ABSTRACT
Many of the health benefits associated with Aloe species have been attributed to the polysaccharides contained in the gel of the leaves. The aim of this study was to investigate the chemical composition as well as the biological evaluation of polysaccharides isolated from the leaves of eight different Aloe species, A. vera (A1), A. arborescens (A2), A. eru (A3), A. grandidentata (A4), A. perfoliata (A5), A. brevifolia (A6), A. saponaria (A7) and A. ferox (A8) grown in El Orman Botanical Garden, Giza, Egypt. Polysaccharides from the plants were isolated using hot extraction method and then hydrolyzed. The polysaccharide hydrolysates were identified using high performance liquid chromatographic technique. Maximum yield of total polysaccharides identified were obtained from A7 (12.04%), A1 (8.51%), A8 (8.03%), A2 (5.32%) and A6 (2.18%) respectively. The isolated polysaccharides were tested for antihyperglycemic activity in alloxan-induced diabetic rats and alpha glucosidase inhibitory activity. Chromatographic investigation of the polysaccharides recorded the presence of 18 saccharides, glucuronic acid, stachyose, galacturonic acid, sucrose, glucose, xylose, galactose, rhamnose, mannose, arabinose, fructose polyol, mannitol and sorbitol in the eight Aloe species, but their quantitative composition differed among the species. Glucuronic acid, stachyose and galacturonic acid were the major detected saccharides. The results of the biological activities revealed significant antihyperglycemic activities with variable degrees. After four weeks of daily administration, polysaccharides isolated from A. vera (A1) and A. arborescens (A2) were the most active with 40 and 44% reduction in blood glucose level, respectively. All the tested polysaccharides showed significant alpha glucosidase inhibitory activity with IC50 (µg/ml) 11.70, 14.60 and 15.80 for A7, A6 and A1 respectively. In conclusion, the tested polysaccharides contribute to the antidiabetic action of these Aloe species.
Key words: Aloe, alpha glucosidase inhibitors, antidiabetic, polysaccharide.
Diabetes is a chronic metabolic disorder characterized by high blood glucose levels (American Diabetes Association, 2009). Type-1 diabetes is an autoimmune disease characterized by T-cell mediated destruction of the pancreatic beta cells. In type-2 diabetes, there is a gradual development of insulin resistance and beta cell dysfunction, strongly associated with obesity and a sedentary lifestyle (Zimmet et al., 2001). Due to a higher incidence of the risk factors, the prevalence of diabetes is increasing worldwide, but more evidently in developing countries (Sherif and Sumpio, 2015). In the Middle East and North Africa Region, 1 in 10 adults have diabetes (Klautzer et al., 2014); the region has the highest prevalence of diabetes (10.9%). The International Diabetes Federation (IDF) estimated that there are 34.6 million people with diabetes in the Middle East and North Africa, a number that will almost double to 67.9 million by 2035 if concerted action is not taken to tackle the risk factors fuelling the epidemic of diabetes throughout the region (IDF, 2013). Egypt is now ranked eighth highest in the world in terms of the disease (IDF, 2013).
Diabetes mellitus is a chronic state of hyperglycemia which results in the development of important complications (Metelko and Brkljacic, 2013), where body cells cannot uptake and utilize glucose; therefore breakdown of fats increase with production of fatty acids and ultimately ketone bodies, this disorder is accompanied by decrease in protein synthesis (American Diabetes Association, 2009). Nevertheless, diabetes mellitus is an illness that is found among the non-transmissible chronic diseases and it is considered a health challenge due to the great number of existing cases, its growing contribution to general mortality, its identification as the most frequent cause of premature disability, its complexity, and the high cost of its treatment (Córdova et al., 2008).
In recent years, several synthetic drugs have been developed to combat against diabetes, but the situation has only marginally improved. Furthermore, these synthetic drugs are not able to combat with all the pathological complications and mostly palliative in their effect. Herbal medications have been used successfully by mankind from ancient time to counteract diabetes and its associated complications (Bailey and Day, 1989). Long-term complications of diabetes include retinopathy and loss of vision; nephropathy cause renal failure; peripheral neuropathy with risk of foot ulcers; and autonomic neuropathy causing gastrointestinal, genitourinary, cardiovascular and sexual dysfunction. Hypertension and abnormalities of lipoprotein metabolism are often found in people with diabetes (American Diabetes Association, 2009).
A number of reviews on medicinal plants used in the management of diabetes in different parts of the world, as well as those used specifically in certain regions, such as in West Africa, Central America and Asia exist (Ezuruike and Prieto, 2014). These include Allium sativa, Gymnema sylvestre, Ocimum sanctum, Pterocarpus marsupium, Trigonella foenum graecum and Tinospora cordifolia. Moreover, most of these plants were clinically tested; the results were recommendation that physicians can depend on herbs in alleviating diabetes and its complications (Ghorbani, 2013; Pandey et al., 2011). Therefore, these herbal plants could be an alternative therapy for diabetes and its complications (Khan et al., 2012).
Polysaccharide hydrocolloids including mucilages, gums and glucans are abundant in nature and are commonly found in many higher plants (Clifford et al., 2002). These polysaccharides constitute a structurally diverse class of biological macromolecules with a broad range of physicochemical properties which are widely used for applications in pharmacy and medicine (Franz, 1989).
The genus Aloe (family Xanthorrhoeaceae, subfamily Asphodeloideae) contains over 500 species of flowering succulent plants, among which the most widely known species is A. vera (L.) Burm. f. or true aloe, which is one of the most important pharmaceutical herbs (Grindlay and Reynolds, 1986; Boudreau and Beland, 2006). Different Aloe species have been used in the treatment of a variety of disorders including infections, dermatologic conditions and also used as a laxative since ancient times in the Greek Herbal of Dioscorides (ca 70 ad) the U.S. pharmacopoeia in 1820 (Davis, 1997; Park and Lee, 2006; Ulbricht et al., 2008). These plants have long meaty thick leaves with twisted sides which end in thorns (Grindlay and Reynold, 1986; Heggars et al., 1993). The substance inside the leaf called gel consists of 99% water with long chain polysaccharide, of acetylated glucomannan kind, and other carbohydrates. It was claimed that the polysaccharides in Aloe vera L. gel had therapeutic properties such as anti-inflammatory, wound healing, promotion of radiation damage repair, antidiabetic and anti-neoplastic activities (Chun-hui et al., 2007). The Aloe leaf structure is made up of three layers: (i) rind, the outer protective layer; (ii) sap, a layer of bitter fluid which helps protect the plant from animals; (iii) mucilage gel, the inner part of the leaf that is filleted out to make A. vera gel. The authors have cut the leaves open and take the inner content so it will include the mucilage and the sap.
During the past 20 years, reports have shown that Aloe preparations have beneficial therapeutic effects on diabetes. Aloe spp. is documented in ethnobotanical survey as one of the potential anti-diabetic plants (Gbolade, 2009). The dried sap, of the Aloe plant had been used for diabetes in the Arabian Peninsula (Ghannam et al., 1986). Its ability to lower the blood glucose was studied in five patients with noninsulin-dependent diabetes and in Swiss albino mice made diabetic using alloxan (Ghannam and Geissman, 1986). The hypoglycemic activity of Aloe species was first demonstrated by Agrawal (1985). Since then, the antidiabetic effects of Aloe preparations have been demonstrated in diabetic patients (Ghannam et al., 1986; Ajabnoor 1990; Bunyapraphatsara et al., 1996; Yongchaiyudha et al. 1996), and in alloxan- or streptozotocin-induced diabetic animal models (Beppu et al., 1993; Rajasekaran et al., 2004, 2005, 2006; Beppu et al., 2006). A. vera gel was administered to STZ-induced diabetic rats decreased fasting blood glucose levels and improved the levels of the antioxidant enzyme (Nwanjo, 2006); moreover aqueous extract prevented the onset of hyperglycaemia in alloxan-induced diabetic rabbits (Akinmoladun and Akinloye, 2007); a polyphenol-rich A. vera extract (350 mg kg-1) with known concentrations of aloin (181.7 mg g-1) and aloe-emodin (3.6 mg g-1) was administered to insulin resistant mice for 4 weeks improved insulin tolerance and fasting blood glucose levels (Perez et al.,2007).
The principle aim of this work was to evaluate the qualitative and quantitative components of the polysaccharide isolated from eight Aloe species growing in Egypt using HPLC analysis. In addition, the possible antidiabetic effect of the purified mucilage against alloxan-induced diabetes in rats and their alpha-glucosidase inhibitory activity were evaluated.
Plant material
The leaves of
Aloe vera L. Burm. f. (A
1)
, Aloe arborescens Mill. (A
2)
, Aloe eru A. Berger (A
3)
, A. Aloe grandidentata Salm-Dyck (A
4)
, Aloe perfoliata L.(A
5),
Aloe brevifolia Mill. (A
6)
, A. saponaria L
. (A
7) and
A. ferox Mill.
(A
8) were collected in April 2013 from El-Orman Botanical Garden, Giza, Egypt. The plants were kindly authenticated by Dr. Mohamed El-Gebaly, Botany Specialist. Voucher specimen 1842013 is kept in the herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Cairo University. Extractions of polysaccharides were conducted in Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt in 2014. HPLC analysis of polysaccharides were performed at Food Technology Research Institute, Agriculture Research center, Ministry of Agriculture and Land Reclamation, Giza, Egypt in 2015.
Extraction of polysaccharides
The polysaccharides were isolated from the fresh leaves of A1, A2, A3, A4, A5, A6, A7 and A8 (Figure 1), by cutting each one of them into small pieces with size range (1-1.5 cm long × 1-2 cm wide). 1 kg of each sample was extracted by hot extraction methods, with 10 L of boiling distilled water, filter while hot through a muslin cloth. The aqueous extract was concentrated to one third to make the aqueous solution saturated with polysaccharides and hence facilitate the polysaccharide precipitation and reduce volume of the used acetone. The polysaccharides were then precipitated in each case by addition of three times of acetone. The polysaccharide was collected by centrifugation at 20°C and 18,000 rpm for 1 h (Megafuge 1.0 R; Heraeus, Haneau, Germany). The mucilage was then vigorously stirred in absolute acetone, filtered and then dried in a vacuum desiccator over anhydrous calcium chloride. The percentage yields of polysaccharides were as follows 0.86, 0.49, 0.20, 0.15, 0.6, 0.25, 1.15 and 0.7% of A1, A2, A3, A4, A5, A6, A7 and A8, respectively.
Chemicals
Authentic sugars for high performance liquid chromatograph (HPLC) (sucrose, D-glucose, D-sorbitol, mannitol, galacturonic acid, glucuronic acid, stachyose, xylose, galactose, rhamnose, mannose and arabinose, all with a purity exceeding 99.0% were purchased from Sigma Aldrich (Steinheim, Germany), and D-fructose was purchased from Merck (Darmstadt, Germany). Alloxan (Sigma Co., USA) freshly dissolved in saline was used for induction of diabetes. Biodiagnostic kit for assessment of blood glucose and glutathione levels (Epico, Egyptian Int. Pharmaceutical Industries Co.,A.R.E.). Carboxymethylcellulose sodium (CMC-Na) was purchased from Acros Organics (NJ, USA), Metformin (1,1-dimethylbiguanide hydrochloride) (Sigma Co., USA). α-Glucosidase enzyme from brewer's yeast (EC 3.2.1.20), the substrate; p-nitrophenyl α-D-glucopyranoside (p-NPG), and Phosphate buffer (pH 6.8) were purchased from Sigma Chemical Co., (St Louis, MO 63103 USA). The positive control, acarbose was purchased from Bayer Pharmaceuticals Pvt., Ltd (USA).
Acid hydrolysis
Ten milligram of each of the mucilage of each plant under investigation were separately heated in 2 ml of 0.5 M sulphuric acid in a sealed test tube for 20 h in a boiling water bath. At the end of the hydrolysis any precipitate was filtered off. The filtrate was freed of sulphate ions by precipitation with barium carbonate. The hydrolsates were separately concentrated under vacuum at a temperature not exceeding 40°C to a syrupy consistency. It was diluted with 10% isopropanol in water to about 10 ml. The individual authentic reference sugars were dissolved in distilled water. All samples were filtered through microfilter (0.45 µm) and stored in vials to be used in HPLC investigation (Gertz, 1990).
HPLC analysis of the polysaccharides
HPLC analysis was used to determine the free sugars in the isolated polysaccharide from the different plants under investigation qualitatively and quantitatively. HPLC analysis was performed on operating system model Shimadzu (SCL-10AVP) equipped with RI detector RID-10A and high pressure pump LC-10ADVP. Separation and determination were performed on column sugar SC1011 (Shodex SUGAR Series) (Particle size 6 µm; length 300 mm; diameter 8 mm) using deionized water as mobile phase with 1ml/min flow rate. Ten milligram of each residue of isolated polysaccharides, as well as of the aforementioned individual authentic sugars was separately dissolved in 1 ml of deionized water. 10 µl of each sample was injected into HPLC using an SGE syringe (Syringe Perfection, Australia). Quantitative determination was based on peak area measurement while qualitative identification was carried out by comparison of the retention times of the peaks with those of the authentic sugars (Figures 2A to D).

Evaluation of the anti-hyperglycemic activity
Animals
Adult male rats of Sprague-Dawley strain (130 to 150 g) were obtained from the laboratory animal facility of the National Research Center, Dokki, Giza. Animals were housed in steel cages under standard conditions, animals were housed in a temperature (20 to 23°C) and humidity (approximately 50%)-controlled colony room on a 12:12-h light: dark schedule. To facilitate measures of food intake, rats were housed conventionally in individual stainless steel hanging wire-mesh cages, and fed with standard pellets, commercial standard chow (18 % protein; Global 2018, Harlan Teklad, Madison, WI) and water ad libitum. All experimental procedures were conducted in accordance with internationally accepted principles for laboratory animal use and care, and were approved by the Ethics Committee (No. 9-031) in accordance with recommendations for the proper care and use of laboratory animals (NIH Publication No. 80-23; revised 1978).
Induction of diabetes mellitus in rats
Diabetes was induced intraperitoneally with a single dose of alloxan (150 mg/kg body weight). Alloxan was first weighed individually for each animal according to the body weight and then solubilized with 0.2 ml saline (154 mM NaCl) just prior to injection. Two days after alloxan injection, rats with plasma glucose levels of 140 mg/dl were included in the study.
Treatment with polysaccharides samples was started 48 h after alloxan injection. Hyperglycemia was assessed after 72 h by measuring blood glucose and after 2 and 4 weeks intervals (Eliasson and Samet, 1969).
Assessment of anti-hyperglycemic effect
The eight polysaccharide samples (A1-A8), isolated from each of the eight tested species were tested for their anti-hyperglycemic activity over 28 days at a dose of 250 mgkg-1 body weight. The doses of the polysaccharides were determined as 250 mg kg-1 on the basis of a preliminary short-term pilot study with a range of variable doses. The control diabetic group in each experiment received a single daily dose of 1% Carboxymethylcellulose sodium (CMC-Na), as a vehicle for the tested sample. The vehicle, metformin and polysaccharides were given orally by gavage as single daily treatments for 4 weeks. At the end of each study period, blood samples were collected from the retro-orbital venous plexus through the eye canthus of anesthetized rats after an overnight fast. Serum was isolated by centrifugation, and the blood glucose level was measured by enzymatic colorimetric method at zero time, at days 14 and 28 from the treatment (Trinder, 1969).
Assay for alpha-glucosidase inhibitory activity
The assay was performed to measure the alpha-glucosidase inhibitory activity of the polysaccharide samples isolated from the eight Aloe species (A1-A8). The enzyme inhibition study was carried out spectrophotometrically in a 96-well microplate reader using a procedure reported by Li et al. (2005). Enzyme assay of 220 µl were prepared using phosphate buffer (50 mM, pH 7). It contained 50 µl p- nitrophenol-a-glucoside (a-NPG) (1.3 mM), 150 µl enzyme (0.026 U) and 20 µl of each polysaccharide sample. The inhibitor was replaced by water in case of incubations performed to determine 100% activity. Blank incubations were performed to cancel the color of the polysaccharide. Polysaccharide (10 mg) was dissolved in 600 µl hot water (90°C). The assays were incubated at 37°C for 7 min and color was measured at λmax410 nm using a Spectra Max 340 (Molecular Devices, USA) spectrometer. Controls contained the same reaction mixture except the same volume of phosphate buffer was added instead of the inhibitor solution. Acarbose (Bayer Pharmaceuticals Pvt., Ltd .USA) was dissolved in water and used as a positive control.
Inhibition %= [(AB - AA)/AB] × 100%
Where AB is the absorbance of the control sample and AA is the absorbance of test sample. The fifty percent inhibitory concentration (IC50 µg/ml) of the active samples against yeast glucosidase was calculated.
Statistical analysis
The statistical comparison of difference between the control group and the treated groups was carried out using two-way ANOVA followed by Duncan's multiple range test.
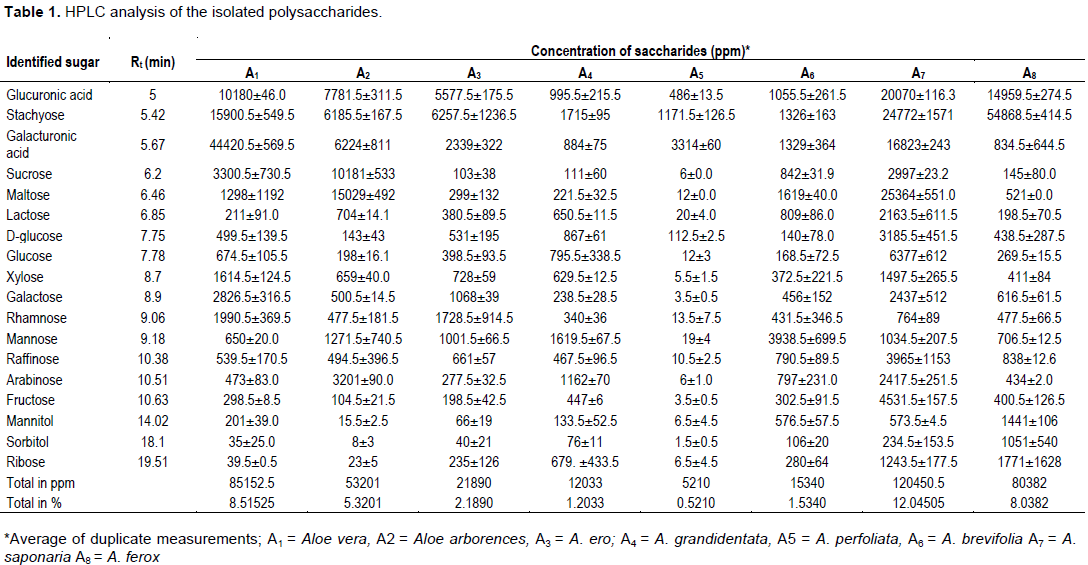
It was obvious from Table 1 and Figures 1 and 2A to D that all the investigated Aloe species were rich in polysaccharides; A7, A1 and A8 had the highest polysaccharide contents (12.0, 8.5and 8.0%) respectively. While A5 and A4 had showed the lowest polysaccharide contents (0.5 and 1.2%) respectively. High concentration of glucuronic acid (2.1, 1.5 and 1.3%), was detected in A7, A8 and A2 respectively. Moreover, Stachyose was major identified sugar as (5.4, 2.6 and 1.7%) in A8, A7 and A1 respectively. Galacturonic acid was prevailed as major sugar (4.5, 1.45 and 0.3%) identified in A1, A7 and A5 respectively. Whereas the lowest concentration of most of the sugars were found in A2, A3, A4 and A6. Chemical composition differs among the species of Aloe. For example, A. barbadensis Miller may contain 2.5 times the aloe-emodin of A. ferox Miller; the time of harvest may a factor into the composition variation. Aloe ferox is similar to A. vera but has many times more nutritional and medicinal value than A. vera (Bhaludra et al., 2013). However, discrepancies exist regarding the composition of polysaccharide species and an understanding of pulp structure in relation to its chemical composition has been lacking (Ni.et al., 2004).
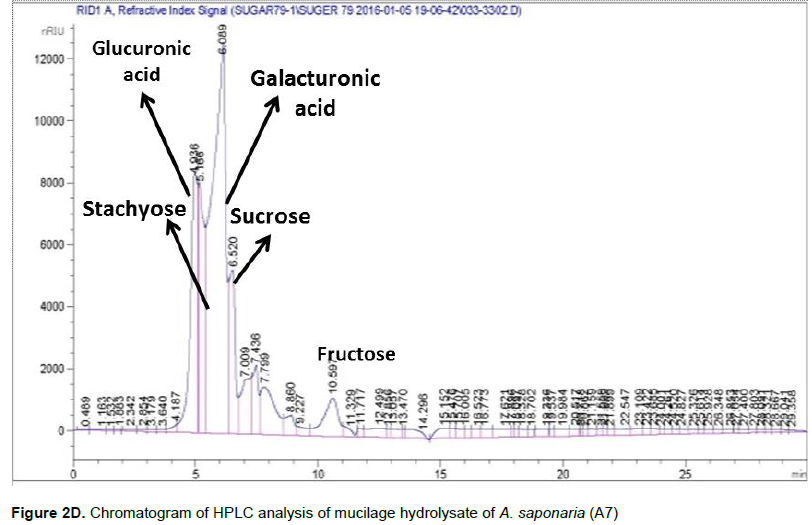
HPLC analysis of the polysaccharides (Table 1) revealed that their composition is more or less similar qualitatively, except for the low detection of stachyose in A1 and A5. While, maltose greatly presented as (2.8 and 2.5%) only in A2 and A7 respectively. On the other hand, the eight samples differ greatly quantitatively in the ratios of the different sugars. Quantitative HPLC analysis revealed that A. saponaria (A7) mucilage hydrolysate contained mainly glucuronic acid, stachyose, galacturonic acid, maltose and glucose in ratio 2.0, 2.4, 1.6, 2.5 and 0.5 % respectively (Figure 2D). A. vera (A1) mucilage hydrolysate contained mainly glucuronic acid, stachyose, galactouronic acid, xylose and galactose in the ratio 1.0, 1.5, 4.4, 0.1 and 0.2% respectively. Glucuronic acid was the dominant sugar in all Aloe species investigated ranging from 486 to 20070 mg kg-1 other quantitatively important saccharides were galactouronic acid stachyose, sucrose, maltose, galactose and glucose.
As many species of Aloe such like A. vera was reported to cause hypoglycemic effects (Ghannam et al., 1986; Ajabnoor, 1990), it was felt that it would be interesting to study the influence of polysaccharides of different Aloe species A. vera, A. arborescens, A. eru, A. grandidentata, A. perfoliata, A. brevifolia, A. saponaria and A. ferox on diabetic rats model that was induced by intraperitoneal injection with alloxan. So, the aim of our study was to shed the light on the antidiabetic effect of the isolated polysaccharide of the eight species under investigation and evaluate their ability to inhibit alpha-glucosidae activity. Although some of the investigated species were reported to have antidiabetic effects such as A. vera and A. arborescens the other species were not tested for this effect.
Oral administration of polysaccharide samples isolated form the eight studied Aloe species (250 mg kg-1 body weight) reduced the blood glucose levels starting from week 2 and continued to week 4 (Table 2). Moreover, the effect of Aloe vera and A. barbadensis was the most pronounced as they induced 40 and 44% reduction in glucose levels, respectively. This was in agreement with the reported literature (Ghannam et al., 1986; Ajabnoor, 1990). Standard samples, glucuronic acid, stachyose, galacturonic acid and mixture of these compounds at a dose of 50 mg kg-1 body weight were exhibited anti diabetic activity, reduced the blood glucose level by 22, 30, 23 and 24% respectively. From results presented (Table 2), synergistic effects of all sugar components of mucilage that isolated from different Aloe species as a hypoglycemic agents (to reduce the blood glucose level) was prevailed.
Our in vitro studies, had shown that the polysaccharide samples isolated form the eight studied Aloe species had moderate inhibitory activities on the alpha -glucosidase enzyme (Table 3), thus they can be used as useful functional foods according to their association with reduced risk of diabetes.
Type 2 diabetes mellitus is a complex disease (Leahy,2005), which is characterized by abnormal hepatic glucose output, insulin resistance and impaired insulin production (Golay, 1988; Fujimoto, 2000). It may be assumed that in individuals with type 2 diabetes, many metabolic pathways are likely to be affected and presumably play a role in their overall metabolic dysfunction.
Thus, the identification of new biomarkers and pathways can improve the characterization of pathophysiological alterations associated with the disease condition (Bain et al., 2009). In non-treated mature-onset diabetics or most subjects with diabetic coma, basal glucuronic acid excretion is reduced (Fishman, 2014). Non treated diabetic patients have been demonstrated to have a reduced glucuronidation capacity after a menthol load, a finding related to the diminished glycogen content of the liver (Fishman, 2014). The glucuronidation capacity is actually reduced in diabetic patients (Muller-Oerlinghausen et al., 1967).
Glucuronidation is the addition of glucouronic acid to a xenobiotic such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids and fatty acid derivatives. The human body uses glucuronidation to make xenobiotics and their metabolites more water-soluble, and, in this way, allow for their subsequent elimination from the body through urine or feces (via bile from the liver) (King et al., 2000). However, glucuronides may be hydrolyzed by β-glucouronidase present in intestinal microflora to the respective aglycone, which may be reabsorbed from the intestine and translocated back to the liver. On the other hand, glucuronic acid is a precursor of ascorbic acid as well as a powerful detoxifier this, binds the toxins entering the liver and eliminates them out of the body via kidneys.
Mucilages are classified as soluble fibers which imbibe water and swell to form gel or colloidal gel. This solution increases the viscosity of the intestinal content and reduces absorption and entraps sugars (Kaczmarczyk et al., 2012; Lattimer and Haub, 2010). Mucilage is not hydrolyzed in intestine to give their constituent sugar rather they pass the intestine intact, absorbs water and swell; the effect which adds bulk to the stool (Satija and Hu, 2012). Partial hydrolysis of plant polysaccharides by intestinal bacteria and subsequent release of glucuronic acid in large intestine may occur; however, kinetic studies show that plant polysaccharides could serve as potential glucuronic acid source. Benefits of plant polysaccharides for diabetic patients are enumerated in many publications. Previous studies reported that polysaccharides could reduce serum lipid in hyperglycemic rats through its antioxidant properties (Xu et al., 2010) and could have antioxidant and antihyperglycemic effects on diabetes mellitus induced by alloxan in rats (Long et al., 2012). In addition, polysaccharides could partially recover the secretory function of islet cells by antioxidant effects. Polysaccharides have antihyperglycemic effect in type 2 Diabetes mellitus and may partially recover the secretary function of islet cells, leading to elevated serum levels of insulin and amylin and improved glucose metabolism regulation (Li et al., 2012).
Postprandial hyperglycemia is the key problem in diabetes mellitus. Ingestion of carbohydrate rich diet causes elevation in blood glucose level by the rapid absorption of carbohydrates in the intestine aided by the action of glycoside hydrolysis which breaks complex carbohydrates into absorbable monosaccharides (Winchester and Flee, 1992).
Thus, use of glycosidase inhibitor such as alpha-glucosidase inhibitors would be a prospective therapeutic agent for the effective management of diabetes. Alpha-glucosidase inhibitors inhibit the disaccharide digestion and impede the postprandial glucose excursion to enable overall smooth glucose profile (Casirola and Ferraris, 2006). Several alpha-glucosidase inhibitors have been isolated from medicinal plants to develop as an alternative drug with increased potency and lesser adverse effects than the existing drugs (Toeller, 1994). Our in vitro studies, had shown that the polysaccharide samples isolated form the eight studied Aloe species had some inhibitory activities on the alpha-glucosidase enzyme (Table 3). They could act by impeding the binding of the substrate to the enzyme. This will reduce hydrolysis of disaccharides and oligosaccharides present in food, which in turn will reduce the amount of free sugars absorbed, consequently, reduces postprandial increase in blood glucose.
Glucuronic acid, and galacturonic acid the major identified sugar acids in all polysaccharide samples of Aloe species could be attributed to the observed anti hyperglycemic effect. Polysaccharides constituents of eight Aloe species have synergistic in vivo anti hyperglycemic activity in alloxan-induced diabetic rats. Moreover, they have moderate alpha-glucosidase inhibitory activity. Therefore, these plant polysaccharides can be used as useful functional foods to control elevated blood glucose levels in diabetic patients. Traditional anti-diabetic plants might provide new antidiabeticcompounds, which can counter the high cost and poor availability of the current medicines for many rural populations in developing counties (Tanak et al., 2006).
The authors have not declared any conflict of interest.
The authors sincerely thank Professor Dr. Mohamed El-Gebaly, Botany Specialist for plants authentication and Dr. Amany Amin Sleem Professor of Pharmacology, National Research Centre, Dokki, Cairo, Egypt for carrying out the pharmacological study.
REFERENCES
|
Agarwal OP (1985). Prevention of atheromatous heart disease. Angiology 36:485-492.
Crossref
|
|
|
|
Ajabnoor MA (1990). Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. J. Ethnopharmacol. 28:215-220.
Crossref
|
|
|
|
|
Akinmoladun AC, Akinloye O (2007). Prevention of the onset of hyperglycaemia by extracts of Aloe barbadensis in rabbits treated with alloxan. Afr. J. Biotechnol. 6:1028-1030.
|
|
|
|
|
American Diabetes Association (ADA) (2009). Diagnosis and Classification of Diabetes Mellitus. Diabet. Care 32:S62-S67.
Crossref
|
|
|
|
|
Bailey LJ, Day C (1989). Traditional plant medicine as treatment for diabetes. Diabet. Care 12:553-564.
Crossref
|
|
|
|
|
Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newqard CB (2009). Metabolomics applied to diabetes research: Moving from information to knowledge. Diabetes 58:2429-2443.
Crossref
|
|
|
|
|
Beppu H, NagamuraY, Fujita K (1993). Hypoglycaemic and antidiabetic effects in mice of Aloe arborescens Miller var. natalensis Berger. Phytother. Res. 7:S37-S42.
Crossref
|
|
|
|
|
Beppu H, Shimpo K, Chihara T, Kaneko T, Tamai I, Yamaji S, Ozaki S, Kuzuya H, Sonoda S (2006). Antidiabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice: Investigation on hypoglycemic action and systemic absorption dynamics of aloe components. J. Ethnopharmacol.103:468-477.
Crossref
|
|
|
|
|
Bhaludra CSS, Bethapudi R R, Murugulla AC, Pullagummi C, Latha T, Venkatesh K, Bheemagani AJ, Pudutha A, and Rani AR (2013). Cultivation, phytochemical studies, biological activities and medicinal uses of Aloe ferox, grandfather of Aloes an important amazing medicinal plant. Int. J. Pharmacol. 9:405-415.
Crossref
|
|
|
|
|
Boudreau MD, Beland FA (2006). An evaluation of the biological and toxicological properties of Aloe Barbadensis (Miller), Aloe Vera. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 24(1):103-54.
Crossref
|
|
|
|
|
Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, Chokechaijaroenporn O (1996). Antidiabetic activity of Aloe vera L. juice. II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine 3:245-248.
Crossref
|
|
|
|
|
Casirola DM, Ferraris RP (2006). Alpha-Glucosidase inhibitors prevent dietinduced increases in intestinal sugar transport in diabetic mice. Metabolism 55:832-841.
Crossref
|
|
|
|
|
Chun-hui L, Chang-hai W, Zhi-liang X, Yi W (2007). Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 42:961-970.
Crossref
|
|
|
|
|
Clifford SC, Arudt SK, Popp M, Jones HG (2002). Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): Localization, composition and physiological roles during droughtâ€stress. Exp. Bot. 53:131-138. Córdova V, Barriguete M, Lara E, Barquera S, Rosas P, Hernández A, León M, Aguilar S (2008). Las enfermedadescrónicas no trasmisibles en México: Synopsis epidemiológicay prevención integral. Salud Pública México 5:419-427.
|
|
|
|
|
Davis RH (1997). Aloe vera: A scientific approach. New York: Vantage Press.
|
|
|
|
|
Eliasson SG, Samet JM (1969). Alloxan induced neuropathies: Lipid changes in nerve and root fragments. Life Sci. 8:493-498.
Crossref
|
|
|
|
|
Ezuruike UF, Prieto JM (2014). The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 155:857-924.
Crossref
|
|
|
|
|
Fishman WH (2014). Metabolic conjugation and metabolic hydrolysis - Vol. 1 Academic press. P 220.
|
|
|
|
|
Franz G (1989). Polysaccharides in pharmacy: Current applications and future concepts. Planta Med. 55:493-497.
Crossref
|
|
|
|
|
Fujimoto WY (2000). The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am. J. Med. 108:Suppl 6a9S-14S.
|
|
|
|
|
Gbolade AA (2009). Inventry of antidiabetic plants in selected districts of Lagos State, Nigeria. J. Ethnopharmacol. 121:135-139.
Crossref
|
|
|
|
|
Ghannam N, Geissman ES (1986). The anti-diabetic activity of Aloes, preliminary clinical and experimental observations. Horm. Res. 24:288-294.
Crossref
|
|
|
|
|
Ghannam N, Kingston M, Al-Meshaal IA, Tariq M, Parman NS, Woodhouse N (1986). The antidiabetic activity of Aloes. Preliminary clinical and experimental observations. Horm. Res. 24:288-294.
Crossref
|
|
|
|
|
Ghorbani A (2013). Best herbs for managing diabetes: A review of clinical studies. Braz. J. Pharm. Sci. 49(3):413-22.
Crossref
|
|
|
|
|
Golay A, Felber JP, Jequier E, DeFronzo RA, Ferrannini E (1988). Metabolic basis of obesity and noninsulin-dependent diabetes mellitus. Diabet. Metab. Rev. 4:727-747.
Crossref
|
|
|
|
|
Gertz CH (1990). HPLC Tips and Tricks. Great Britain, Oxford, P 608.
|
|
|
|
|
Grindlay D, Reynolds T (1986). The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J. Ethnopharmacol. 16:117-151.
Crossref
|
|
|
|
|
Heggars JP, Pelly RP, Robson MC (1993). Beneficial effect of Aloe in wound healing. Phytother. Res. 7:48-52.
Crossref
|
|
|
|
|
International Diabetes Federation (2013). IDF Diabetes Atlas, 6th edition. Brussels. Available at: http://www.idf.org/diabetesatlas.
|
|
|
|
|
Kaczmarczyk MM, Miller M J, Freund GG(2012). The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes, cardiovascular disease and colon cancer. Metabolism 61:1058-1066.
Crossref
|
|
|
|
|
Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, Pillai KK (2012). A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied Sci. 4(1):27-42.
Crossref
|
|
|
|
|
King C, Rios G, Green M, Tephly T (2000). Current Drug Metabolism. "UDP-glucuronosyltransferases". Curr. Drug Metab. 1(2):143-61.
Crossref
|
|
|
|
|
Klautzer L, Becker J, Mattke S (2014). The curse of wealth - Middle Eastern countries need to address the rapidly rising burden of diabetes. Int. J. Health Pol. Manag. 2(3):109-114.
Crossref
|
|
|
|
|
Lattimer JM, Haub MD (2010). Effects of dietary fiber and its components on metabolic health. Nutrients 2:1266-1289.
Crossref
|
|
|
|
|
Leahy JL (2005). Pathogenesis of type 2 diabetes mellitus. Arch. Med. Res. 36:197-209.
Crossref
|
|
|
|
|
Li T, Zhang XD, Song YW, Liu JW (2005). A Microplate-Based Screening Method for α-Glucosidase Inhibitors. Nat. Prod. Res. Dev. 10:1128-1134.
|
|
|
|
|
Li X, Yu Z, Long S, Guo Y, Duan D (2012). Hypoglycemic effect of Laminaria japonica polysaccharide in diabetes mellitus mouse model. ISRN Endocrinol. 4p.
Crossref
|
|
|
|
|
Long S-H, Yu Z-Q, Shuai L, Guo Y-L, Duan D-L, Xu X-Y, Li X-D (2012). The hypoglycemic effect of the kelp on diabetes mellitus model induced by alloxan in rats. Int. J. Mol. Sci. 13(3):3354-3365.
Crossref
|
|
|
|
|
Metelko Z, Brkljacic N (2013). Prevention of diabetic foot. Acta Med. Croat. 67:35-44.
|
|
|
|
|
Muller-Oerlinghausen B, Hasselblatt A, Jahns R (1967). Impaired hepatic synthesis of glucuronic acid conjugates in diabetic rats. Life Sci. 6(14):1529-1533.
Crossref
|
|
|
|
|
Ni Y, Turner D, Yates KM, Tizard I (2004). Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. Immunopharmacol. 4(14):1745-55.
Crossref
|
|
|
|
|
Nwanjo HU (2006). Antioxidant activity of the exudate from Aloe barbadensis leaves in diabetic rats. Biokemistri 18:77-81.
|
|
|
|
|
Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S (2011). Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 3(4):504-512.
Crossref
|
|
|
|
|
Park Y, Lee S (2006). New Perspectives on Aloe. New York: Springer Verlag. pp. 1-5.
|
|
|
|
|
Perez YY, Jiménez-Ferrer E, Zamilpa A, Hernández-Valencia M, Alarcón-Aguilar FJ, Tortoriello J, Román-Ramos R (2007). Effect of a polyphenol-rich extract from Aloe vera gel on experimentally induced insulin resistance in mice. Am. J. Chin. Med. 35:1037-1046.
Crossref
|
|
|
|
|
Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S (2006). Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin. Exp. Pharmacol. Physiol. 33:232-237.
Crossref
|
|
|
|
|
Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S (2004). Hypoglycemic effect of Aloe vera gel on streptozo- tocin-induced diabetes in experimental rats. J. Med. Food 7:6166.
Crossref
|
|
|
|
|
Rajasekaran S, Sivagnanam K, Subramanian S (2005). Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J. Pharm. Pharmacol. 57:241-246.
Crossref
|
|
|
|
|
Satija A, Hu F (2012). Cardiovascular Benefits of Dietary Fiber. Curr. Atheroscler. Rep. 14:505-514.
Crossref
|
|
|
|
|
Sherif S, Sumpio BE (2015). Economic development and diabetes prevalence in MENA countries: Egypt and Saudi Arabia comparison. World J. Diabet. 6(2):304-311.
Crossref
|
|
|
|
|
Tanak M, Misawa E, Ito Y, Habara N, Nomaquchi K, Yamada M,Toida T, Hayasawa H,Takase M, Inaqaki M, Higuchi R (2006). Identification of five phytosterols from Aloe vera gel as antidiabetic compounds. Biol. Pharm. Bull. 29(7):1418-1422.
Crossref
|
|
|
|
|
Toeller M (1994). Alpha-Glucosidase inhibitors in diabetes: Efficacy in NIDDM subjects. Eur. J. Clin. Investig. 24:31-35.
Crossref
|
|
|
|
|
Trinder P (1969). Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 22(2):158-161.
Crossref
|
|
|
|
|
Ulbricht C, Armstrong J, Basch E. Basch S, Bent S, Dacey C, Dalton S, Foppa I, Giese N, Hammerness P, Kirkwood C, Sollars D, Tanguay-Colucci S, Weissner W (2008). An evidence-based systematic review of Aloe vera by the Natural Standard Research Collaboration. J. Herb Pharmacother. 7:279-323.
Crossref
|
|
|
|
|
Winchester B, Fleet GW (1992). Amino-sugar glycosidase inhibitors: versatile tools for glycobiologists. Glycobiology 2:199-210.
Crossref
|
|
|
|
|
Xu X Y, Qin L H, Guo Y L, Luan L J (2010). Effects of Laminaria japonica polysaccharide to reduce serum lipids and anti-inflammation in hyperlipenmia rats. Acta Anat. Sin. 41(5):693-697.
|
|
|
|
|
Yongchaiyudha S, Rungpitarangsi V, Bunyapraphatsara N, Chokechaijaroenporn O (1996). Antidiabetic activity of Aloe vera L. juice.I. Clinical trial in new cases of diabetes mellitus. Phytomedicine 3(3):241-243.
Crossref
|
|
|
|
|
Zimmet P, Alberti KGMM, Shaw J (2001). Global and societal implications of the diabetes epidemic. Nature 414:782-787.
Crossref
|
|