Full Length Research Paper
ABSTRACT
INTRODUCTION
The concept of food having medicinal value has been reborn as 'functional foods'. The list of health benefits accredited to functional foods continue to increase and the gut is an obvious target for the development of functional foods, because it acts as an interface between the diet and all other body functions. One of the most promising areas for the development of functional food components lies in the use of probiotics. Probiotics, are live microorganisms that, when administered in adequate amounts, confer health benefits on the host (FAO/WHO, 2002). One of the most accepted approaches through which the gut microbiota composition can be influenced is through the use of probiotics; which are life microbial dietary additives.
Besides the nutritional values, ingestion of lactic acid bacteria (LAB) and their fermented foods has been suggested to confer a range of health benefits including immune system modulation, increased resistance to malignancy and infectious illness (Soccol et al., 2010). Vergin in 1954 suggested that the microbial imbalance in the body caused by antibiotic treatment could have been restored by a probiotic rich diet; a suggestion cited by many as the first reference to probiotics as they are defined nowadays. Similarly, Vasiljevic and Shah (2008) recognized detrimental effects of antibiotic therapy and proposed the prevention by probiotics. The idea of health-promoting effects of LAB is by no means new, as Metchnikoff proposed that lactobacilli may fight against intestinal putrefaction and contribute to long life (Brant and Todd, 2014). Such microorganisms may not necessarily be constant inhabitants of the gut, but they should have a “beneficial effect on the health status of man and animal” (Belhadj et al., 2010). For the gastrointestinal ecosystem, the most important microbial species that are used as probiotics are LAB.
Lactic acid bacteria (LAB) are the most prominent non-pathogenic bacteria that play a vital role in our everyday life, from fermentation to preservation, food and vitamin production, and to prevention of certain diseases and cancer due to their probiotics properties. These microorganisms are one of the prominent groups of bacteria which inhabit the gastrointestinal tract, and the importance of these non-pathogenic bacteria has been more noticed (Krishnendraet al., 2013). Several lactobacilli have been noted to have nutritional benefits, improved lactose utilization, have anti-cholesterol and anti-carcinogenic, and protection against other diseases (Krishnendraet al., 2013). Especially, Lactobacillus spp. are well known producers of antimicrobial compounds especially bacteriocins which have high antimicrobial activity (Aweenet al., 2012). The production of these compounds by intestinal microflora is one of the most important mechanisms responsible for the antagonistic activity against intestinal pathogens and therefore it is essential to examine this property in isolates that are candidates for probiotics (Bilkovaet al., 2011). Effective probiotics should possess antimicrobial activity particularly to the pathogens of the gastrointestinal tract (Klayraunget al., 2008).
Palm wine is an alcoholic beverage produced from the sap of various palm tree species. The drink is particularly common in parts of Africa, South India and the Philippines. In Africa, the sap is most often taken from oil palms such as Elaeisguineensis, or from Raffia, kithul or Nipa palms (Ukhum et al., 2005). Besides fermenting yeast belonging to various genera e.g Saccharomyces, Candida, Endomycopsis, Hausenula, Pichia, Saccharomy codes and Schizosaccharomyces (Ezeronye and Legras, 2009; Chandrasekhar et al., 2012), the dominant bacterial population of palm wine was previously described as lactic acid bacteria-strains of Lactobacillus plantarum, Leuconostocmesenteriodes and L. mesenteroidessubsp. dextranicum. Palm wine is milky-white and effervescent because of the presence of live bacteria and yeast (Ezeronye, 2009) resulting from natural fermentation. The sap of palm tree has been shown to be a rich medium capable of supporting the growth of various types of microorganisms. In general, the methods of palm wine tapping and collection of palm sap, including air and the environment as a whole, influence the microbial content of the sap (Amoa-Awwaet al., 2007; Naknean et al., 2010).
Palm wine plays an important role in many ceremonies in Cameroon, parts of Nigeria such as among the Igbo people, and elsewhere in Central and Western Africa. Guests at weddings, birth celebrations and funeral wakes are served generous quantities. The wine is often infused with medicinal herbs to treat a wide variety of physical complaints.
The widely used probiotic bacteria reported in literature were isolated from gastro intestinal tract, but very few are from exogenous origin such as palm wine. This study aimed at investigating the probiotic potential of lactic acid bacteria from palm wine.
MATERIALS AND METHODS
Sample collection
Thirty samples of fresh palm sap were collected in sterile wide-mouth bottles directly from the farmers and transported to the laboratory for processing. The samples were kept at room temperature for 48 h for fermentation to take place. After which they were carefully processed under aseptic conditions.
Isolation and phenotypic identification of lactic acid bacteria
LAB was isolated from palm wine by pour plate method using De Man Rogosa and Sharpe (MRS) agar. For this purpose, 1 ml of each sample was added to 9 ml of saline solution (NaCl, 0.85%). 1 ml aliquot of the 10-4 and 10-5 dilutions was aseptically disposed on sterile plates. About 15 ml of MRS agar was poured onto it and allowed to solidify. All plates were incubated at 30°C for 48 h under anaerobic conditions. After the incubation, a preliminary catalase test was carried out. Catalase negative discrete colonies which appeared on the plates with distinct morphological differences such as color, shape and size were picked and purified 2-3 times by re-streaking on fresh MRS agar. The pure colonies were further characterized using Gram staining test and cell morphology examinations. Catalase negative and Gram positive isolates were preserved in 15% glycerol at -80°C until identification. Carbohydrate fermentation patterns of LAB were determined using API 50 CHL kit (bioMerieux, France). The APILAB PLUS database software was used to interpret the results.
Antimicrobial activity of LAB
The antimicrobial activity of LAB was determined by modifying the disc diffusion method of Hamdan and Mikolajcik (1974). Sterile filter discs (diameter; 6 mm) were dipped into the cultured MRS broth of LAB incubated at 30°C for 24 h in a shaker (187 rpm) and placed on solidified Mueller-Hinton agar (LIOFILCHEM DIAGNOSTICI) seeded with 14 h cultures of indicator microorganisms. The plates were kept at 4°C for 3 h to permit diffusion on the assay material, and incubated at 37°C for 16 h. Some of the discs were dipped in un-inoculated MRS broth which served as negative control. Also, antibiotic discs of Ofloxacin and Azithromycine were placed on solidified Muller-Hinton agar (LIOFILCHEM DIAGNOSTICI) seeded with 14 h cultures of indicator microorganisms and incubated under the same conditions. These served as positive control for the tests on Salmonella enteric subsp. enterica and E. coli, respectively. Their zones of inhibition (clear zones around the discs) were evaluated. This was done by using a ruler to measure the diameter of the disk plus the surrounding clear area in millimeters (mm).
Tolerance to acidic conditions
The lactic acid bacteria isolates were cultured in MRS broth for 18 h. The LAB cells were harvested by centrifugation for 10 min at 5000 rpm and 4°C. Pellets were washed trice in phosphate-saline buffer (PBS at pH 6.2). The pH was adjusted by a pH meter with the use of HCl 1 N to pH 1.0, 2.0, 3.0 and 6.2 (control pH). The cell pellets (107-108 CFU/ml) were resuspended in 10 ml of PBS (pH 1.0, 2.0, 3.0 and 6.2) and incubated at 30°C for 1, 2, 3 and 4 h. The cells were enumerated by plating 100 µL aliquot of the inoculated PBS solutions at the various tested times, for 24 h. The experiments were performed in duplicates.
Bile tolerance
These lactic acid bacteria isolates were cultured in MRS broth, for 16-18 h. The LAB cells were harvested by centrifugation for 10 min at 5000 rpm and 4°C. Pellets were washed trice in phosphate-saline buffer (PBS at pH 6.2) and resuspended in PBS (pH 6.2). Two sets of MRS broth were prepared containing 0.15 % (w/v) oxgall-bile and the other 0.30% (w/v) oxgall-bile. Also, one set of MRS broth was prepared without oxgall-bile. This served as the control. These sets of MRS broth were inoculated with 100 µl aliquot of the LAB suspensions (107-108 CFU/ml) and incubated for 1, 2, 3 and 4 h. Then, viable bacteria counts were obtained after 24 h incubation at 37°C (Barakat et al., 2011). The experiments were performed in duplicates. In both cases, the survival percentage of LAB was calculated by the following formula:

RESULTS
Isolation and selection of LAB
Twenty catalase negative and Gram positive bacteria were isolated from fermented palm sap and considered as presumptive LAB. All belong to the genus Lactobacillus. Five isolates (A, B, D, G and I) were selected on the basis of their potential to inhibit potent food borne pathogens (S. enterica, E. coli BL21. E coli XL 1B, E. coli 109 JM, E. coli DH 5α).Further identification was done using biochemical tests summarized in Table 1. The isolates A and B, were identified as strains of L. pentosus. D and G were identified as strain of L plantarum 1. I was identified as L. brevis1.
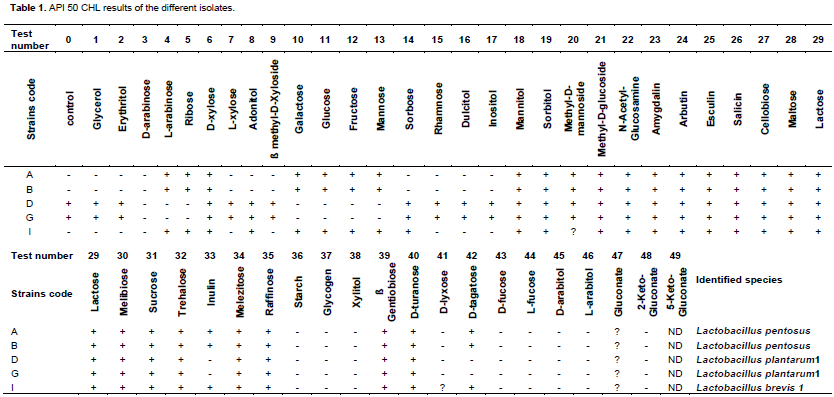
Antimicrobial activity of LAB
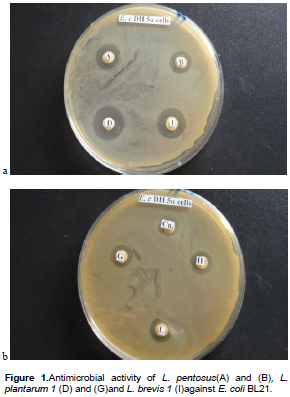
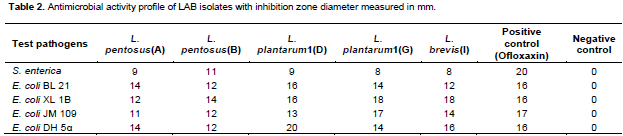
Figure 1 shows some halos of inhibition of pathogenic strains by broth culture of lactic acid bacteria isolated. The inhibition of some test pathogens by the positive control (Ofloxaxin) is presented in Figure 2. Antimicrobial activities of the LAB isolated from palm wine samples are summarized in Table 2. They are expressed in term of diameter of the zones of inhibition (in mm). Only L. Pentosus (B) had moderate activity against S. entericawith an inhibition zone of 11 mm. All the LAB isolates had activity against the four strains of E. coli. For E. coli Bl 21, L. plantarum1(D) had the highest antimicrobial activity with inhibition zone of 16 mm. For E. coli XL 1B, L. plantarum1(G) and L. brevis1(I) had the highest activity with inhibition zones of 18 mm. For E. coli JM 109, L. plantarum1(G) had the highest activity against it with inhibition zone of 17 mm. For E. coli DH 5α, L. plantarum1(D) had the highest antimicrobial activity with inhibition zone of 20 mm. Overall, for all the test pathogens, the highest activity was demonstrated on E. coli DH 5α by Lactobacillus plantarum1(G).
Tolerance of LAB to acid and bile
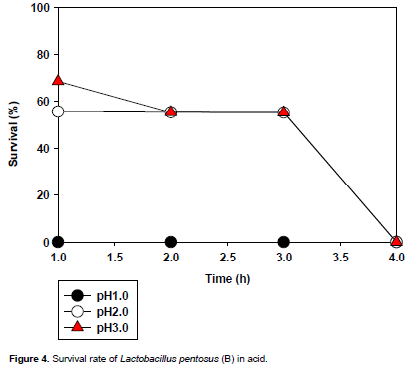
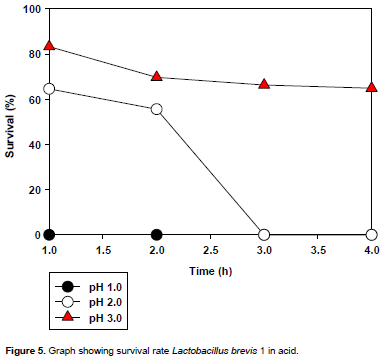
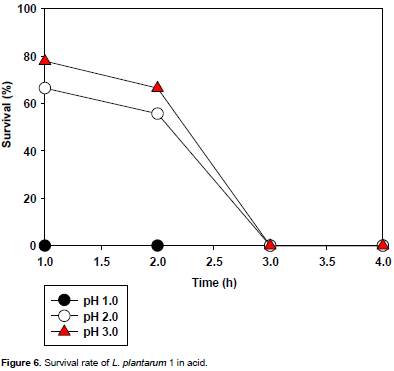
The acid tolerance of the selected LAB isolates is presented in Figures 3 to 6. All the isolates did not survive the acidic condition of pH 1.0. L. plantarum could not tolerate the acidic conditions (pH 2.0 and 3.0) for 3 h (time of interest); however L. pentosusand L.brevis1tolerated the acidic conditions (pH 2.0 and 3.0) for 3 h with L. brevis1showing the highest tolerance to pH 3.0 for 3 h of incubation.
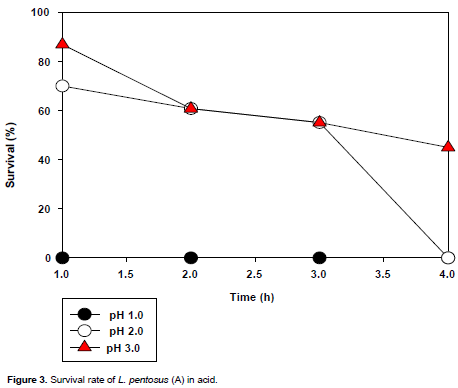
L. pentosus (A) did not tolerate the acidic condition, pH 1.0 (Figure 3), as no survival was observed at that point on the graph. It tolerated the acid condition of pH 2.0, it had a survival rate of about 70% at the first hour, which dropped slightly to 60% at the second hour and to about 55% at the third hour which then dropped drastically to zero at the fourth hour. It also tolerated the acidic condition of pH 3 as its survival rate was about 84% at the first hour and decreased to 60% at the second hour which dropped slightly to 52% at the third hour, then later sloped gently to about 48% at the fourth hour.
For L. pentosus (B) (Figure 4), it had a similar reaction to L. pentosus (A). Also, it did not tolerate pH 1.0 as its survival rate remained zero throughout the experiment. It tolerated pH 2.0 as its survival rate stood at about 57% for three hours after which it dropped drastically to zero at the fourth hour. Again it tolerated pH 3.0 as its survival rate was 70% and decreased gradually to 57% at the second hour where it remained constant till the third hour and dropped significantly to zero at the fourth hour.
L. plantarum1(D) (Figure 5), had some major different reactions from the L. pentosusstrains. Again it showed no tolerance at pH 1.0 as it produced a zero survival rate till the fourth hour. It tolerated pH 2.0 with a survival rate of about 53% which slightly decreased to 50% at the second hour and dropped drastically to zero at the third hour where it remained constant to the fourth hour. It had a similar reaction in pH 3.0, where it had a tolerance of about 55% which decreased gradually to 52% at the second hour and dropped drastically to zero at the third hour and remained constant to the fourth hour.
For L. plantarum 1(G) (Figure 6), it had a similar reaction to L. plantarum 1 (D). Again it did not tolerate pH 1.0, as it showed a zero survival rate right up to the fourth hour. But it produced a higher survival rate in pH 2.0 of about 64% which decreased gradually to 50% at the second hour and drastically to zero at the third hour, where it remained constant to the fourth hour. In pH 3 it also had a higher tolerance as its survival rate increased to about 78% which gradually dropped to 50% at the second hour and drastically to zero at the third hour, where it remained constant to the fourth hour.
L. brevis1 (Figure 6) did not tolerate pH 1.0 as shown by its survival rate of zero throughout the experiment. It tolerated pH 2.0 with a survival rate of 62 % which decreased slightly to 55 % at the second hour and drops drastically to zero at the third hour and remained constant to the fourth hour. In pH 3.0, it had a survival rate of about 82% which dropped to 70% at the second hour and remained almost constant at that point till the fourth hour.
The tolerance to bile is shown in Figures 7 and 8. The entire LAB isolates survived 1 to 4 h incubation in MRS broth containing 0.15 and 0.30% (w/v) bile with higher survival rates in 0.15% (w/v) concentration than in 0.30% (w/v), as compared to the control without bile.
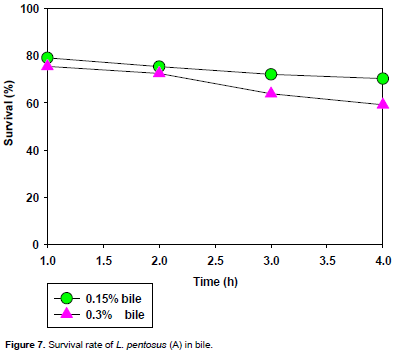
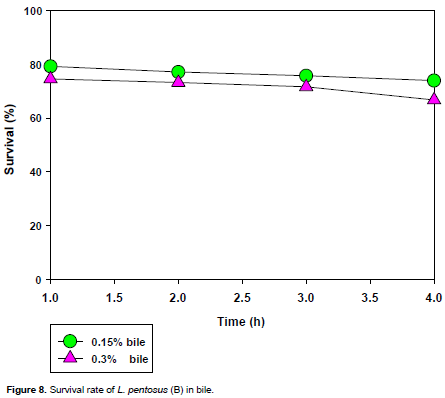
For L. pentosus(A) and(B)and L. plantarum 1(G), they survived in 0.15% (w/v) bile concentration with a survival rate of about 80% and decreased slightly to 60% at the third hour where it remained constant to the fourth hour. For L. plantarum 1(D), it survived the 0.15% (w/v) bile concentration with a survival rate of about 83% which dropped slightly at the second hour to about 75% and remained constant to the fourth hour at that level. It also survived the 0.30% (w/v) bile concentration with a survival rate of about 75% which remained constant to the second hour and dropped slightly to about 60% at the third hour where it remained constant to the fourth. For L. brevis1(I), it survived the 0.15% (w/v) bile concentration with a growth rate of about 82% which remained slightly constant to the third hour and dropped to about 65% at the fourth hour.
DISCUSSION
L. pentosus, L. plantarum1and L. brevis1 were isolated from our samples. Earlier, Amoa-Awua et al. (2007) identified L. plantarum and L. mesenteriodesin palm wine while Uzochukwu et al. (1994) isolated L. mesenteroides, L. dextranicum and Lactobacillus spp. from palm wine samples in Nigeria. Palm wine can harbour heavy microbial loads because it is rich in simple sugars which the microorganisms use as substrates for growth. Lactic acid bacteria, especially Lactobacillus spp. also prefer such basic sugars and predominate in fermented palm wine during fermentation. They also produce organic acids and antimicrobial substances which inhibit the growth of most other bacteria.
In this study, only L. pentosus (B) had slight effect on the S. entericasubsp. entericatest pathogen while all the isolates, L. plantarum1 (D) and (G), L. Pentosus (A) and (B) and L. brevis 1, were active against different strains of E. coli (BL21, JM 109, XL1B and DH 5α strains). There are many strains among Lactobacilli with documented probiotic ability (Nikolic et al., 2008), thus they have important applications in the prevention of infection. Similar properties were observed in vitro for inhibitory activity of different lactobacillion Clostridium difficile, Campylobacter jejuni and E. coli (Hassan et al., 2012).
The inhibitory action of L. pentosus, L. plantarum 1 and L. brevis1 could be due to their capacity to produce lactic acid, bacteriocins, hydrogen peroxyde (H2O2) and deacetyl which could kill pathogens. Lactic acid bacteria produce lactic acid and the antimicrobial effect of lactic acid is due to undissociated form of acid which penetrate
the membrane and liberate hydrogen ion in the neutral cytoplasm thus leading to inhibition of vital cell functions (Krishnendraet al., 2013).
Being resistant to low pH is one of the major selection criteria for probiotic strains (Çakır, 2003). Resistance to pH 3 is often used for in vitro assays to determine the resistance to stomach pH (Ekundayo, 2014). Food usually stays in the stomach for 3 h (Kavitha and Devasena, 2013), and this time limit was taken into account since to reach the small intestines, probiotics have to pass through the stressful conditions of stomach (Çakır, 2003). Although, in the stomach, pH can be as low as 1.0, in most in vitro assays, pH 3.0 has been preferred due to the fact that, a significant decrease in the viability of strains is often observed at pH 2.0 and below (Kavitha and Devasena, 2013). For selection, the strains resistant to low pH, PBS of pH 1.0, 2.0, and 3.0 were used. The time that food takes during digestion in the stomach is 3 h, thus the screening of isolates resistant to pH 1.0, 2.0 and 3.0 during a period of 1 to 4 h was carried out.
The findings in this study concerning the lower survival rate in pH 2.0 than in pH 3.0, was similar to results reported elsewhere (Vaiseeet al., 2014). Also Sahadevaet al. (2011) and Boke et al. (2010) reported that the viability of Lactobacillus strains was significantly reduced at pH 2.0 as compared to pH 3.0. One of the most important standard for selection of LAB as probiotic candidates is the potential viability at low pH (Allamehet al., 2012). Normally, LAB are capable of inducing an acid tolerance response (ATR) in response to acid treatment (Maria et al., 2001). The systems induced by this response include pH homeostasis, protection and repair mechanisms. Thus, the L. pentosus (A and B) and L. brevis1, have a higher capacity of initiating these mechanisms which will eventually make them more liable to resist the acidic conditions.
Although, the bile concentration of the human gastro intestinal tract varies, the mean intestinal bile concentration is believed to be 0.3% (w/v). Concentrations of 0.15 and 0.3% (w/v) of bile salts have been recommended as a suitable concentration for selecting probiotic bacteria for human use (Hatice et al., 2010). To evaluate the potential of using LAB as effective probiotics, it is generally necessary to evaluate their ability to resist the effects of bile acid. Oxgall is a natural dried bovine bile component containing both conjugated and unconjugated bile salts (Barakatet al., 2011). The time at which food stays in small intestine is suggested to be 4 h (Kavitha and Devasena, 2013). Bile salts are released into the small intestine after ingestion of fatty foods and have a detergent-like function, which may disrupt the lipids and fatty acids of bacterial cell membranes (Pennacchia et al., 2004). Certain microorganisms, including several species of Lactobacillus, can reduce this detergent effect by hydrolyzing bile salts with the bile salt hydrolase (BSH) enzyme (Erkkilä and Petäjä, 2000; Gotcheva et al., 2002). Hence, L. pentosus, L. plantarum1and L. brevis1,are capable of hydrolyzing bile salts with the BSH enzyme and reducing the detergent effect of bile salts making them able to survive in bile. They also have the ability to use up the glucose produced by the bile salts to enhance their survival, by providing the ATP pool required (Corcoran et al., 2005). This permits optimal H+ extrusion by providing Fo-F1-ATPase. Thus, L. pentosus, L. plantarum 1 and L. brevis 1 are protected from being killed or damaged, by these mechanisms. The thicker protective coating of exocellular polysaccharides (EPS) enables them to better withstand stomach acid and bile salts (Hatice et al., 2010). Moreover, the protective effect of the food matrix may prevent these LAB strains from bile exposure hence, giving rise to their increased bile resistance (Begley et al., 2005). Vasiee et al. (2014) reported a good survival rate (about 60%) of Lactobacillus strains which tolerated bile salts of 0.3% (w/v) concentration. Mourad et al.(2006) also showed the survivability of L. Plantarum strains in conditions of high bile salt concentration and low pH values.
CONCLUSION
This study demonstrates that, fermented palm wine is a potential source of LAB with probiotic properties, especially their antimicrobial activity against food borne pathogenic bacteria. The inhibition of Salmonella in this study is a promising finding suggesting a probable application of such LAB in the treatment of foodborne infections. Probiotics microorganisms are emerging tools in the prevention and fight against infections of the human system and the problem of antibiotic resistance. Thus, could help improve the health situation of the public.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Research Foundation for Tropical Diseases and the Environment (REFOTDE), Buea, Cameroon. The authors thank Prof. Wanji Samuel for his guidance and support and the members of the REFOTDE laboratory for their assistance in the progress of this work. They also thank the University of Buea for the quality assistance.
REFERENCES
| Allameh SK, Daud H, Yusoff FM, Saad CR, Ideris A (2012). Isolation, identification and characterization of Leuconostocmesenteroidesas a new probiotic from intestine of snakehead fish (Channastriatus). Afr. J. Biotechnol. 11(16):3810-3816. | ||||
|
Amoa-Awua WK, Sampson E, Tano-Debrah K (2007). Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeisguineensis) in Ghana. J. Appl. Microbiol. 102:599-606. Crossref |
||||
|
Aween MM, Hassan Z, Muhialdin BJ, Noor HM, El-jamel YA (2012). Evaluation on Antibacterial Activity of Lactobacillus acidophilus strains isolated from Honey. Am. J. Appl. Sci. 9(6):807-817. Crossref |
||||
|
Barakat OS, Ibrahim G, Tawfik N, El-Kholy W, Gad EA (2011). Identification and probiotic characteristics of Lactobacillus strains isolated from traditional Domiati cheese. Int. J. Microbiol. Res. 3(1):59-66. Crossref |
||||
|
Begley M, Gahan CG, Hill C (2005). The interaction between bacteria and bile.Federation Eur. Microbiol. Soc. 29(4):625-651. Crossref |
||||
| Bilkova A, Sepova HK, Bukovsky M, Bezakova L (2011). Antibacterial potential of Lactobacilli isolated from a lamb. Vet. Med. 7(56):319-324. | ||||
|
Brant RJ, Todd RK (2014). Impact of genomics on the field of probiotic research: historical perspectives to modern paradigms. A. V. Leeuwenhoek 106:141-156. Crossref |
||||
| ÇakırÄ°(2003).Determination of some probiotic properties on Lactobacilli and Bifidobacteria.Ankara University Thesis of PhD; pp. 24. | ||||
| Chandrasekhar K, Sreevani S, Seshapani P, Pramodhaakumari J (2012). A Review on Palm wine. Int. J. Res. Biol. Sci. 1:1-6. | ||||
|
Corcoran BM, Stanton C, Fitzgerald GF, Ross RP (2005). Probiotic Lactobacilli in Acidic Environments Is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 71(6):3060-3067. Crossref |
||||
| Ekundayo FO (2014) Isolation and identification of lactic acid bacteria from rhizosphere soils of three fruit trees, fish and ogi. Int. J. Curr. Microbiol. Appl. Sci. 3(3):991-998. | ||||
|
Erkkilä S, Petäjä E (2000). Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci. 55:297-300. Crossref |
||||
|
Ezeronye OU, Legras JL (2009). Genetic Analysis ofSaccharomyces Cerevisiae Strains Isolated From Palm Wine In Eastern Nigeria. Comparison with other African Strains. J. Appl. Microbiol. 106(5):1569-1578. Crossref |
||||
| FAO/WHO (2002).Report of a Joint Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO) expert consultation on guidelines for the evaluation of probiotics in food.Accessed April 2006. http:// www.who.int/foodsafety/fsmanagement_probiotic_guidelines. | ||||
|
Hamdan IY, Micolajcik EM (1974). Acidolin: an antibiotic produced by Lactobacillus acidophilus. J. Antibiot. 8:631-636. Crossref |
||||
| Hassan H, Ali E, Karim M, Javad H (2012). Investigation of antibacterial, acid and bile tolerance properties of Lactobacilli isolated from Koozeh cheese. Vet. Res. For. 3(3):181-185. | ||||
| Hatice B, Belma A, Gulcin A (2010). The Role of Resistance To Bile Salts And Acid Tolerance of Exopolysaccharides (Epss) Produced By Yogurt Starter Bacteria. Arch. Biol. Sci. 62(2):323-328. | ||||
| Kavitha JR, Devasena T (2013). Isolation, Characterization, Determination of Probiotic Properties of Lactic Acid Bacteria from Human Milk.J. Pharm. Biol. Sci. 7 (3):1-7. | ||||
|
Klayraung S, Viernstein H, Sirithunyalug J, Okonogi S (2008). Probiotic properties of Lactobacilli isolated from Thai traditional food. Sci. Pharm. 76(3):485-503. Crossref |
||||
| Krishnendra S, Nama SD, Priyanka P, Priyanka S, Neelofar S, Jitendra N (2013). Antagonistic Activity of Lactic Acid Bacteria from Dairy Products. Int. J. Pure Appl. Biosci. 1(1):28-32. | ||||
|
Maria DA, Luca B, Vitaliano P, Cocconcelli PS, Marco G (2001). The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147:1863-1873. Crossref |
||||
| Mourad K, Nour-Eddine K (2006).In vitro preselection criteria for probiotic Lactobacillus plantarumstrains of fermented olives origin.Int. J. Probio. Prebio. 1(1):27-32. | ||||
| Naknean P, Meenune M, Roudaut G (2010). Characterization of palm sap harvested in Songkhla province, Southern Thailand. Int. Food Res. J. 17:977-986. | ||||
|
Nikolic M, Terzic-Vidojevic A, Jovcic B, Begovic J, Golic N, Topisirovic L (2008). Characterization of lactic acid bacteria isolated from Bukuljac, a homemade goat's milk cheese. Int. J. Food Microbiol. 122(1-2):162-170. Crossref |
||||
|
Pennacchia C, Ercolini D, Blaiotta G, Pepe O, Mauriello G, Villani F (2004). Selection of Lactobacillus strains from fermented sausages for their potential use as probiotics. Meat Sci. 67:309-317. Crossref |
||||
| Soccol CR, Vandenberghe LP, Spier MR, Medeiros AB, Yamaguishi CT, Lindnen JD, Pandey A, Thomaz-Soccol V (2010). The Potential of Probiotics.A Rev. Food Technol. Biotechnol. 48:413-434. | ||||
| Ukhum ME, Okolie NP, Oyerinde AO (2005). Some mineral profile of fresh and bottles palm wine-a comparative study. Afr. J. Biotechnol. 4:829-832. | ||||
|
Uzochukwu SVA, Balogh E, Ngoddy PO (1994). The role of microbial gums in the colour and consistency of palm wine. J. Food Qual. 17:393- 407. Crossref |
||||
| Vasiee AR, Tabatayazdi YF, Mortazvi A, Edalatian MR (2014). Isolation, identification and characterization of probiotic Lactobacilli spp. from Tarkhineh. Int. Food Res. J. 21(6):2487-2492. | ||||
|
Vasiljevic T, Shah NP (2008). Review: probiotics - from Metchnikoff to bioactives. Int. Dairy J. 18:714-728. Crossref |
||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0