ABSTRACT
Ephedra is likely one of the oldest medicinal plants still currently in use. In folk medicine, extracts of Ephedra foeminea are commonly used to treat cancer patients. In relation to its traditional use, the aim of the present study was to determine the cytotoxic activity in vitro of E. foeminea extracts on cancer and non-cancer cells. Cell viability was determined using XTT assay, induction of apoptosis by cell sorting and caspase-3 inhibition, and the effects on cell cytoskeleton structure were detected using cell transfection utilizing different cytoskeleton markers. Chemical profiling, analysis of active extracts and identification of compounds was done using high pressure liquid chromatography (HPLC) and gas chromatography-mass spectroscopy (GCMS). E. foeminea leaf ethanol extract and E. foeminea fruit juice reduced cancer cell viability in vitro, whereas the water extract reduced cytotoxic activity in all cell lines. The extract's cytotoxic activity was conveyed at least partially via the induction of caspase 3-dependent cell apoptosis, and enhanced by the addition of Taxol. Both E. foeminea ethanol leaf extract and fruit juice affected actin-stained but not tubulin-stained filaments. Ethanol extract promoted the formation of invadopodia-like structures and fruit juice promoted the formation of large focal adhesion points in the treated cells. Active sub-fractions of E. foeminea extracts were found to contain several compounds including trans-sinapyl alcohol and trans-sinapaldehyde derivative.
Key words: Ephedra foeminea, plant extract, cancer cells, actin, invadopodia, apoptosis.
Abbreviation:
Z-VAD-FMK, N-Benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone; MMP9, matrix metallopeptidase 9; DDW, double distilled water; EtOH, ethanol; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Ephedra is a ubiquitous
genus of
gymnosperm shrubs that grow in temperate and subtropical regions usually on shores or in sandy soils under direct sunlight throughout
North and Central America,
Europe,
Africa and
Asia (Ickert-Bondmet et al., 2009). It is probably one of the oldest medicinal plants still currently in use, featured prominently in traditional Chinese medicine for over 2000 years (Abourashed et al., 2003). The main species used in Chinese medicine is
Ephedra sinica Stapf, traditionally known in Chinese as 'ma huang' (Gurley et al., 1998). Premodern Chinese, Native Americans and Mormons boil the green leaves in water and serve this extract as tea to treat respiratory congestion and asthma (Normile, 2003).
The clinical interest in the folk use of Ephedra increased during the 20th century especially for the treatment of cancer. Importantly, an Ephedra foeminea decoction (that is, water extract) has been used widely by cancer patients to treat their ailments (Ben-Arye et al., 2016). Recently, in a research on the use of complementary and alternative medicines in Palestinian populations, it was reported that 68% of breast cancer patients were supplementing treatment with herbal remedies, with E. foeminea as the primary ingredient (45.7%) (Ali-Shtayeh et al., 2016). Moreover, E. foeminea only recently became one of the most commonly utilized plants by the Palestinian population- from 0.0% in 2011 to 55.2% in 2014, primarily due to local media reporting of E. foeminea decoctions as an effective herbal remedy for cancer patients (Ali-Shtayeh et al., 2016). These water extracts are generally prepared by boiling the leaves for an hour (Ben-Arye et al., 2016), although in some cases, the fruits are consumed as well. Reports show that some breast cancer patients even completely replace modern chemotherapy treatment with E. foeminea herbal extracts (Ben-Arye et al., 2016). Hence, it is extremely important to examine the effectiveness of E. foeminea against cancer in general, and breast cancer in particular.
In the last 15 to 20 years, the use of Ephedra has been restricted due to potentially hazardous effects (Abourashed et al., 2003). Indeed, the aerial parts of different Ephedra species contain active alkaloids (Yeung, 1980), including the phenylpropylamino alkaloids ephedrine and pseudoephedrine. Ephedrine is a sympathomimetic agent (Astrup et al., 1995; Kobayashi et al., 2003) and its consumption may lead to increased cardiac rate and contractility as well as overstimulation of the central nervous system (Abourashed et al., 2003; Krizevski et al., 2012). However, E. foeminea species specifically lacks both ephedrine and pseudoephedrine (Abourashed et al., 2003; Krizevski et al., 2012). In this paper, the efficacy of E. foeminea extracts against cancer cells in vitro in relation to its folk-use was examined. The effect of different extracts of E. foeminea (leaf ethanol extract vs. water extract) and fruit juice on viability of cancer and non-cancer cells was evaluated and reported.
Water extraction (decoction)
Leaves from male and female plants of Ephedra foeminea were collected in the Samaria Mountains (coordinates 32.087661° N, 35.274732° E WGS84) in Central Israel. The soils in the region are terra rossa; plants were collected during August 2016 (temperatures ranged from 22to 24°C) when plants were flowering. Botanical samples were verified by Dr. Jotham Ziffer-Berger, Director of the Herbarium of the National Natural History Collections, The Hebrew University of Jerusalem, Israel. Ephedra extracts were prepared according to instructions received from Y. Sharvit, a local herbalist. Leaves were weighed in a ratio of 43:7.8 g of male to female plants. To the leaves, 1 L of double distilled water (DDW) was added and the mixture was placed in a closed cooking pot. The mixture was brought to boil on a heating plate at medium heat; then reduced and left to simmer at a lower temperature for 2 h. After simmering, the pot was removed from the heating plate and left to cool at room temperature. The extract was then filtered using a 2.5 ml syringe with a 0.22 µm teflon filter and stored at -20°C. Diluted extract for cell treatment contained 30% of the extract and 70% of Dulbecco's Modified Eagle Medium (DMEM, Biological Industries [BI] Ltd., Cat # 01-055-1A, Kibbutz Beit-Haemek, Israel).
Fruit juice preparation
Ephedra fruits were prepared according to the instructions received again from Y. Sharvit. Thirty grams of fruit were crushed using a mortar and pestle, producing 6 ml of Ephedra fruit juice. The juice and crushed material was transferred to 50 ml falcon tubes and placed in a centrifuge at 6500 rpm for 5 min. The supernatant was then stored at -20°C. The homogenous solution was filtered using a 2.5 ml syringe with a 0.45 µm teflon filter and diluted with 1 L of DDW (0.6% v/v). For higher concentrations, the volume of DDW was adjusted accordingly.
Ethanol extraction
Ethanol extraction was developed in the laboratory based on the protocol of Nagappan (2012). Two grams of E. foeminea leaves (from male and female plants at the same ratios as mentioned earlier) were crushed using a mechanical grinder and then transferred to a 50 ml falcon tube. 20 ml of 70% ethanol (EtOH) was added and the mixture was incubated overnight at 28°C with shaking at 180 rpm. The following day, the tube was centrifuged for 5 min at 2500 rpm and the supernatant was removed under vacuum in a rotary evaporator and stored at -20°C. The extract was reconstituted by adding 160 µl of 70% EtOH to the dried sample and incubated at room temperature for 10 to 15 min. One milliliter of DDW was added to the tube. Following vortex, the homogenized solution was filtered using a 2.5 ml syringe with a 0.45 µm teflon filter. The resulting filtrate was used for the experiments described below.
Heat inactivation of E. foeminea ethanol extract and fruit juice
Extracts (ethanol and fruit juice) were heated in order to examine the possible presence of heat-labile components. Heat treatment of the extracts was carried out by heating the filtered extract of each sample for 1 h at 90°C in a closed tube and left to cool for 2 h.
Cell culture
Human (Homo sapiens) cell lines used in this study included: MDA-MB-231-mammary gland/breast cells derived from metastatic site (ATCC® HTB-26™); A549 lung carcinomatous cells (ATCC® CRM-CCL-185™); HaCaT, keratinocytes from histologically normal skin (Creative Bioarray CSC-C8977H); HCT116, epithelial colorectal carcinoma cells (ATCC® CCL-247™). Cells were grown at 37°C in a humidified 5% CO2- 95% air atmosphere. All tissue culture media and serum were purchased from Biological Industries (BI) Ltd., Israel. MDA-MB-231, A549 and HaCaT were maintained in DMEM, and HCT116 were maintained in McCoy's 5A medium. Both media were supplemented with 10% FBS, 1% Penicillin-Streptomycin Solution, 1% L-glutamine and 0.002% plasmocin.
XTT indirect viability and caspase-3 dependent assays
Cells were seeded into 96-well plates at a concentration of 10,000 cells per well in triplicate in DMEM. The following day, DMEM was replaced with fresh medium containing either plant extracts, solvents used for extraction, or only fresh medium for control. Cells were incubated with the different treatments for 2 days and then 2,3,-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium inner salt (XTT) reduction was used to quantify viability according to the manufacturer’s instruction (BI, Kibbutz Beit-Haemek, Israel). Cells were incubated with XTT reagent for 2 h at 37°C in a humidified 5% CO2 – 95% air atmosphere. Absorbance was recorded by a photometer SPEKTRAFluor Plus (Tecan, Salzburg, Austria) at 490 nm with 650 nm reference wavelength. Cell survival was estimated from the equation: % cell survival = 100 × (At-Ac) (treatment) / (At-Ac) (control), where At and Ac are the absorbencies (490 nm) of the XTT colorimetric reaction in treated and control cultures, respectively, minus non-specific absorption measured at 650 nm. Absorbance of medium alone was also deducted from specific readings. Dose-effect curves were determined for the extracts.
For dose response assays, data points were connected by non-linear regression lines of the sigmoidal dose-response relation. GraphPad Prism (Version 6 for Windows, GraphPad Software Inc., San Diego, USA) was employed to produce dose-response curves and IC50 doses for ethanol extract and fruit juice on MDA-MB-231 and HaCaT cells by performing nonlinear regression analysis.In order to test whether the cell death events observed are mediated by caspase-3 activity Z-VAD-FMK (N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone. Abcam, Cat # ab120382, Cambridge, United Kingdom), a cell-permeable pan-caspase inhibitor that irreversibly binds to the catalytic site of caspase proteases was used. A final concentration of 100 µM of the caspase inhibitor was added to treated wells to examine the differences in cell proliferation and viability in the presence of Z-VAD-FMK. Cell proliferation and viability was determined by comparing those treated with E. foeminea extracts with or without Z-VAD-FMK. For all experiments, a DDW control (30% v/v water in XTT reaction mix) and ethanol control (0.17% v/v ethanol in XTT reaction mix) were tested and showed no effect (data not shown).
Actin and tubulin staining
For actin and tubulin staining, 10,000 cells in a total volume of 500µl were seeded on confocal plates. Plates were incubated at 37°C in a humidified 5% CO2-95%. After 24 h, in order to transfect the cells, the medium in each plate was replaced with a fresh medium (500 µl) containing 5 µl of CellLight Actin-RFP (Life Technologies, Cat # C10502, Eugene, Oregon, USA) or Tubulin-GFP (Life Technologies, Cat # C10509, Eugene, Oregon, USA) and plates were incubated under the same conditions for 16-24 h. Successful transfection was determined by confirming fluorescence in 1-2 plates, then cells were washed 3 times with 1.5 ml of DMEM and incubated under the same conditions. After 2 h, the medium was replaced with 700 µl of fresh medium containing the appropriate treatment and plates were incubated for 90 min under the same conditions. Fluorescence intensity of red/green fluorescent proteins (RFP/GFP) were examined using live-cell imaging and photographed with a Leica TCS SP8 laser scanning confocal microscope (Germany) with LAS_X software, equipped with an OPSL 488 nm laser for GFP excitation and OPSL 552 nm for RFP excitation and HC PLAPO 63X 1.2 N.A. objective.
Annexin V/PI staining
Apoptosis was assessed using Alexa Fluor® Dead Cell Apoptosis Kit with Annexin V and PI (Life Technologies, Cat # V13241, Eugene, Oregon, USA). Staining was done according to manufacturer instructions. In brief, cells were seeded in 6-well plate culture dishes at density of 5 x 105 cells per well in DMEM. The following day, the medium was replaced with medium containing E. foeminea extracts at the desired concentrations (as indicated below). Treated samples were incubated for 90 min at 37°C in humidified 5% CO2–95% air atmosphere. Cells in each well were collected separately using trypsin. Then tubes were centrifuged for 8 min in 1400 rpm and cells were resuspended and washed twice with 1 ml of PBS. The cells in each sample were counted. If necessary, the number of cells was adjusted to a concentration of 2 x 106 cells/ml in 1X Annexin binding buffer. Otherwise, cells were resuspended in 200 µl of 1X Annexin binding buffer and transferred to FACS tubes. Cells were stained using 5 µl of Alexa Fluor® 488 or FITC solution and 1 µl of propidium iodide (PI) working solution followed by incubation at room temperature for 15 min in the dark. Then 400 µl of Annexin V binding buffer were added to each tube and flow cytometry was performed using a GALLIOS flow cytometer. Cells were considered to be apoptotic if they were Annexin V+/PI- (early apoptotic) and Annexin V+/ PI+ (late apoptotic). Live cells were Annexin V-/PI-.
RNA extraction, cDNA synthesis and quantitative PCR (qPCR)
Cells were seeded into a 6-well plate at a concentration of 2,000,000 cells/ml per well. After 24 h incubation at 37°C in a humidified 5% CO2–95% air atmosphere, cells were treated with ethanol extract at a dilution of x5 for 1.5 and 3 h. Non-treated cells or cells treated only with ethanol served as controls. Cells were harvested and total RNA was extracted using TRI reagent (Sigma-Aldrich) according to the manufacturer’s protocol. The RNA pellet was dissolved in nuclease-free sterile water and RNA was purified and concentrated using an RNeasy MiniElute Cleanup Kit (Qiagen, USA) as per the manufacturer’s instructions. For cDNA synthesis, 2.5 µg of total RNA and 0.1 µM of random hexamer primers (Promega, USA) were heated for 5 min at 65°C and snap-chilled on ice. The following components were added to the reaction mixture: 0.2 mM dNTP mixture (Invitrogen, USA), 200 U of superscript II-reverse transcriptase (RT) enzyme (Invitrogen, USA), 40 U of Ribolock RNAse inhibitor (Thermo Fisher-Scientific), reverse transcriptase (RT) buffer (1x final concentration), and nuclease-free sterile water (VWR, Amersco Life Sciences, OH, USA) to a reaction volume of 21 µl. The reaction was incubated at 42°C for 60 min followed by incubation at 70°C for 10 min. qPCR was used to determine the gene transcription of matrix metallopeptidase 9 (MMP9) in treated and non-treated MDA-MB-231 cells as described above.
Expression of MMPs characterizes cells undergoing induction of invadopodia, whereas MMP9 is produced mainly by the MDA-MB-231 cells and contributes to metastatic progression (Mehner et al., 2014). The qPCR was performed using components supplied in the KAPA SYBR FAST qPCR kits (Kapa Biosystems, USA) and genes specific primers. For MMP9 (GenBank accession no. NM_004994.2), the following primers were used: (forward) 5'-TTGACAGCGACAAGAAGTGG-3' and (reverse) 5'-TCACGTCGTCCTTATGCAAG -3'. GAPDH served as the reference gene with forward (5'- CAGCCTCAAGATCATCAGCA-3') and reverse (5'- TGTGGTCATGAGTCCTTCCA-3') primers. The reaction mixture consisted of the following components: 2x Master Mix with integrated antibody-mediated hot start, SYBR-Green I fluorescent dye, MgCl2, dNTPs, stabilizers, 2 µl of the template, and PCR-grade water to a final volume of 10 µl. The qPCR analysis was carried out on a Rotor-Gene 6000 instrument (Corbett-Qiagen, Valencia, CA, USA) according to the following program: 3 min at 95°C, followed by 49 cycles of 95°C for 3 s, 60°C for 20 s and 72°C for 1 s. The threshold cycle (Ct) was calculated by the Rotor-Gene 6000 instrument software. The values of the steady-state level of gene transcripts were determined by the 2ΔΔCT method (Arocho et al., 2006), as a ratio between target gene (MMP9) versus the reference gene (GAPDH) and/or treated cell samples versus non-treated cells. A value above or below 1 represents an increase or decrease respectively in the steady-state level of gene transcripts for the examined conditions. Means ± SE (n = 3) were calculated for three biological replicates for each examined treatment.
High pressure liquid chromatography (HPLC) and fractionation
The concentrated Ephedra fruit juice, ethanol extract and water extract were filtered through 0.45 µm syringe filters. 350 µl of sample was loaded in HPLC for profiling and separation (Snyder et al., 2012). The separation of the sample was carried out with Varian Prostar HPLC system coupled with Varian 410 Autosampler, 210 pump, 320 UV/Vis detector. The separation was performed on a Purospher RP-18 endcapped column (250 mm × 4.6 mm I.D.; Merck KGaA, Darmstadt, Germany) with a guard column (4 mm × 4 mm I.D.). Solvent gradients were formed by varying the proportion of solvent A (0.1% acetic acid in DDW) to solvent B (methanol) with the flow rate of 1.0 ml min-1. Initially, solvent B was maintained at 10% for 10 min and then increased to 55% in 5 min.
From 55%, solvent B increased to 65% in 25 min and then maintained at 65% for 5 min and then reduced to 10% in 5 min and equilibrated for 5 min. Compound peaks were detected with two different wavelengths of 280 and 320 nm. Fractions were collected every 3 min from a total run of 30 min. Fractions were then dried using vacuum drying and lyophilization. After drying, fractions were taken for XTT assays as described above. Sub-fractions from Fraction 7 of the ethanol extract were obtained using solvent gradients formed by isocratic proportion of 60% from solvent A and 40% of solvent B with a flow rate of 0.5 ml min-1 for 25 min, and were collected based on peaks. Sub-fractions were then dried using vacuum drying and lyophilization. After drying, sub-fractions were taken for XTT assays as described above.
Gas chromatography-mass spectroscopy (GCMS)
Sub-fractions 7.1 to 7.9 of fraction 7 were dried using vacuum drying and lyophilization. After drying, samples were reconstituted in 100 µl of 100% EtOH and then loaded into the GCMS for analyses (Halket et al., 2004). The analysis was carried out using an Agilent 7890B gas chromatograph coupled to a 5977A mass spectrometer (electron multiplier potential 2 KV, filament current 0.35 mA, electron energy 70 eV, and the spectra were recorded over the range m/z 40 to 500). An Agilent 7683 autosampler was used for sample introduction. 1 μl of each sample was injected to the GCMS using a 1:10 split ratio injection mode. Helium was used as a carrier gas at a constant flow of 1.1 ml s-1. An isothermal hold at 50°C was kept for 2 min, followed by a heating gradient of 6°C min-1 to 300°C, with the final temperature held for 4 min. Solvent delay of 3 min was applied. A 30 m, 0.25 mm ID 5% cross-linked phenylmethyl siloxane capillary column (HP-5MS) with a 0.25 μm film thickness was used for separation and the injection port temperature was 200°C. The MS interface temperature was 280°C. Peak assignments were carried out with the aid of library spectra (NIST 14.0) and compared with published data and MS data obtained from the injection of standards purchased from Sigma-Aldrich.
Statistical analysis
Results are presented as mean + SE of replicate analyses and are either representative of or include at least two independent experiments. Means of replicates were subjected to statistical analysis by Tukey-Kramer test (P ≤ 0.05) using the JMP statistical package and considered significant when P ≤ 0.05.
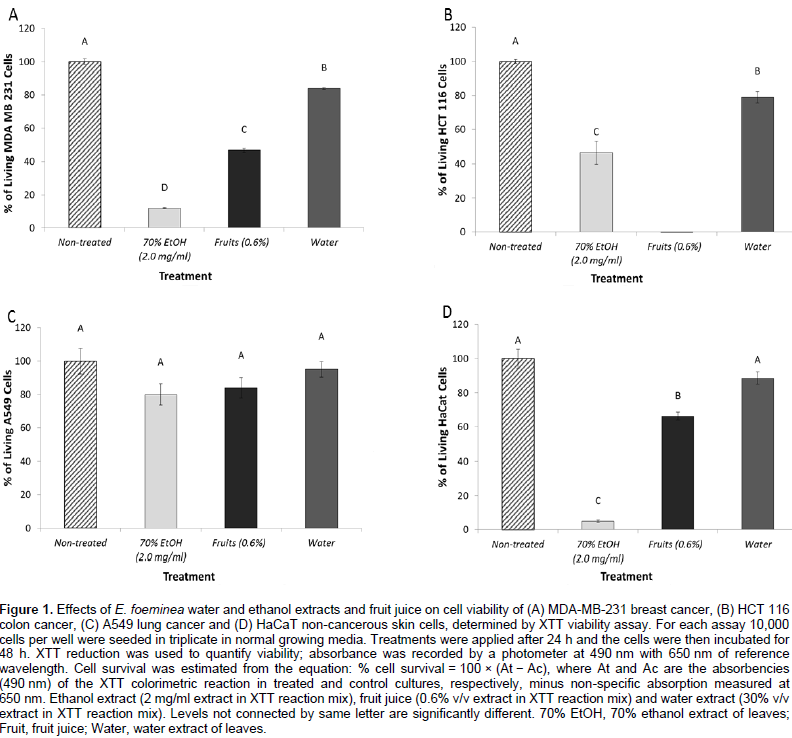
Reduction of cell viability of cancer cells by E. foeminea extracts
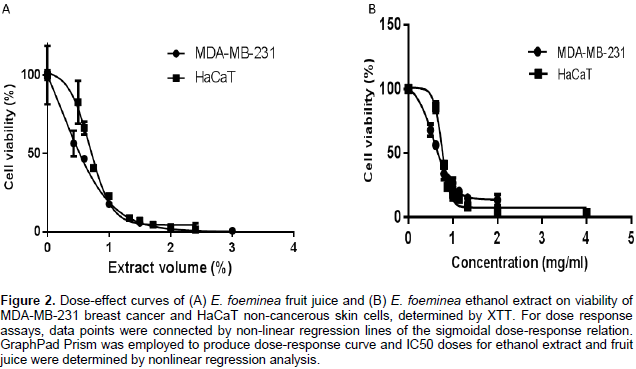
The E. foeminea leaf ethanol extract and fruit juice significantly reduced MDA-MB-231, HCT116 and HaCaT cell viability (Figure 1A, B and D, respectively). However, at these concentrations, they were not effective on A549 cell viability (Figure 1C). Water extract was the least active of the extracts on the different cell lines, and even at a relatively high concentration (30% v/v extract in XTT reaction mix) lacked significant activity on A549 and HaCaT cell viability (Figure 1C and D, respectively). Fruit juice was more effective on MDA-MB-231 than HaCaT cell lines with IC50 0.54 ± 0.07 and 0.69 ± 0.05%, respectively (Figure 2A). Ethanol extract was also more effective on MDA-MB-231 than HaCaT cell lines with IC50 0.57 ± 0.02 and 0.75 ± 0.01 mg/ml, respectively (Figure 2B).
The activity of E. foeminea fruit juice and ethanol extract on cancer cell viability is heat sensitive
Heat inactivation (90°C for 1 h) of E. foeminea fruit juice led to a significant reduction in activity in 1.5, 0.6 and 0.4% concentrations (v/v) (Figure 3A). Heat treatment of the ethanol extract affected activity to a much lesser extent; a significant reduction in activity was recorded in the 2.0 mg/ml concentration and to some extent, in the 0.8 mg/ml concentration (Figure 3B).
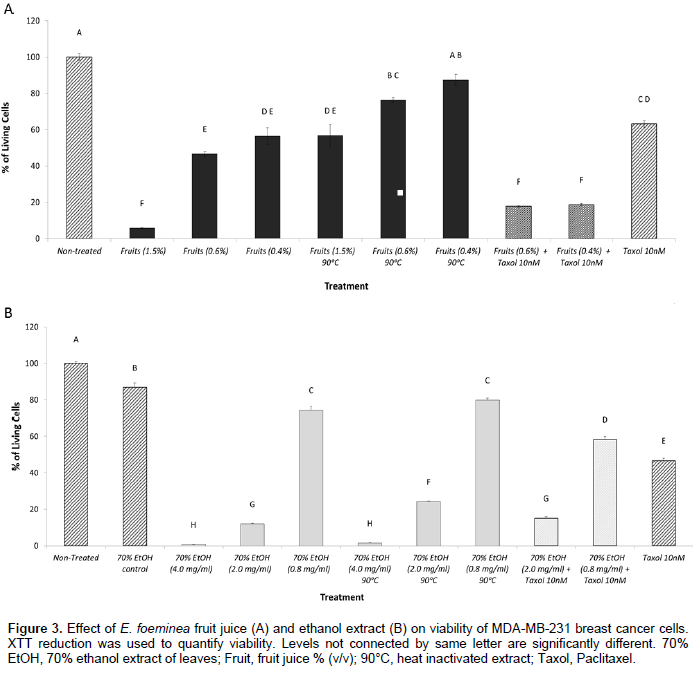
The activity of E. foeminea ethanol extract and fruit juice on cancer cell viability as enhanced by Taxol
The addition of Taxol at a concentration of 10 nM (Mayzlish-Gati et al., 2015) to fruit juice at 0.6 and 0.4% concentration (v/v) showed reduced cell viability in comparison with activity of Taxol only or fruit juice only (in the corresponding concentrations) on cell viability (Figure 3A). Addition of Taxol at a concentration of 10 nM to the ethanol extract at concentration of 0.8 mg/ml showed reduced cell viability in comparison with the activity of Taxol only or ethanol extract only (in the corresponding concentration) on cell viability (Figure 3B).
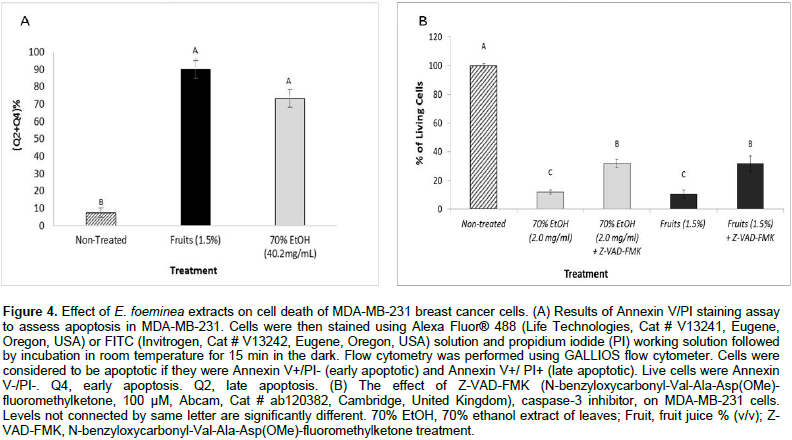
Induction of cell death via apoptosis by E. foeminea extracts
Cell sorting by FACS based on Alexa Fluor® 488/Annexin V staining showed that treatment with high concentrations of the two different extracts, E. foeminea ethanol extract and fruit juice, lead respectively to a large proportion of cells that are in early (4.9 ± 0.7 and 43.3 ± 2.1) or late (68.2 ± 4.4 and 46.6 ± 3.8) apoptosis in comparison with the non-treated cells (FACS sorted at Q4 or Q2, respectively) (Figure 4A). The addition of Z-VAD-FMK caspase inhibitor I to E. foeminea ethanol extract and fruit juice led to a significant decrease in activity on cancer cell viability (Figure 4B).
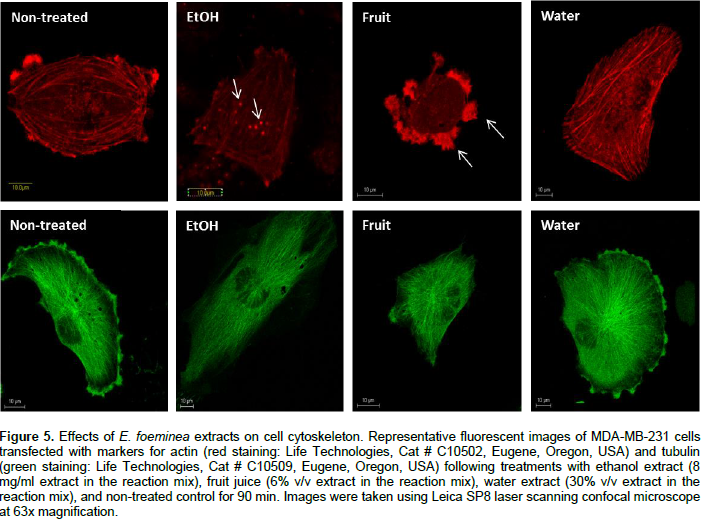
Effect of E. foeminea extracts on actin and tubulin filaments
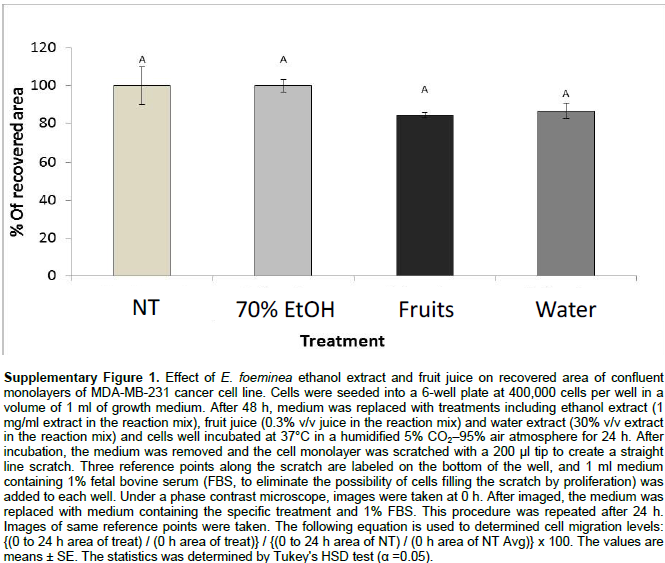
Staining for actin and tubulin was carried out to determine the effect of E. foeminea ethanol extract, water extract and fruit juice on the organization of cytoskeleton filaments. Treatment of MDA-MB-231 cells with E. foeminea ethanol extracts (8.0 mg/ml for 90 min) caused reduction in focal adhesion points and induced formation of actin structure similar to invadopodia, characterized by bright points of actin staining distributed in MDA-MB-231 cells (Figure 5). Exposure of MDA-MB-231 cells to E. foeminea fruit juice (6% v/v, for 90 min) led to reduction in actin filaments and formation of what appears to be large focal adhesion points, whereas water extract did not show any effect on the actin filaments (Figure 5). No change in tubulin filaments were apparent following treatment with the E. foeminea extracts or fruit juice (Figure 5). Together, these results suggest that E. foeminea fruit juice and ethanol extract affect the organization of actin-stained but not tubulin-stained filaments. Despite their effect on actin, in a 2-D migration assay, E. foeminea extracts did not show significant effect on the cell migration of MDA-MB-231 breast cancer cells as compared to the control (Supplementary Figure 1).
Induction of matrix metallopeptidase 9 (MMP9) expression by E. foeminea ethanol extract
The steady state level of matrix metalloproteinases
9 (MMP9) mRNA in MDA-MB-231 cells was significantly increased by 6.96 ± 1.00 fold following treatment with E. foeminea ethanol extract.
Chemical profiling and analysis of E. foeminea extracts
Chemical profiling of the active E. foeminea ethanol extract and fruit juice was carried out by separating the chemical constituents of ethanol extracts and fruit juice in HPLC based on the multistep gradient program described in the materials and methods section. Subsequently, activity of the fractions on cell viability was tested using the XTT assay. The results demonstrated that Fraction 7 in both ethanol extract and fruit juice have high activity and significantly reduced MDA-MB-231 cell viability (Figure 6). However, the activity of other fractions was different between the two extracts. Fractions 6 and 8 had high activity in the ethanol extract, but only medium to low activity in the fruit juice. Fraction 2 also had high activity in the fruit juice, and medium activity in the ethanol extract (Figure 6).
Sub-fractionation of the active ethanol Fraction 7 in both E. foeminea ethanol extract and fruit juice was carried using the isocratic program described in the materials and methods section. Subsequently, activity of the sub-fractions on cell viability was tested using XTT assay. The results demonstrate that several sub-fractions, especially 7.2, 7.3 and 7.5, had high activity and significantly reduced MDA-MB-231 cell viability (Figure 7).The active sub-fractions were analyzed for identification of compounds using GCMS. Results are presented in Table 1. Only sinapyl-related compounds (trans-sinapyl alcohol, trans-sinapyl alcohol derivative and trans-sinapaldehyde derivative, respectively) were present in all the active fractions examined (Table 1); sugar present in all fractions were used as internal standard. Notably, sub-fraction 7.1, inactive for cell viability, did not contain sinapyl-related compounds.
The effect of different extracts of E. foeminea leaves and fruit juice on cancer and non-cancer cells, and on the cell cytoskeleton was examined. The results based on in vitro assays raise concerns that the folkloric use of E. foeminea decoction (water extract) does not have a significant effect on cancer cell viability. However, both E. foeminea leaf ethanol extract and fruit juice have a significant ability to reduce cell viability. It is possible that the water extraction method of boiling the leaves reduces its activity, and that some heat-labile compounds are being inactivated in the process. Indeed, some of the components in E. foeminea fruit juice and to a lesser extent, the ethanol extract, were found to be heat-labile; activity of the ethanol extract was reduced once heated. However, the lack of activity of the E. foeminea water extract stands in stark contrast to the recent sporadic reports of improvement in the medical condition of cancer patients following its use. The inefficacy of E. foeminea water extract was also reported in Ben‑Arye et al. (2016).
The reduction in cancer cell viability by E. foeminea ethanol extract and fruit juice was shown on two different types of cancer cell lines, MDA-MB-231 (breast cancer) and HCT116 (colon cancer), but not on A549 (lung cancer) cells. A somewhat reduced effect was also recorded on non-cancer skin cells (HaCaT). The fact that ethanol extract and fruit juice significantly reduced HaCaT viability may suggest that despite the potential beneficial activity of the E. foeminea ethanol extract and fruit juice on cancer cells, they may have adverse cytotoxic effects on non-cancer cells and tissues. The ineffectiveness of all extracts on A549 cell line may result from the insensitivity of this cell line to the cytotoxic compounds in E. foeminea extracts. However this needs to be further investigated.The cytotoxic activity of E. foeminea ethanol extract and fruit juice is mediated, at least partially, by induction of apoptosis via a caspase 3-dependent pathway. This cytotoxic activity was enhanced by Taxol, and vice versa, that of Taxol was enhanced by the extract and juice, suggesting that they may act together to enhance cancer cell death.
E. foeminea ethanol extracts caused changes in the organization of actin filaments, leading to the formation of invadopodia-like structures. Invadopodia are
actin-rich protrusions of the
plasma membrane associated with cell migration and metastasis (Yamaguchi, 2012). Part of the activity of invadopodia in invading extracellular membranes is due to localized proteolytic activity of MMPs (Yamaguchi, 2012), involved in tumor metastasis (Foda and Zucker, 2001). In particular, MMP9 was found to be produced mainly by the MDA-MB-231 tumor cells and to significantly contribute to metastatic progression (Mehner et al., 2014). The fact that treatment with the
E. foeminea ethanol extract leads to a marked increase in MMP9 expression suggests that this extract may indeed induce formation of invadopodia-like structures. Fruit juice did not lead to the formation of invadopodia-like structures, but rather to the formation of what might be large focal adhesion points. The latter are also found as a result of treatment with Taxol. In human umbilical vein endothelial cells (HUVECs), it was shown that Taxol inhibits cell migration, at least in part, by disruption of the regulated formation and turnover of focal adhesions (Kamath et al., 2014). However, despite the effect on actin, and actin’s involment in cell migration (Yamaguchi and Condeelis, 2007), there was no significant effect on cell migration by any of the
E. foeminea extracts or fruit juice.
Taxol affects tubulin by inhibiting the dynamic instability of microtubules (Schiff et al., 1979; Yvon et al., 1999). However, no changes in tubulin structure could be detected in MDA-MB-231 cells treated with E. foeminea extracts suggesting that the effect of the extracts are mainly on actin structures. Taken together, Taxol and E. foeminea extracts may affect different components, that is, microtubules and actin, respectively, leading to enhanced aberrations of cell cytoskeleton.
When E. foeminea ethanol extract and fruit juice were fractioned, common active fractions and fractions with different activity levels were identified in the two E. foeminea extracts, suggesting differences in chemical composition. To further identify active compounds in E. foeminea, the active ethanol fraction (Fraction 7) was sub-fractionated and subjected to GCMS analysis, which revealed the presence of several compounds. Only sinapyl-related compounds (trans-sinapyl alcohol, trans-sinapyl alcohol derivative and trans-sinapaldehyde derivative) were present in all active fractions. Interestingly, derivatives of sinapyl alcohol were previously found in Ligularia nelumbifolia (Zhao et al., 1994), a plant commonly used in the Chinese medicine for reducing inflammation, for the treatment of coughs, for curing apoplexy and for the treatment of tuberculosis (Zhao et al., 1994). Moreover, it was shown that derivatives of sinapyl alcohol are cytotoxic against several cancer cell lines (Zhao et al., 2002; Zou et al., 2006). It might be that these compounds are responsible for the cytotoxic activity against cancer cells of the E. foeminea ethanol extract. However, further research is needed to fully characterize the activity of sinapyl-related compounds, to exclude or include the activity of additional compounds in E. foeminea (e.g., those present in fruit juice) and to better understand mode of cytotoxic activity in E. foeminea.
The leaf ethanol extract and fruit juice of E. foeminea have a significant ability to reduce cancer cell viability. This is mediated at least partially via induction of caspase 3 dependent-cell apoptosis and may be induced by sinapyl-related compounds found in E. foeminea ethanol extract. However, cytotoxic activity was low in the water extract widely used in folk medicine. Importantly, the use of this plant for treating cancer may be dangerous, partially due to effects of the extracts on cell cytoskeleton and gene expression associated with cancer cells invasiveness and metastasis.
The authors have not declared any conflict of interests..
M. M. was supported by a scholarship from The Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel. R. N. and G. S. were supported by the ARO Postdoctoral Fellowship. The authors thank Dr. Jotham Ziffer-Berger of the Herbarium, The National Natural History Collections, Hebrew University of Jerusalem, Israel, for botanical authentication of the plants used in this study.
REFERENCES
|
Abourashed EA, El-Alfy AT, Khan IA, Walker L (2003). Ephedra in perspective – a current review. Phytother. Res. 17:703-712.
Crossref
|
|
|
|
Ali-Shtayeh MS, Jamous RM, Salameh NM, Jamous RM, Hamadeh AM (2016). Complementary and alternative medicine use among cancer patients in Palestine with special reference to safety-related concerns. J. Ethnopharmacol. 187:104-122.
Crossref
|
|
|
|
|
Arocho A, Chen B, Ladanyi M, Pan Q (2006). Validation of the 2-ΔΔCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn. Mol. Pathol. 15:56-61.
Crossref
|
|
|
|
|
Astrup A, Breum L, Toubro S (1995). Pharmacological and clinical studies of ephedrine and other thermogenic agonists. Obes. Res. 3:537-540.
Crossref
|
|
|
|
|
Ben-Arye E, Mahajna J, Aly R, Ali-Shtayeh MS, Bentur Y, Lev E, Deng G, Samuels N (2016). Exploring an herbal "wonder cure" for cancer: a multidisciplinary approach. J. Cancer Res. Clin. Oncol. 142(7):1499-1508.
Crossref
|
|
|
|
|
Foda HD, Zucker S (2001). Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov. Today 6:478-482.
Crossref
|
|
|
|
|
Gurley BJ, Wang P, Gardner SF (1998). Ephedrineâ€type alkaloid content of nutritional supplements containing Ephedra sinica (maâ€huang) as determined by high performance liquid chromatography. J. Pharm. Sci. 87:1547-1553.
Crossref
|
|
|
|
|
Halket JM, Waterman D, Przyborowska AM, Patel RK, Fraser PD, Bramley PM (2004). Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 56:219-243.
Crossref
|
|
|
|
|
Ickert-Bond SM, Rydin C, Renner SS (2009). A fossilâ€calibrated relaxed clock for Ephedra indicates an Oligocene age for the divergence of Asian and New World clades and Miocene dispersal into South America. J. Syst. Evol. 47:444-456.
Crossref
|
|
|
|
|
Kamath K, Smiyun G, Wilson L, Jordan MA (2014). Mechanisms of inhibition of endothelial cell migration by taxanes. Cytoskeleton (Hoboken) 71:46-60.
Crossref
|
|
|
|
|
Kobayashi S, Endou M, Sakuraya F, Matsuda N, Zhang XH, Azuma M, Echigo N, Kemmotsu O, Hattori Y, Gando S (2003). The sympathomimetic actions of l-ephedrine and d-pseudoephedrine: direct receptor activation or norepinephrine release? Anesth. Analg. 97:1239-1245.
Crossref
|
|
|
|
|
Krizevski R, Bar E, Shalit OR, Levy A, Hagel JM, Kilpatrick K, Marsolais F, Facchini PJ, Ben-Shabat S, Sitrit Y, Lewinsohn E (2012). Benzaldehyde is a precursor of phenylpropylamino alkaloids as revealed by targeted metabolic profiling and comparative biochemical analyses in Ephedra spp. Phytochemistry 81:71-79.
Crossref
|
|
|
|
|
Mayzlish-Gati E, Laufer D, Grivas CF, Shaknof J, Sananes A, Bier A, Ben-Harosh S, Belausov E, Johnson MD, Artuso E, Levi O, Genin O, Prandi C, Khalaila I, Pines M, Yarden RI, Kapulnik Y, Koltai H (2015). Strigolactone analogs act as new anti-cancer agents in inhibition of breast cancer in xenograft model. Cancer Biol. Ther. 16:1682-1688.
Crossref
|
|
|
|
|
Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES (2014). Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 5:2736-2749.
Crossref
|
|
|
|
|
Nagappan R (2012). Evaluation of aqueous and ethanol extract of bioactive medicinal plant, Cassia didymobotrya (Fresenius) Irwin & Barneby against immature stages of filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2(9):707-711.
Crossref
|
|
|
|
|
Normile D (2003). The new face of traditional Chinese medicine. Science 299:188-190.
Crossref
|
|
|
|
|
Schiff PB, Fant J, Horwitz SB (1979). Promotion of microtubule assembly in vitro by Taxol. Nature 277:665-667.
Crossref
|
|
|
|
|
Snyder LR, Kirkland JJ, Glajch JL (2012). Practical HPLC method development. John Wiley & Sons.
|
|
|
|
|
Yamaguchi H (2012). Pathological roles of invadopodia in cancer invasion and metastasis. Eur. J. Cell Biol. 91:902-907.
Crossref
|
|
|
|
|
Yamaguchi H, Condeelis J (2007). Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773:642-652.
Crossref
|
|
|
|
|
Yeung AY (1980). Encyclopedia of common natural ingredients. John Willey & Sons, New York, USA. pp. 166-167.
|
|
|
|
|
Yvon AMC, Wadsworth P, Jordan MA (1999). Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell. 10:947-959.
Crossref
|
|
|
|
|
Zhao Y, Hao X, Lu W, Cai J, Yu H, Sevénet T, Guéritte F (2002). Syntheses of two cytotoxic sinapyl alcohol derivatives and isolation of four new related compounds from Ligularia nelumbifolia. J. Nat. Prod. 65:902-908.
Crossref
|
|
|
|
|
Zhao Y, Zhongjian J, Yang L (1994). Sinapyl alcohol derivatives and other constituents from Ligularia Nelumbifolia. Phytochemistry 37:1149-1152.
Crossref
|
|
|
|
|
Zou HB, Dong SY, Zhou CX, Hu LH, Wu YH, Li HB, Gong JX, Sun LL, Wu XM, Bai H, Fan BT, Hao XJ, Stöckigt J, Zhao Y (2006). Design, synthesis, and SAR analysis of cytotoxic sinapyl alcohol derivatives. Bioorg. Med. Chem. 14:2060-2071.
Crossref
|
|