported by Anjarwalla et al. (2016) and Kalinganire et al. (2007) showed that grafting methods (Wedge and Side veneer) did not have significant effect on survival of Adansonia digitata grafts. The lack of significant influence of method and season on survival coupled with significant decline in survival across all seasons suggests that death of grafts could be due to other parameters that were not measured. Several biotic and abiotic factors that affect plant development have been reported to hinder survival of grafts in the nursery (Ozturk and Serdar, 2011). Poor environmental conditions like above optimum temperatures coupled with high relative humidity may also affect the development of grafts and cause rotting at the graft union (Karadeniz, 2006). Several fungal rots were observed at the point of union during assessments. The abiotic factors reported to be affecting development of plant grafts include poor craftsmanship that could cause poor alignment of rootstock with scions; thus leading to early deaths (Ozturk and Serdar, 2011). Although abiotic factors such as poor craftsmanship was limited by retaining the same experienced craftsmen across all seasons of grafting, disturbance of the point of union could not be avoided completely during the regular assessments.
There were significant differences (F 2, 159) =3.60; p=0.029) on the number of grafts which had fully developed terminal shoots among the three grafting methods. The wedge method had significantly higher number of successes as compared to SI and SA (Figure 3d). The grafts from wedge technique were about 1.5 times higher in number of fully developed terminal shoots than those from SA techniques (Table 1). Similarly, grafts from SI developing buds were about 1.3 times higher than those from SA (Table 1). This shows that wedge grafting method gave the highest graft take-off in terms of the percentage of terminal shoots that developed.
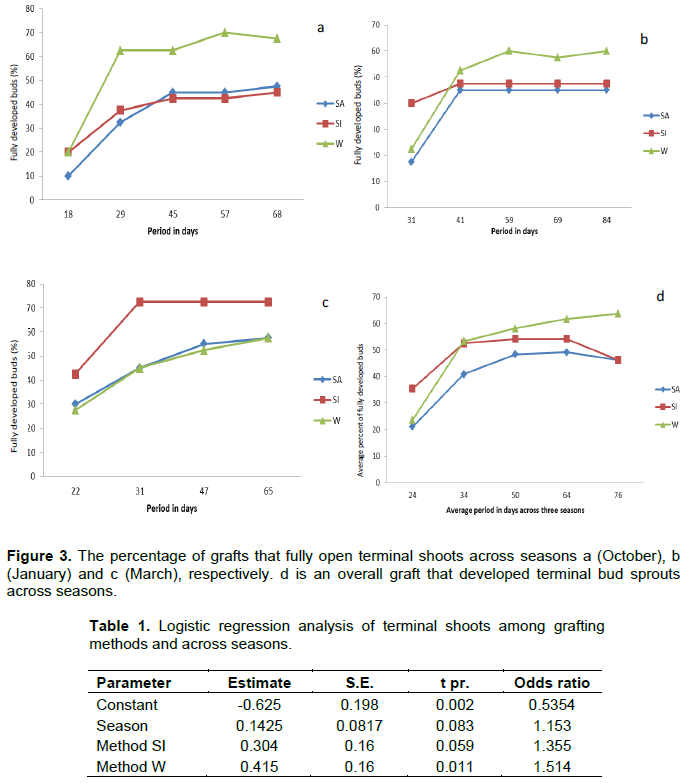
Wedge grafts had significantly high number of terminal shoots as compared to the other grafting methods. The findings suggest that grafting method affected formation of terminal shoots (Figure 3d).
This finding is in tandem with the past findings by Kimondo (2010) that wedge graft of V. payos was the best among the grafting methods tested. Several similar studies comparing side grafting with wedge grafts on other fruit tree species in the tropics have given varied results. This is because success rate of a particular grafting method is partly influenced by the species under trial (Munjuga et al., 2014; Anjarwalla et al., 2016). For instance, findings from grafting of Allanblackia floribunda indicated that grafts take-off in side tongue (80%) and side veneer (52%) was higher than Wedge/ top cleft (50%) (Asaah et al., 2011), whereas studies on grafting of
Adansonia digitata indicated higher graft take for wedge grafts than side veneer grafts (Anjarwalla et al., 2016). However, it is important to note that there is a slight difference between the side grafts mentioned in these past studies and the side/splice grafts tested in the present study and cannot be wholly by compared design. Also, the ecological condition and phenology as well as the genetic make-up of these other species are completely different from that of
V. payos. There are no documented evidence comparing the performance between or among splice, wedge (Top cleft) and saddle grafts. Existing studies in the tropics have mainly compared wedge, side veneer, side tongue, whip and tongue grafting methods (Sanou et al., 2004; Akinnifesi et al., 2008; Anjarwalla et al., 2016).
The differences in the success level among various grafting method stems mainly from the variation in the level of cambium contact among the three grafting methods (Garner, 2013). Cambium contact of the rootstock and scion is a key requirement for the formation of callus and enhancing re-union. The possible explanation to the better performance of wedge in this experiment over others could be due to the following factors:
Splice or side grafts are done by making a diagonal or slanting cut in the rootstock and the scions brought together. The diagonal cut is necessary to immobilize these parts until the formation of a strong union as well as a cambium contact (Garner, 2013). A simple diagonal cut like in splice graft tends to slide under pressure while a notched splice which is achieved in the whip and tongue or wedge graft where the use of interlocking tongues enabled grafters to set scion in stable position before tying using para film or grafting tape to enhance cambial contact. Therefore, this explains why in general, the opening of the terminal bud and subsequent growth of grafts were higher in wedge grafts than in splice grafts.
Their pressure exerted within the tissues due to compression or elasticity of the wood is greater in the interlocked tongue (wedge or whip and tongue) than in a mere slanting cut found in the splice (Garner, 2013). This argument partly explains the higher success rate recorded in the opening of terminal shoots of wedge grafts with interlocked tongues as opposed to splice grafts which lack the interlocked tongues. Enough pressure between stock and scion is paramount to prevent them from moving independent of each other. The same pressure is also essential to orient the plane of cell division to develop spatially organized tissue (Barnett and Asante, 2000). Since natural elasticity of the wood causes this pressure, wedge or whip and tongue have been reported to have this compression more on the scions than splice graft due to the presence of interlocking tongues in wedge grafts. Splice graft has to depend solely on the craft man’s ability to tie the para film or grafting tape to achieve this compression. This ability may vary from one craft man to another therefore explaining the better performance of the wedge grafts over splice and saddle.
Effect of season on development of terminal shoots
At the end of experiment, in October, 68% of wedge grafts developed terminal shoot, while 48% of both saddle and side grafts developed terminal shoots (Figure 3a). In January, however, there were 60% of wedge opening terminal shoots, 48% splice and 45% saddle opening terminal shoots (Figure 3b). In March, 73% of saddle grafts developed terminal buds, and 58% each of side and saddle grafts developed terminal buds (Figure 3c). Overall, wedge grafting was high in October and January but in March, splice type of graft was high (Figure 3a, b and c). However, inferential analysis shows that there were no significant differences in the number of terminal shoots that developed across seasons (F2, 165 =2.64; p=0.072). This means that there was no sufficient evidence to show that grafts that develop into terminal shoots varied with season.
Empirical evidence on the effect of grafting season on graft take has indicated mixed results. In the present study, the lack of significant differences in the numbers of terminal buds formed across seasons was similar to the findings of previous studies by Kimondo (2010) which indicated no difference in graft success of V. payos across seasons in April, May and July. In another instance of grafting baobab, a relatively high (63%) success rate was achieved when grafting was done between October-December after rains had begun and the tree leaves had flushed the buds (Anjarwalla et al., 2016). On the other hand, grafting success of mango fruit tree in the tropics have been reported to be possible any time of the year as long as the temperature is optimum for callusing (Karadeniz, 2006). All these studies point to the indication that the season of scion collection does not significantly cause variation in the number of grafts successfully developing terminal shoots and lateral branches. However, the findings contradicts the expectations that the best graft in the tropics can be achieved in the season transitioning from dry to rainy when the species is breaking dormancy and has highest growth hormone concentrated on the buds before leaf buds burst (Hartmann et al., 2002). The expectations in this case was that October grafts would emerge the best and significantly high in achieving grafts take-off (callus formation and subsequent development of terminal buds) in October when scions of V. payos are either breaking dormancy or the plant tissues are actively undergoing cell division and probably have high growth hormone concentrated on the buds before bursting (Hartmann et al., 2002). Instead, it indicates that scions collected and grafted in the month of March gave the best performance (73% splice, 58% each of wedge and saddle grafts). While the scions in October may have been in a different physiological state to enhance callusing and positive graft take-off, the lack of significant difference in the number of terminal shoots that sprout across seasons can be explained partly by the relatively uniform optimum climatic conditions for callusing.
Ambient temperature and relative humidity have been reported to influence greatly graft take-off (Karadeniz, 2006). The optimum temperatures for callusing of tropical evergreen fruit trees like mangoes have been recorded between 24 - 28°C though temperature as high as 38°C can be tolerated within the tropics and have less significant effect on callusing (Karadeniz, 2006). Whereas, higher temperatures above 40°C do affect negatively, survival and callusing of mangoes (Karadeniz, 2006). Additionally higher relative humidity has also been reported to influence callusing of grafts before they develop terminal buds ( Karadeniz, 2006). The temperatures in this experiment varied slightly (Figure 1). The high temperatures in March (Figure 1) may partly explain the high performance of grafts in March. But all the same, there was no much variation in the temperatures across all seasons of grafting. There was, however, high variation in the mean relative humidity during the experiment with November recording the highest (78%) and March the lowest of 53% (Figure 1).
Additionally, hormonal concentration in plants which varies across seasons is known to be one of the greatest causes of variation in graft take-off across season (
Tanaka et al., 2006). The concentration level of hormones across seasons of grafting was beyond the scope of this study on
V. payos.
Effect of grafting method and season on the number of lateral buds, and mean growth of terminal and lateral buds
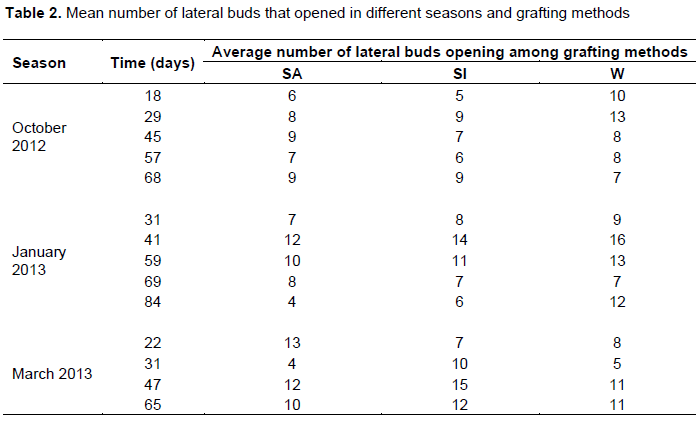
No significant differences were observed across seasons and grafting methods on the number of lateral buds opening (Table 2). There was however significant differences in heights of terminal shoot among different grafting methods (Table 3). Wedge grafting method recorded the tallest terminal shoot as compared to saddle and side grafts. Pairwise comparisons showed that the terminal growth in saddle and side grafts did not differ significantly (p>0.05) Table 3. There were similarly significant differences in terminal shoot height across all seasons (Table 4). Those grafts from scions collected and performed in the month of October showed the tallest mean terminal shoots as compared to those collected and done in January. Grafts done in January had their mean shoot heights significantly taller than those in March (Table 4). Also, generally grafting done in October exhibited the longest average lateral buds (5.46 cm) across all grafting methods (Table 5). Wedge grafts had the longest lateral buds (6.28 cm) in October. There were significant differences across all seasons and method of grafts (Table 4). Grafts done in the month of October had the best growth inlateralbudsforall grafting methods followed by January and March (Table 5).
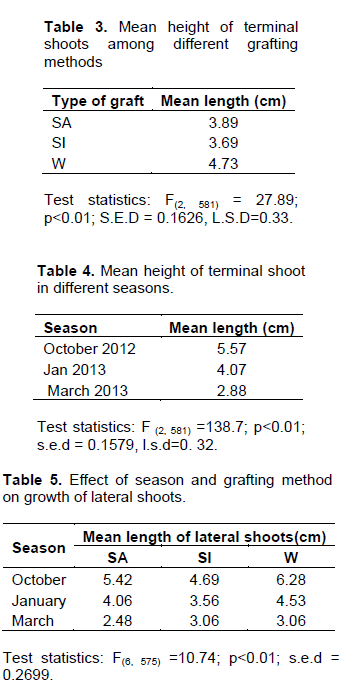
Although there are no empirical studies showing directly the effect of seasonality and method of grafting on the growth of terminal and lateral shoots of wild fruits from the tropics, factors affecting plant development across seasons could explain this partly (Hartmann et al., 2002; Chipojola et al., 2013). According to Chipojola et al. (2013), light, temperature, relative humidity, growth hormone concentrations, carbohydrate concentration/ reserve in the grafted materials could be varying across seasons and is likely to affect shoot growth and plant vigor (Chipojola et al., 2013). For example, auxin levels diminish from the period when plants are transitioning from dormancy to active growth season (Hartmann et al., 2002; Chipojola et al., 2013). High auxin hormones concentrations enhance phenomenon known as apical dominance which enhances terminal growth while suppressing the formation and growth of lateral buds
(Tanaka et al., 2006; Dun et al., 2006) . In this study, the highest terminal growth was recorded in the month of October (Table 4).
This may point to the argument that auxin concentration in the plants is highest during the period of transition from dormancy to active growth. In this case, October grafts may have had higher hormone concentration in their apical buds as compared to the rest of the seasons (Hartmann et al., 2002). This argument also partly explains why there were no significant differences in the number of lateral buds formed across seasons (Table 2). Contrarily, the significant effect of season on the length of lateral shoots may be attributed largely to the different growth condition (Figure 1) that varied across seasons than may be attributed to endogenous growth hormone.
The significant differences on growth of terminal and lateral shoot growth under different grafting methods with wedge method leading (Table 5) may be due to high rates of wedge graft take-off success as compared to other grafting method. It could be argued that with ease in graft take-off and the subsequent healing of graft union of a particular method, leading to faster growth, new shoots are enhanced.
CONCLUSION AND RECOMMENDATIONS
The present study reveals that wedge grafting method exhibited the best performance in V. payos as compared to splice and saddle grafts. This is because of internal pressure exerted through compression of the wood as compared to saddle and splice is higher. This results show that graft take-off of V. payos in Kitui was successful in all season of the year tested: the buds are just breaking dormancy in October, the crown has full foliage and crown bear flowers in January, or even during the season when the tree bears fruits in March. However, owing to the variation in growth vigor of the shoots observed across seasons, it can be suggested that October would be the best season that favor growth of successful grafts.
To further strengthen these findings, further research could include scions collected before October when the trees are dormant (September or August) to find out if there will be a significant influence on graft take-off. Season of grafting varied due to the physiological state of the scions, and the environmental conditions under which species were kept. They also do influence graft survival and success of various species, it is imperative to improve the climatic conditions for grafting V. payos.
Survival reduced significantly over the period of grafting. Incidences of rots at the graft union were noted to have contributed greatly to deaths. To improve graft survival, there is need to improve grafts handling during maintenance and assessments of V. payos.
The authors have not declared any conflict of interests.
REFERENCES
|
Akinnifesi FK, Sileshi G, Mkonda A, MhangoJ, Chilanga T, (2007). Germplasm Supply, Propagation and Nursery Management of Miombo Fruit Trees. Indigenous fruit trees in the tropics: domestication, utilization and commercialization. CABI.341-68.
|
|
|
|
Anjarwalla P, Ofori D, Owino A, Matuku D, Adika W, Njogu K, Kehlenbeck K (2016). Testing different grafting methods for vegetative propagation of baobab (Adansonia digitata L.) in Kenya to assist its domestication and promote cultivation. For. Trees Livelihoods 26(2):85-95.
Crossref
|
|
|
|
|
Asaah E, Tchoundjeu Z, Ngahane W, Tsobeng A, Kouodiekong L, Jamnadass R, Simons A (2011). Allanblackia floribunda: a new oil tree crop for Africa: amenability to grafting. New Forests 41(3):389-398.
Crossref
|
|
|
|
|
Barnett JR, Asante AK (2000). The formation of cambium from callus in grafts of woody species. Savidge RA, Barnett JR, Napier R, ed (s). Cell and molecular biology of wood formation. Experimental Biology Reviews. BIOS Scientific Publishers Ltd.: Oxford, UK pp. 155-167.
|
|
|
|
|
Beentje HJ (1994). Kenya Trees, Shrubs and Lianas. National Museums of Kenya, Nairobi, Kenya. 722 p.
|
|
|
|
|
Bhollai LD (2013). Assessments of an iron formation deposit in Ikanga area, Kitui County, South eastern Kenya, MSc. Thesis. College of Biological & Physical Sciences, Department of Geology Thesis University of Nairobi.
|
|
|
|
|
Chipojola FM, Mwase WF, Kwapata MB, Njoloma JP, Bokosi JM, Maliro MF (2013). Effect of tree age, scion source and grafting period on the grafting success of cashew nut (Anacardium occidentale L.). Afr. J. Agric. Res. 8(46):5785-5790.
|
|
|
|
|
Dun EA, Ferguson BJ, Beveridge CA (2006). Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol. 142(3):812-819.
Crossref
|
|
|
|
|
Garner RJ (2013). The grafter's handbook. Chelsea Green Publishing.
|
|
|
|
|
Hartmann HT, Kester DE, Davies FT, Geneve RL (2002). Plant propagation principles and practices. Prentice Hall, Upper Saddle River, New Jersey 849 p.
|
|
|
|
|
Karadeniz T (2006). Relationships between graft success and climatic values in walnut (Juglans regia L.). J. Cent. Euro. Agric. 6(4).
|
|
|
|
|
Kalinganire A, Weber JC, Uwamariya A, Kone B (2007). Improving rural livelihoods through domestication of indigenous fruit trees in the parklands of the Sahel. Fruit Trees 10:186-203.
Crossref
|
|
|
|
|
Kimondo JM, Agea J, Okia GA, Abohassan RA, Ndeunyema ET, Teklehaimanot Z, Mulatya J (2012). Physiochemical and nutritional characterization of Vitex payos (Lour.) Merr: An indigenous fruit tree of Eastern Africa. J. Hortic. 4(10):161-168.
|
|
|
|
|
Kimondo JM (2010). The potential for Optimization of Vitex payos as a Dry land Resource in Kenya. PhD Thesis, School of Environment, Natural Resources and Geography, University of North Wales, Bangor.
|
|
|
|
|
Mahopatra D, Mishra S, Sutar N (2010). Banana and its by-product utilisation: an overview. J. Sci. Ind. Res. 69(5):323-329.
|
|
|
|
|
Mbora A, Jamnadass R, LillesØ JB (2008). Growing high priority fruits and nuts in Kenya: Uses and Management. The world Agroforestry centre. Nairobi,Kenya P 61.
|
|
|
|
|
Mudge K, Janick J, Scofield S, Goldschmidt EE (2009). A history of grafting. Hortic. Rev. 35:437-493.
Crossref
|
|
|
|
|
Munjuga M, Kariuki W, Njoroge JB, Ofori D, Jamnadass R (2014). Effect of rootstock type, scion source and grafting methods on the healing of Allanblackia stuhlmannii grafts under two nursery conditions. Afr. J. Hortic. Sci. 7.
|
|
|
|
|
Muok BO, Owuor B, Dawson I, Were J (2001). The potential of indigenous fruit trees; result of a study in Kitui District, Kenya. Agroforestry today 12:13-15.
|
|
|
|
|
Ozturk A, Serdar U (2011). Effects of different nursery conditions on the plant development and some leaf characteristics in Chestnuts (Castanea sativa Mill.). Aust. J. Crop Sci. 5(10):1218.
|
|
|
|
|
Pina A, Errea P (2005). A review of new advances in mechanism of graft compatibility-incompatibility. Sci. Hortic. 106:1-11.
Crossref
|
|
|
|
|
Sanou H, Kambou S, Teklehaimanot Z, Dembélé M, Yoss H, Sina S, Bouvet JM (2004). Vegetative propagation of Vitellaria paradoxa by grafting. Agrofor. syst. 60(1):93-99.
Crossref
|
|
|
|
|
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006). Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45(6):1028-36.
Crossref
|
|