ABSTRACT
The government of Cameroon is responsible for distributing hybrid cocoa seeds to farmers in Cameroon. These high-yielding and pod rot-resistant hybrids are obtained from self-incompatible commercial clones used for seed production by manual pollination of freshly opened flowers. The experimental procedure for the propagation technique of these clones has never been reported. The objectives of this study are to assess the effect of clone, cutting source and growth regulator concentration on the growth and rooting of cocoa (Theobroma cacao L.) stem cuttings. The experiment consisted of 4 clones (SNK16, ICS40, UPA143 and T79/501; two cutting sources: B1 = orthotropic and B2 = Plagiotropic) and four concentrations of growth regulator (4-indol-3-butyric acid) concentrations (D0 = 0 mg, D1 = 12.5 mg, D2 = 25 mg and D3 = 37.5 mg), thus a 4 × 2 × 4 factorial experiment in a completely randomized block design with 3 replicates was designed. Cuttings were set and monitored weekly for shoot sprouting for 10 weeks and rooting at the 10th week. The following parameters were measured: survival rate, number of cuttings with shoots, number of produced shoots, leaves length, number and length of produced roots. Clone, cutting source and growth regulator concentration significantly affected survival rate, shoot sprouting and rooting of cocoa cuttings at p = 0.05. Overall, cuttings started producing shoots 3 weeks after setting (WAS) and at 10 WAS all the cuttings had rooted. Assessment of cuttings in Clementine propagators showed a survival rate of approximately 75%, with orthotropic cuttings showing higher results than plagiotropic cuttings, whereas, D1 (12.5 mg) was the overall effective growth regulator (IBA) concentration that induced the highest number of roots from all the clones. UPA143 was the clone with highest value for all the factors assessed. The results will be valuable in management decision when producing planting materials by stem cuttings.
Key words: Theobroma cacao, vegetative propagation, clone, cutting source, growth regulator concentration, clementine propagator, shoot and root growth.
Cocoa was introduced in Cameroon in the 19th century and has since played a major role in the economic development of the country (Champaud, 1966). Cocoa is a cash crop for all producing countries, and is an important source of income for farmers. In Cameroon, cocoa is one of the main export products, and represents approximately 28% of non-oil exports and 40% of exports from the primary sector (Ondoua et al., 2016). Cameroon produces about 280,000 tons of cocoa beans annually and is considered as the 5th high cocoa producer in the World (FAOSTAT, 2015). This has been possible through consistent government effort carried out by the Cocoa Development Cooperation (with French acronym SODECAO) which provides farmers with highly productive hybrids that are also adapted to the climatic conditions in Cameroon. These high-yielding and pod rot-resistant hybrids are obtained by manual pollination of freshly opened flowers from self-incompatible commercial clones planted in seed orchards of SODECAO. These commercial clones are imported from Trinidad and Brazil and propagated vegetatively for the establishment of seed orchard. At the start of the program, seed orchards were established by grafting because that was the only available method valorized during that period for the propagation of cocoa. Faced with graft incompatibility and variability of seedlings, there is usually insufficient production of clones for the seed orchards. Vegetative propagation by cuttings can resolve the above problems although it was abandoned in Cameroon about 40 years ago (personal communication).
Vegetative propagation is used to obtain an exact copy of the genome of a mother plant. This is achieved through the use of meristematic, undifferentiated cells that can differentiate into organs required to form a whole new plant (Wiesman and Jaenicke, 2002). The typical approach is propagation by stem cuttings, in which roots are induced to form on a piece of stem detached from a donor plant (Libby, 2004). Vegetative propagation is one of the used techniques in propagating superior commercial cocoa clones (Tee and Lamin, 2014). The technique for producing rooted cuttings was first elaborated by Pyke (1933) and was further developed in the 1950s (Evans, 1951). Several authors (Archibald, 1955; McKelvie, 1957; Hall, 1963) had also made important contributions to the vegetative propagation of cocoa by stem cuttings in Ghana. The physiological principle involved in propagating cocoa clones by stem cuttings promotes the development of adventitious roots from the pericycle region at the stem base just above the cut, in a high humidity environment (Laliberté and End, 2015; De Klerk et al., 1999; Rasmussen et al., 2009). Modern scientific investigations have improved the management techniques of cuttings. A number of studies on hormone application, cutting stock origin (Toxopeus, 1970; Kevers et al., 1997; Lily and Ramadasan, 1979; Koko et al., 2011), number of leaves on cuttings (Amoah, 1986), effect of light, temperature and humidity (Lily and Ramadasan, 1979), rooting media (Amoah, 1986; Lily and Ramadasan, 1979; Kouamanan, 2001) and water requirement (Koko et al., 2011) for successful cutting establishment had been reported. According to Wiesman and Jaenicke (2002), several endogenous and exogenous factors such as water and energy status, hormonal balance, mineral and health status of cuttings, age of the cutting, propagation environment and stock plant management influence the success of this process. Leakey (2004) reported that adequate stock plant management improved the rooting ability of cuttings by providing the appropriate morphological and physiological conditions for shoot development. There are even more sophisticated techniques such as micropropagation by tissue culture in cocoa (Troare et al., 2003; Chantrapradist and Kanchanapoom, 1995). Vegetative propagation of cocoa by cuttings was initiated by the Institute of Agricultural Research for Development (IRAD) in the past few years but the experimental procedure for the propagation technique has never been reported. This will assist in the management process in the production of clones. The aim of this research is to vegetatively propagate selected clones of cocoa by stem cuttings. Specifically, to assess the effect of growth regulators concentration and source of cuttings on bud sprouting and rooting of selected cocoa clones used in Cameroon.
The study was carried out at the nursery of the Institute of Agricultural Research for Development (IRAD) Nkoemvone (2.81122°N and 11.13972°E), situated 15 km from Ebolowa, the capital of the South Region of Cameroon. The site is located in a bimodal rainfall zone, dominated by ferralitic soils. The mean annual temperature is 25°C, with the least monthly temperature of 22.8°C recorded in July, whereas the highest monthly temperature of 28.6°C is recorded in April. Mean annual rainfall ranges from 1550 to 2000 mm with highest precipitation occurring between April and May as well as between September and October.
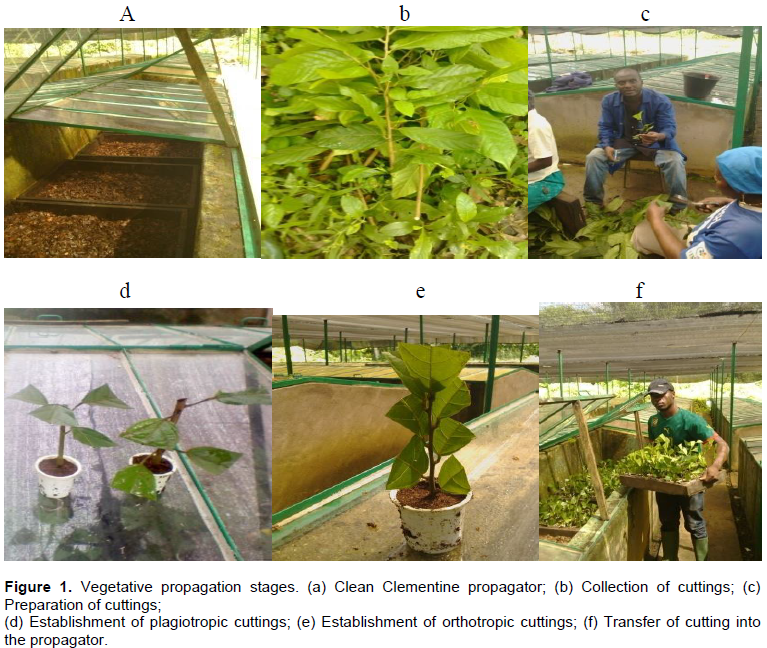
The Clementine propagator was used for this trial (Figure 1a). These propagators consist of a series of 10 propagators coupled together and adjacent to another series which serves for hardening plants. These propagators were constructed in cement bricks and are 1 m high, 6 m long and 1.5 m wide. At the interior is a 15 cm wide and 25 cm deep pipe used for watering and drainage. They have a transparent glass cover to allow penetration of light for photosynthesis in the cuttings, and are conceived to maintain a permanent relative humidity of approximately 100%. Successful rooting of cocoa cuttings requires a humid environment (Hartmann et al., 2002).
Preparation of materials, setting of cuttings and observation of trial
Propagators were cleaned and cleared of all dirt particles, and filled with gravel up to 10 cm. Wooden propagation trays were then filled with sawn wood chips previously treated with a systemic fungicide, cleanomil, which contains copper oxide (600 g/kg) and metalaxyl (120 g/kg) as active ingredients; as well as a systemic insecticide, parastar, containing imidiachloprid (20 g/l) and lambda-cyhalothrine (20 g/l) as active ingredients. 50g of each of the fungicide and insecticide were dissolved in 15 L of water in a watering can before application. Propagation trays were placed in the propagators. Decomposed saw dust was treated in the same manner like the sawn wood chips and filled into perforated alkathene plastic pots of dimension, 24 × 14 cm three days before cuttings were set.
The plant genetic materials used for this trial consisted of 4 clones including Upper Amazonian Forastero (UPA143 and T79/501) as well as Trinitario (SNK16 and ICS40) found in the SODECAO seed orchards which were used for the production of high-yielding and pod rot-resistant hybrids.
Two cutting sources, orthotropic (B1) from the main stem and plagiotropic (B2) from branches were used in this trial. Young and healthy cocoa shoots were collected from tree bases and trunks (for orthotropic cuttings, B1) and from secondary and tertiary branches (for plagiotropic cuttings, B2) in the cocoa seed orchard (Figure 1b). The latter were collected early (before 7 a.m.) in the morning. Each shoot/branch was reduced to a cutting of about 15 cm in length and 1 cm diameter (Figure 1c). Each cutting had a slanting upper surface to ease run-off during watering (Tchoundjeu, 1989). The leaves were reduced to four and each halved to about 80 cm2 surface areas to reduce water loss through evapotranspiration and maintain photosynthesis for cutting survival (Longman, 1993).
The growth regulator, 4-indol-3-butyric acid (IBA) (10 mg per tablet) was applied to cuttings at four different concentrations of: D0: 0 mg of IBA; D1: 12.5 mg of IBA in ½ a liter of water; D2: 25 mg of IBA in ½ a liter of water; D3: 37.5 mg of IBA in ½ a liter of water.
Each cutting was quickly dipped into the growth regulator solution of appropriate concentration for about 30 s before setting in treated decomposed saw dust in the perforated alkathene plastic pot (Figure 1d and e). Cuttings were set about 3 cm deep. Pots were then placed in germination trays in propagators filled with sawn wood chips to ensure their stability (Figure 1f). The trial was a 4 × 2 × 4 factorial experiment in a completely randomized block design, with 3 replicates. Each treatment consisted of 30 cuttings, with a total of 2880 cuttings set for the trial (that is 30 × 32 = 960 × 3 repetitions = 2880). Cuttings were watered on a daily basis in the morning and any fallen leaves and dead cuttings were removed.
Data collection and analysis
Survival rate (%) was assessed on 2880 cuttings. Because of the destructive nature of the assessment when plants are lifted to collect rooting data, a Z-sampling method was used on each treatment for data collection on the shoot and root parameters which reduced the sample to 960 cuttings (that is, 10 × 32 = 320 × 3 repetitions = 960). Foliar growth (number of cuttings with shoots, number of produced shoots and leaves length) and root growth (number and length of roots per cutting) were assessed at the 10th week.
The survival rate and shoot sprouting were collected in 2 weekly intervals for a period of 10 weeks from when cuttings were set, while rooting was assessed at the end of the 10th week. The rooting media were flooded in water to ease lifting of cuttings and to prevent the roots from breaking. A cutting was considered to have rooted if it had a root of at least 1 cm (Atangana et al., 2006). A rooted cutting was assessed for number of roots by counting, whereas root lengths were measured using a ruler. Number of cuttings with shoots and number of produced shoots were counted while the leaves lengths were measured from the petiole base through the mid rib to the tip using a ruler. The number of life cuttings at the time of data collection was used to estimate survival. Data were input on Microsoft Excel and analyzed using the Statistical Package for Social Sciences (SPSS) Version 16. Univariate analysis of variance with 3 factors (clone, cutting type and growth regulator concentration) was carried out, whereas the Duncan multiple range test was used to separate means at 5% level of significance.
Effects of clone, cutting source and growth regulator concentration on survival rate
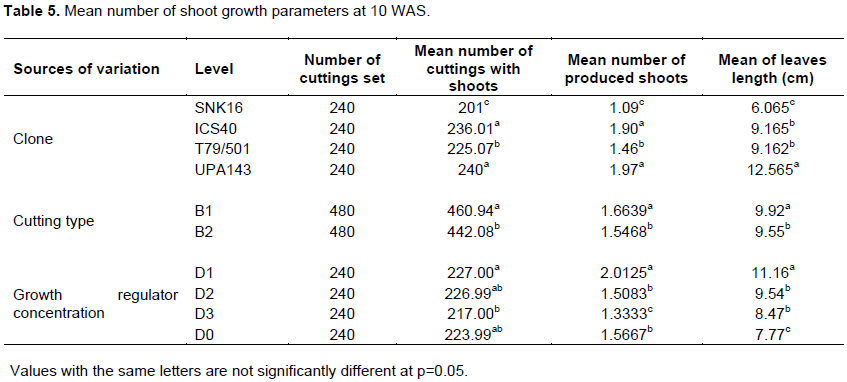
Mortality rate had no particular trend with time among the clones, cutting sources and growth regulator concentrations. Higher mortality rate was observed in Trinitario clones (ICS40 6 and SNK16 2) which recorded dead cuttings in the 1st week of the trial while mortality in Upper Amazon clones (UPA143 and T79/501) started at the 3rd week. The highest mortality was observed at the 3rd and 5th WAS on all the clones. The mortality rate was always higher in Trinitario than in Upper Amazon clones, notwithstanding the week (Figure 2). The survival rate was significant for clone and cutting type at P = 0.05 (Table 1) UPA143 had a significantly higher survival rate than the other clones while T79/501 showed significantly higher survival rate than ICS40 and SNK16 which were not significantly different. Orthotropic cuttings showed a significantly higher survival rate than plagiotropic cuttings, whereas growth regulator concentration had no effect on cutting survival with D0 having the highest rate of survival (59.37) (Table 2).
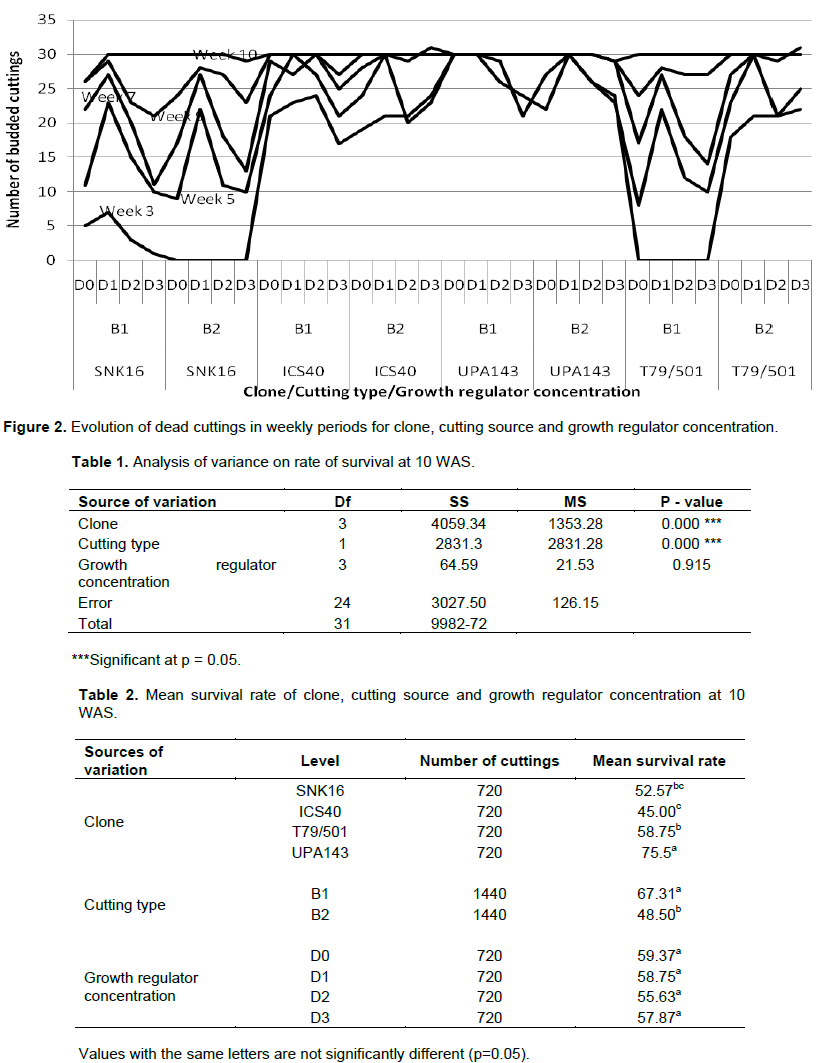
Generally, orthotropic cuttings showed a better survival rate than plagiotopic cuttings confirming the result of Liabeuf (1946) on the vigor of orthotropic cuttings. Regarding the method of propagation, setting cuttings in Clementine propagators were less successful (76 % survival) than in plastic tunnels (Koko et al., 2011), with 80% survival rate, although the difference is minimal.
Effects of clone, cutting source and growth regulator concentration on number of cuttings with shoots
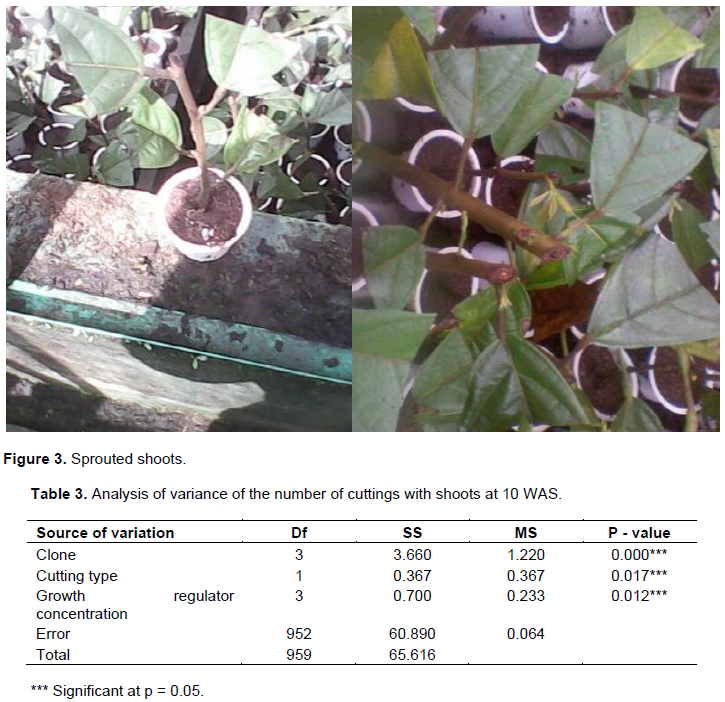
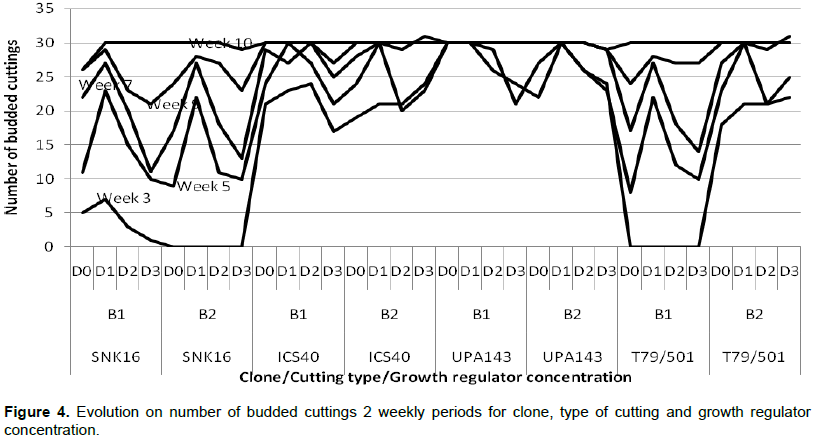
According to the results, all 4 clones produced young shoots at 3 WAS of cuttings and at 10 WAS where almost all the cuttings had shoots (Figure 3) with 100% in clone UPA143. The analysis of variance results showed significant differences in all the sources of variation (Table 3). All cuttings (100%) of clone UPA143 showed the presence of flushing at 3 WAS, followed by clone ICS40 with 77.5% of cuttings having shoots, whereas T79/501 and SNK16 clones produced shoots on 35 and 7% of cuttings, respectively (Figure 4 and Table 5).
Effect of clone, cutting source and growth regulator concentration on number of shoots produced per cutting
Significant differences were observed on the clones, cutting sources and the growth regulator concentrations with respect to the number of produced shoots. Clone UPA143 had a significantly higher number of shoots than ICS40 and T79/501 clones which showed no significant difference, but produced a significantly higher number of shoots than SNK16 clone (Tables 4 and 5). There was an increase in the number of buds produced per clone, growth regulator concentration and cutting type with time (from weeks 3 to 10). Growth regulator concentration D1 (12.5 mg of IBA in half a liter of water) induced more buds notwithstanding the clone, cutting type or week of assessment. Clone UPA143 produced the highest number of shoots, despite the cutting type or growth regulator concentration, followed by clone ICS40 (where orthotropic cuttings produced more buds than plagiotropic cuttings), clone T79/501 (with plagiotropic cuttings producing slightly more buds that orthotropic cuttings) and lastly, clone SNK16 (where there was only a minimal difference in bud production between orthotropic and plagiotropic cuttings, with the former having more buds). Bud production was observed to be largely influenced by genetic factors, although adequate growth regulator application and use of appropriate cutting type could also play an important role (Figure 5 and Table 4). The trend in the number of cuttings with shoots was similar to that of the number of produced shoots for clone and type of cuttings. However, the trend in growth regulator concentration was different where concentration (D2) had the highest number of cuttings with shoots but produced less shoots as compared to the concentration (D0) though not significantly different (Table 5).
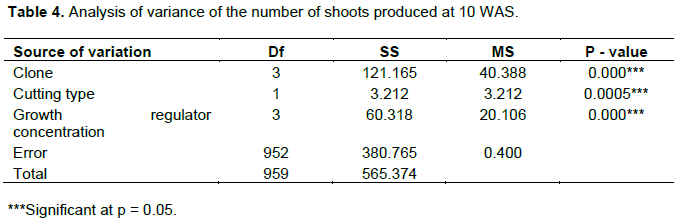
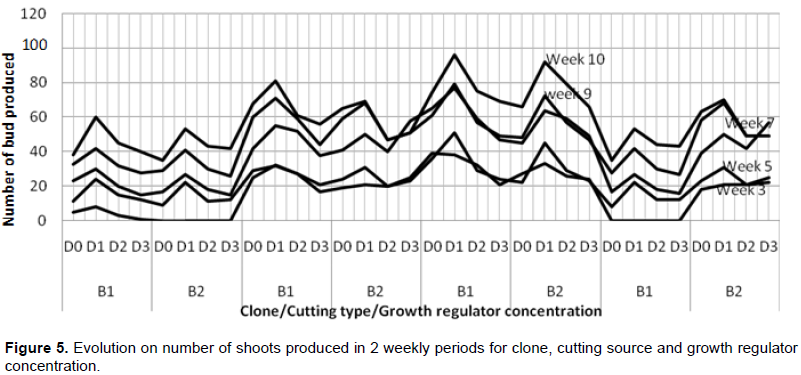
Effect of clone, cutting type and growth regulator concentration on leaf length
The results showed that clone, cutting type and growth regulator concentration had highly significant effects on leaf length (Table 6). Clone UPA143 had significantly longer leaves than the other clones, with a mean leaf length of 4.5 cm at week 10 with growth regulator concentration D1 (12.5 mg of IBA in ½ a liter of water) and orthotropic cuttings, whereas the least mean leaf length was recorded in clone SNK16 at 5 WAS with the control treatment for growth regulator concentration (D0) and plagiotropic cuttings.
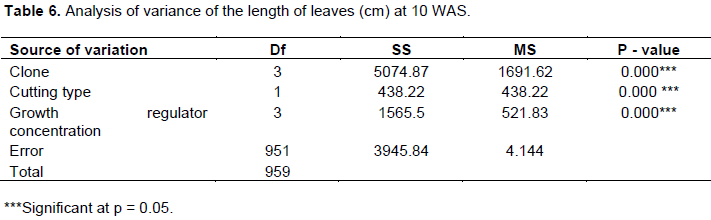
Orthotropic cuttings (B1) produced longer leaves at 10 WAS for most clones and all growth regulator concentrations. There was only a slight difference in leaf lengths between both cutting types with clone UPA143. Growth regulator concentration D1 induced the longest leaves, notwithstanding the clone, cutting source or number of weeks after setting of cuttings. This showed that leaf length of rooted cocoa cuttings vary with clone, cutting type and used growth regulator concentration. Analysis of variance showed a highly significant difference among the studied factors at 5% level of significance (Table 6). A comparison of clones for leaf length at 10 WAS using Duncan multiple range test showed a similarity between ICS40 and T79/501, with difference in their means very close to 0. On the contrary, there was a significant difference among UPA143 and the rest of the clones, confirming that leaf length of cocoa cuttings are influenced by clones (Table 5). Maximum leaf production was attained by all clones at 10 WAS. The LSD for growth regulator concentration revealed that 50% of cuttings in the control treatment (D0) had leaf lengths of less than 8 cm, which was inferior to those of D3, D2 and D1 by 9, 10 and 11 cm, respectively. Difference in the growth regulator concentrations indicated a difference at 5% confirming, the effect of growth regulator concentration on leaf length of cocoa cuttings at 10 WAS. According to Himme (1956), leaf lengths of cocoa cuttings vary with respect to cutting origin, in line with the results of the present study which revealed that orthotropic cuttings produced longer leaves than plagiotropic cuttings. Growth regulator concentration also influenced leaf lengths of cocoa cuttings in accordance with Charrier (1969) who pointed out that leaf growth of cocoa cuttings varies with the applied hormone concentration.
The obtained results were in line with those of Amoah (2006a) who observed that the effect of clone was very predominant in the course of rooting, with different degrees of leaf production between clones. Koko et al. (2011) also reported that the Upper Amazon clones produced leaves earlier than Trinitario clones. However, Koko et al. (2011) however observed leaves on Upper Amazon clones 5 WAS, in contrast to the present study in which sproutings were observed at 3 WAS for Upper Amazon clones and 5 WAS for Trinitario clones. All used clones for the trial were grown under the same environmental conditions; therefore differences in results are possibly genetic. This observation was in line with that of Nanda et al. (1968) who reported that success in cocoa propagation using cuttings from different clones can vary considerably according to their genetic constitutions.
Effect of clone, cutting type and growth regulator concentration on number of produced roots per cutting
Analysis of variance revealed a highly significant difference among the tested factors at the significance level of 5% (Table 7). Clone UPA143 produced a significantly higher number of roots, notwithstanding the cutting type or growth regulator concentration (Figure 6). Orthotropic cuttings produced more roots than plagiotropic cuttings, whereas IBA concentration (D3) induced the greatest number of roots on orthotropic cuttings of clones UPA143 and SNK16 (Table 9). Generally, D1 and D2 induced many roots for all clones and cutting types. Duncan multiple range test reveals a significant difference in the number of roots produced by cocoa cuttings treated with different IBA concentrations.
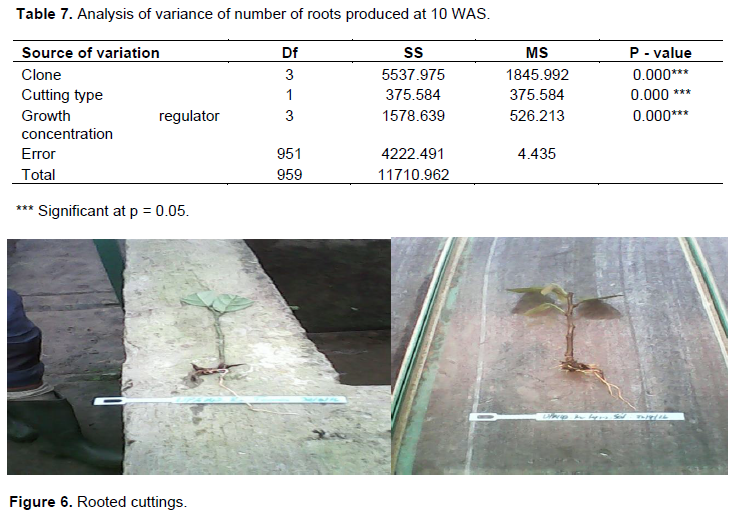
A comparison of clones using the Duncan multiple range test showed that there was no significant difference in root number of cocoa cuttings between clones T79/501 and ICS40 at p=0.05 (Table 9). This confirmed that clones affect root production in cocoa cuttings although there may be similarities between some clones. A Tuckey plot of cutting type showed that orthotropic cuttings produced between 3 and 19 roots, whereas plagiotropic cuttings produced between 1 and 17 roots. The median value for root number of orthotropic cuttings was 10, meaning that at least 50% of the latter cuttings produced at least 10 roots. On the other hand, the median value for plagiotropic cuttings was 8, thus 50% of them produced at least 8 roots. A Duncan multiple range test of cutting types showed a significant difference in the number of produced roots between orthotropic and plagiotropic cuttings at p=0.05.
All 3 factors (clone, cutting type and growth regulator concentration) had positive effects on rooting of cocoa cuttings, in line with Liabeuf (1946) who observed an increase in root production on cocoa cuttings treated with IBA as well as Himme (1956) in a study on cocoa root system. Archibald (1953) observed considerable variation in rooting behavior among cuttings from different clones, different trees of the same clone, different parts of the same tree and different parts of the same shoot due to internal factors, with photosynthetic efficiency of the leaf being a key determinant in the cutting survival. Hall (1963) and Toxopeus (1964) observed significant differences among clones in rooting ability. The latter authors found that Upper Amazon and Trinitario clones perform higher than Amelonado in rooting response.
Tee and Lamin (2014) observed that IBA application on cocoa cuttings in different substrates positively influenced rooting, flushing and cuttings survival of some cocoa clones (KKM22 and MCBC1) in a non-mist propagator, but not others (LKMS1, PBC123 and BR25) which showed low (2.79-6.43%) rooting rates. On the contrary, Mbah and Retallick (1992) observed that different IBA concentrations did not improve rooting in Balanites aegyptiaca cuttings. Shiembo et al. (1996) reported that applications of different IBA concentrations made no significant difference to rooting of Irvingia gabonensis cuttings. However, the latter growth regulator improved root number in Ricinodendron heudelotii cuttings but did not affect the rooting percentage (Shiembo et al., 1997). This showed that IBA application had different effects on the rooting of different tropical tree species.
Effect of clone, cutting type and growth regulator concentration on root length of cocoa cuttings
Clone UPA143 had the longest roots at 10 WAS, notwithstanding the cutting type, whereas ICS40, SNK16 and T79/501 showed average root growth. The longest root at 10 WAS (13.6 cm) was produced by orthotropic cuttings (B1) with growth regulator treatment D1 (12.5 mg) (Figure 6). Analysis of variance of the effect of each factor on cocoa root length showed that there were highly significant differences at p=0.05 for each factor (Table 8). A comparison of clones with respect to root length using LSD showed there were significant differences at p=0.05 except between ICS40 and T79/501. The median values for root length of cocoa cuttings for the factor IBA concentration varied from a minimum of 5 cm for dose D0 to 8 cm for dose D1. D2 and D3 registered 6 and 5.5 cm, respectively (Figure 6).
Clone UPA143 had the longest mean root length, followed by ICS40, T79/501 and SNK16 in a decreasing order. Orthotropic cuttings (B1) produced longer roots than plagiotropic cuttings, whereas growth regulator concentration D1 induced longer mean roots than the others (D2, D3, and D0) in a decreasing order (Table 9).
Results of analysis of variance for the factors investigated in this trial showed significant differences at p=0.05. Additionally, individual analysis of each factor showed significant differences among clones, cutting types and growth regulator concentrations. This confirmed that root length of cocoa cuttings vary depending on clone, cutting origin and growth regulator concentration. Liabeuf (1946) obtained similar results after treatment of cocoa cuttings with IBA. Apart from growth regulator treatment, clone and cutting type had significant effects on root production and root length. Clones, foliar surface, physiological age, anatomic traits, nutritional and biochemical factors exerted a strong influence on rooting. According to Amoah (2006b), Upper Amazon and Trinitario clones root well than the clone Amelonado, the reasons being genetic.
The effect of four clones, two cutting sources and four IBA concentrations has provided important information on cocoa propagation. Orthotropic cuttings (B1) showed a better survival rate (67.31%) than plagiotopic cuttings (B2), confirming the result of other scientists elsewhere on the vigor of orthotropic cuttings.
A classification of the different clones showed survival rates of 75.5% for UPA143, 58.75% for T79/501, 52.57% for SNK16 and 45% for ICS40 after 10 weeks in the propagator. Clones ICS40 and T79/501 had similar results for root number meaning the effect of clone could be limited in some cases, whereas orthotropic cuttings and growth regulator concentration D1 showed significantly higher mean values for all parameters assessed.
Vegetative propagation by stem cuttings is a technique that had been abandoned in Cameroon, in favor of grafting for the establishment of cocoa seed orchards. However, stem cuttings have the advantage of providing many plants within a short time for the creation of such seed orchards. Future research will be the evaluations of all the clones available in the research stations of IRAD in different media.
The authors have not declared any conflict of interests.
REFERENCES
|
Amoah FM (1986). Studies on the rapid propagation of cacao (Theobroma cacao L.) (Ph D Thesis). Wye College, University of London. 280.
|
|
|
|
Amoah FM (2006a). Review of vegetative propagation of cacao (Theobroma cacao L.) by rooted cuttings. 1. Physiological considerations. Ghana J. Agric. Sci. 39:209-216.
|
|
|
|
|
Amoah FM (2006b). Review of vegetative propagation of cacao (Theobroma cacao L.) by rooted cuttings. 2. Environmental and technical considerations. Ghana J. Agric. Sci. 39:217-226.
|
|
|
|
|
Archibald JF (1953). Factors involved in the rooting response of cuttings, Proceedings of the West African international cocoa research conference, 12-16 December 1953, Tafo, Gold Coast 40-42.
|
|
|
|
|
Archibald JF (1955). The propagation of cocoa by cuttings, Technical Bulletin, (West African Research Institute), 3.
|
|
|
|
|
Atangana AR, Tchoundjeu Z, Asaah EK, Simons AJ, Khasa DP (2006). Domestication of Allanlackia floribunda: Amenability to vegetative propagation. For. Ecol. Manag. 237:246-251.
Crossref
|
|
|
|
|
Champaud J (1966). Distribution du nombre de graines par cabosse chez plusieurs clones de cacaoyer: un caractère à prendre en compte pour l'amélioration des rendements. Dans : 16ème Conférence internationale sur la recherche cacaoyère. Lagos: Cocoa Producers' Alliance, Bali (Indonésie).
|
|
|
|
|
Chantrapradist C, Kanchanapoom K (1995). Somatic embryo formation from cotyledonary culture of Theobroma cacao L. J. Sci. Soc. Thailand 21:125-130.
Crossref
|
|
|
|
|
Charrier A (1969). Contribution à l'étude de la morphogenèse et de la multiplication vegetative du cacaoyer (Theobroma cacao L.). Café Cacao Thé 13:97-114.
|
|
|
|
|
De Klerk GJ, Van der Krieken W, De Jong JC (1999). Review–The formation of adventitious roots: New concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 35:189-199.
Crossref
|
|
|
|
|
Evans H (1951). Investigations of the vegetative propagation of cocoa. Trop. Agric. (Trinidad), 28:7-12.
|
|
|
|
|
FAOSTAT (2015). Statistiques de la production cacaoyere au Cameroun.
|
|
|
|
|
Hall TRH (1963). The cuttings production and rooting potential of some WACRI cocoa clones. Trop. Agric. (Trinidad) 40(3):223-228.
|
|
|
|
|
Hartmann HT, Kester DE, Davies FT, Geneve RL (2002). Plant propagation principles and practices. Prentice Hall ENG. Cliff, New Jersey 07632.
|
|
|
|
|
Himme V (1956). Etude du systeme radiculaire du cacaoyer au Congo belge et Ruanda urundi. Bulletin d'information de l'INEAC 58p.
|
|
|
|
|
Kevers C, Hausman JF, Faivre-Rampant O, Evers D, Gaspar T (1997). Hormonal control of adventitious rooting: Progress and questions. J. Appl. Bot. Angew. Bot. 71:71-79.
|
|
|
|
|
Koko L, Koffi N, Konan A (2011). Multiplication végétative du cacaoyer (Theobroma cacao L.) par la technique de bouturage direct sous tunnel plastique. J. Appl. Biosci. 46:3124-3132.
|
|
|
|
|
Kouamanan OK (2001). Multiplication vegetative du cacaoyer : le bouturage. Mémoire de maitrise, Universite d'Abobo-Adjame (Cote d'Ivoire) P 43.
|
|
|
|
|
Laliberté B, End M (2015). Supplying new cocoa planting material to
|
|
|
|
|
Leakey RRB (2004). Physiology of vegetative propagation. In: (Burley J, Evans J, Youngquist JA (eds.) Encyclopedia of forest sciences. Academic Press, London, UK. pp. 1655-1668.
Crossref
|
|
|
|
|
Liabeuf J (1946). Rapport annuel des activités de la station cacaoyère de Nkoemvone. pp. 6-9.
|
|
|
|
|
Libby WJ (2004). Propagation technology for forest trees. In: Burley J, Evans J, Youngquist JA (eds) Encyclopedia of Forest Sciences. Academic Press, London, UK. pp. 237-244.
Crossref
|
|
|
|
|
Lily VG, Ramadasan A (1979). Changes in phenolic content in coconut leaf in relation to the development of leaf rot. Indian Phytopath. 32:112-113.
|
|
|
|
|
Longman KA (1993). Rooting cuttings of tropical trees: tropical trees: Propagation and Planting Manuals. Vol.1, 138. Illustrated by Wilson RHF. Commonwealth Science.
|
|
|
|
|
Mbah JM, Retallick SJ (1992). Vegetative propagation of Balanites aegyptiaca (L.) Del. Commonwealth For. Rev. 71(1):52-56.
|
|
|
|
|
McKelvie AD (1957). W.A.C.R.I. breeding material, Proceedings of the cacao breeding conference, Tafo, Ghana, 1-3 October 1956, 10-15.
|
|
|
|
|
Nanda KK, Purohit AN, Anand VK (1968). Seasonal rooting response of stem cuttings of some forest tree species to auxins. Indian For. pp 154-162.
|
|
|
|
|
Ondoua JM, Mony RD, Siegfried D, Ngotta BJB, Taffouo VD, Kenne M, Ekodeck GE (2016). Myrmecofauna of cocoa trees infested by Loranthaceae genus Phragmanthera in SODECAO seed fields of Nkoemvone (South of Cameroon). J. Entomol. Nematol. 8(3):19-27.
Crossref
|
|
|
|
|
Pyke EE (1933). The vegetative propagation of cacao. II softwood cuttings, Second annual report on cacao research, Trinidad 2:3-9.
|
|
|
|
|
Rasmussen A, Smith TE, Hunt MA (2009). Cellular stages of root formation, root system quality and survival of Pinus elliottii var. elliottii × P. caribaea var. hondurensis cuttings in different temperature environments. New For. 38:285-294.
Crossref
|
|
|
|
|
Shiembo PN, Newton AC, Leakey RRB (1996). Vegetation propagation of Irvingia gabonensis, A West African fruit tree. For. Ecol. Manag. 87(1-3):185-192.
Crossref
|
|
|
|
|
Shiembo PN, Newton AC, Leakey RRB (1997). Vegetative Propagation of Ricinodendron heudelotii, a West African Fruit Tree. J. Trop. For. Sci. 9(4):514-525.
|
|
|
|
|
Tchoundjeu Z (1989). Vegetative Propagation of the Tropical Hardwoods of Khaya ivorensis (A. Chef) and Lovoa trichilioides (Harm). Thesis Submitted to the University of Edinburgh for the Degree of Doctor of Philosophy, 261.
|
|
|
|
|
Tee YK, Lamin K (2014). Vegetative propagation in Cocoa (Theobroma cacao): Effects of propagation environment and rooting substrates on rooting behaviour of cocoa stem cuttings, In: Enhancing strategic plant physiological research and technologies for sustainable resources. Proc. Int. Conf. Plant Physiol. 26-28 Indonesia.
|
|
|
|
|
Toxopeus H (1964). F3 Amazon in Nigeria. The Cocoa Research Institute of Nigeria, Annual report 13-23.
|
|
|
|
|
Toxopeus H (1970). Seasonal trend of the rooting success of cutting of cacao clnes in Nigeria and the relations with the establishment ability. Euphytica 19(4):426-429.
Crossref
|
|
|
|
|
Troare A, Maximova SN, Guiltinan MJ (2003). Micropropagation of Theobroma cacao L. using somatic embryo-derived plants. Soc. In vitro Biol. 1-7.
|
|
|
|
|
Wiesman Z, Jaenicke H (2002). Introduction to vegetative tree propagation: concepts and principles. In: Jaenicke H, Beniest J (eds): Vegetative Tree Propagation in Agroforestry: Training Guidelines and References. International Centre for Research in Agroforestry (ICRAF), Nairobi, Kenya, 148.
|
|