ABSTRACT
The aim of this study was to investigate the immunomodulatory activities of protein fractions isolated from Delphinium staphysagria seeds used in Moroccan traditional medicine. D. staphysagria protein extract has been separated on sephadex column chromatography. The immunomodulatory activities were tested using primary culture of rabbit lymphocytes (splenocytes and thymocytes), by evaluation of macrophage phagocytosis and by studying the hemagglutination effect of proteins separated by chromatography. Three protein fractions were isolated on sephadex column. At 0.1mg/ml, these fractions didn’t show any cytotoxic effect against lymphocytes. The fraction F1 with the high molecular weight of 115 kDa exhibited a mitogenic action on splenocytes and positive hemagglutinating activity associated to inhibition of macrophage phagocytosis capacity. The second protein fraction F2 was about 11 kDa inhibited phagocytosis without affecting lymphocytes proliferation. The third fraction F3 was the smallest with 2.25 kDa indicated a stimulatory effect on both splenocytes and thymocytes proliferation without hemagglutinating activity but inducing decrease of macrophage phagocytosis. In conclusion, protein fractions isolated from D. staphysagria have no cytotoxic effect. The fraction F3 acted as an immunostimulating by enhancing proliferation of thymocytes and splenocytes while the F1 stimulated preferentially splenocytes via probably lectin pathway. All fractions studied decreased phagocytosis impairing the antigen presentation to lymphocytes.
Key words: Delphinium staphysagria, protein fractions, immunomodulation, mitogenic effect, hemagglutination, MTT assay.
A number of disorders such as viral infections, various cancers and autoimmune diseases can be managed with immunomodulators drugs from medicinal plants (Patwardhan et al., 1990; Patil et al., 2012). Delphinium plants are a large species within the family Renonculaceae, largely distributed throughout the northern hemisphere region, such as Asia, Europe, and North America, while a few occur in equatorial Africa (Tang and Feng, 1981). Delphinium, an important genus of the family Ranunculaceae, is well known for its potential uses in medicine (Benn and Jacyno, 1983). It’s recognized as a rich source of biologically active and structurally complex diterpenoid and norditerpenoid alkaloids with febrifuge, sedative, cardiotonic and analgesic activities (Riedle and Nasir, 1991; Raza et al., 2001) .
The traditional medicinal system in Morocco describes certain plants which strengthen the immune system (Bellakhdar, 1997, 2008; Merzouki et al., 1997). Delphinium staphysagria seeds are also used for toothaches, hair toning and reducing hair loss (Merzouki et al., 2000). Fraidi et al. (2013, 2014) have shown that D. staphysagria seeds are a promising source of potential antioxidant, analgesic and anti-inflammatory activity. In Morocco, many medicinal plants have been studied, and they have shown significant immunomodulatory activities (Daoudi et al., 2008, 2012, 2013; El Hamsas et al., 2012) which can be used in the treatment of various ailments. Thus, the aim of this study was to evaluate the effect of protein fractions isolated from D. staphysagria seeds on cell immunity by evaluating the proliferation of lymphocytes, splenocytes and macrophages phagocytosis.
All chemicals products used in this study were purchased from Sigma (St Louis, MO, USA).
Animal
Male rabbits weighing 2.5 kg were housed under a 12 h light/dark cycle in a temperature-controlled room (22 to 24°C) and used for in vitro investigations. Rabbits had free access to standard chow and water. The animal experiments were used according to national ethical laws.
Plant materiel and extraction
The plant materials selected for this study were D. staphysagria seeds, which were bought from herbalists in the Fez Bouleman region. First, the plant seeds were washed twice with distilled water, air dried, and ground into a fine powder. Then 10 g were dissolved in 100 ml of phosphate buffered saline (PBS, 150 mM, pH 7.4) and stirred during two hours. The suspension was centrifuged (15 min at 4500 rpm, 4°C) and the supernatant was sterilized by filtration through 0.45 µm nitrocellulose filters. Total protein extract was prepared from the aqueous extract after precipitation by the addition of cold ammonium sulfate at 40%.
Sephadex-chromatography column
To characterize the extracted proteins of D. staphysagria seeds for their immunomodulatory effect, a chromatochraphic separation of protein extract was applied followed by a study of the activity of different purified fractions. Total protein extract was dialyzed, and then separated on sephadex (G-100) chromatography column (40 × 2 cm). Elution was performed by PBS at rate of 120 ml per hour. Fractions of 2 ml were collected and their protein concentration was determined by measuring absorbance at 280 nm.
Cell material and culture
Cell suspensions used in this study were obtained from sacrificed rabbit. The spleen and thymus were removed aseptically from the animals and then suspension was prepared by pressing the organs through a fine wire mesh. The cell suspension was washed by centrifugation repeated in RPMI medium and the red blood cells were lysed by 154 mM ammonium chloride. The number of viable cells was determined microscopically by a trypan blue exclusion test. The culture used RPMI medium (without glucose) supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 10% (v/v) FCS (fetal calf serum) and antibiotics (ampicillin 100 U/ml and streptomycin 100 mg/ml).
Phagocytosis test
The phagocytosis test was realized as notified by EL Hamsas et al. (2010b). Macrophages were obtained from spleen cell preparation. Briefly, 100 µl of spleen cells suspension at 106 cells/ml was added in 96 well plates that were incubated at 37°C for 3 h for adherence of macrophages. Thereafter, supernatant was removed and every well-plate was washed twice with sterile PBS. The phagocytosis test was conducted using neutral red as indicator of macrophage phagocytosis activity. In every well-plate, 100 µl of RPMI (with 10% (v/v) FCS Fetal Calf serum) containing 0.075% of neutral red and 10 µl of plant extracts (or PBS in the blank) were added and then plates were incubated for 2 h. Finally, after removing the supernatant and washing them three times, the reaction was stopped with a solution containing acetic acid (1M) /ethanol (1:1 v/v). Macrophage phagocytosis activity was evaluated by measuring absorbance at 540 nm.
Cell proliferation assay
Cell proliferation was evaluated by the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetra-zolium bromide (MTT) assay (Mossman, 1983; Daoudi et al., 2008; EL Hamsas et al., 2010a). Cell suspension was plated at 5000 cells/well in 96 well plates and incubated at 37°C in a humidified incubator, under an atmosphere of 95% air and 5% CO2 for 72 h. Protein fractions were added to cells before their incubation at the concentration of 0.1 mg/ml. Thereafter, 10 µl of MTT solution (5 mg/ml in PBS) was added. After 3 h of incubation, the formazan formed in cells was solubilized by 100 µl of dimethyl sulfoxide (DMSO). The optical density was measured at 570 nm.
Hemagglutination assay
The erythrocytes were isolated from the rabbits. The blood was collected from the neck of the animals after sacrification. The erythrocytes cells were obtained from successive washes of the blood with 0.9% NaCl. Then, erythrocyte suspension was adjusted to 2% (v/v) in 0.9% NaCl. Hemagglutination assay was performed using the method of Kabat and Mayer (1961). Assays were performed in 96 wells plates. Hemagglutination was monitored macroscopically and confirmed microscopically. The experiments were performed by mixing 25 μl of the erythrocyte suspension with 25 μl of protein fractions and 50μl of PBS in total volume of 100 μl. Positive control was performed using concanavaline-A at 7 μg/ml. In all groups, the wells were carried out in triplicate and incubated for a period of 30 min at 37°C.
Statistical analysis
Each experimental condition was realized at least in triplicate (n = 3). Data were expressed as the mean ± SEM. Statistical analysis was carried out using student’s t-test. Differences were considered statistically significant at p<0.05.
Total protein extract obtained from aqueous extract after ammonium sulfate precipitation at 40% was separated on Sephadex (G100) chromatography column. As shown in Figure 1, three fractions (peaks) were obtained. The molecular weight of fractions F1, F2 and F3 were about 115 KDa, 11kDa and 2.25 kDa, respectively. The effect of these three fractions was tested on lymphocytes proliferation (splenocytes and thymocytes), on macrophage phagocytosis activity and for agglutination of blood cells. Cells proliferation was evaluated by MTT assay and phagocytosis action by neutral red assay.
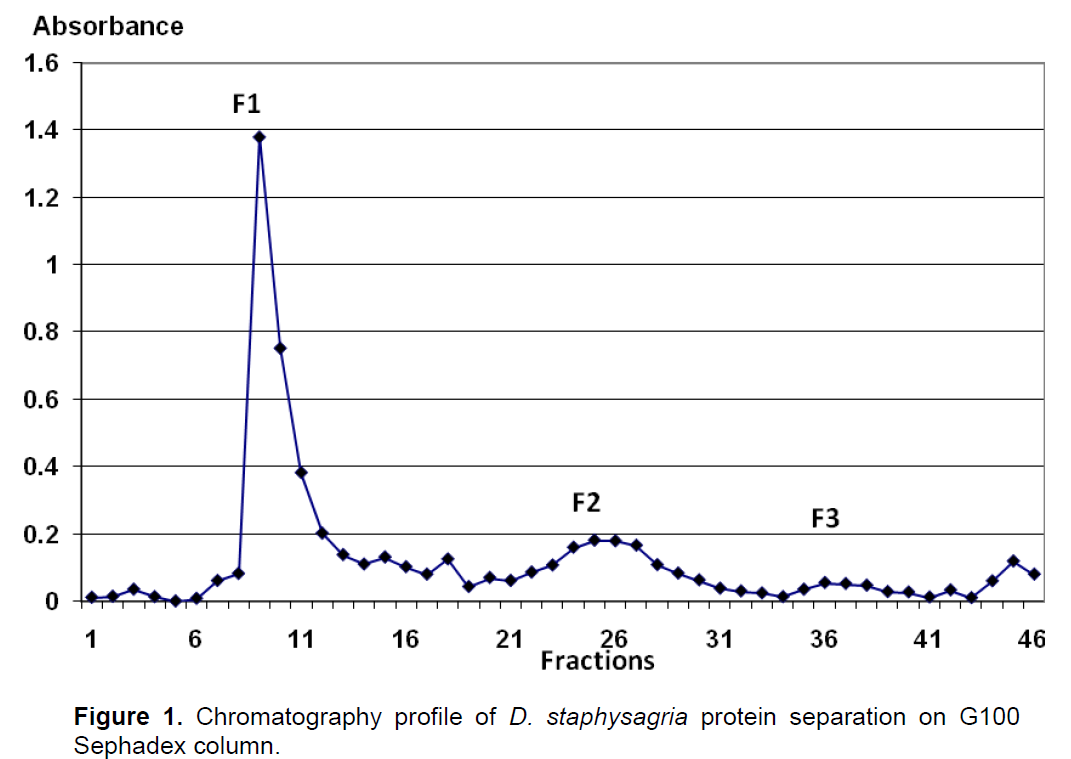
Effect of protein fractions on lymphocytes proliferation
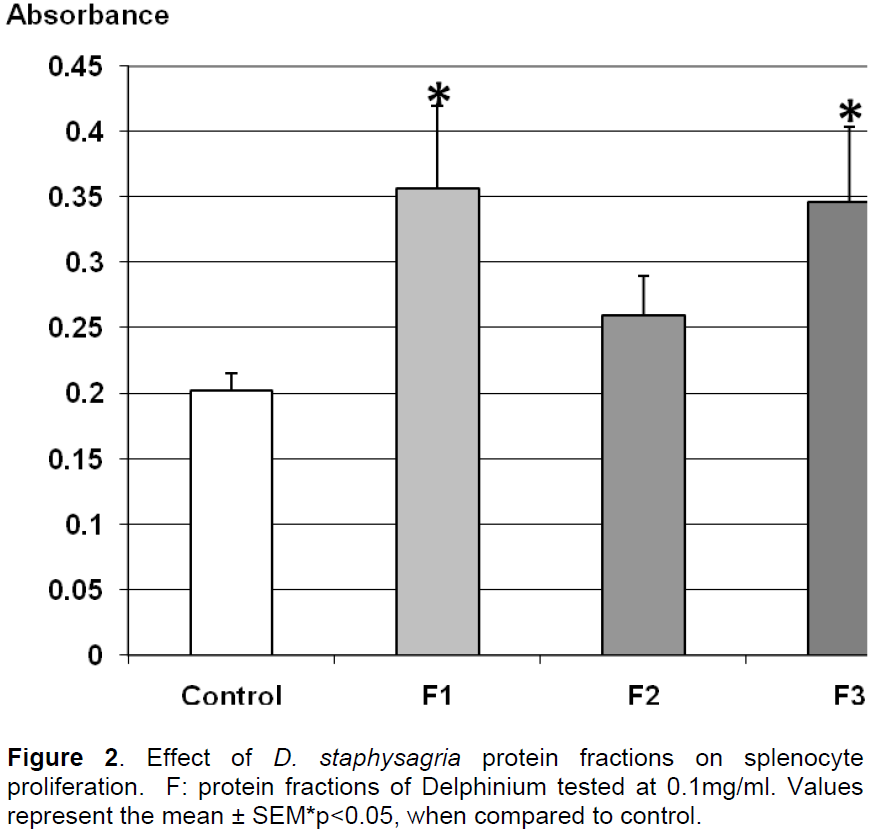
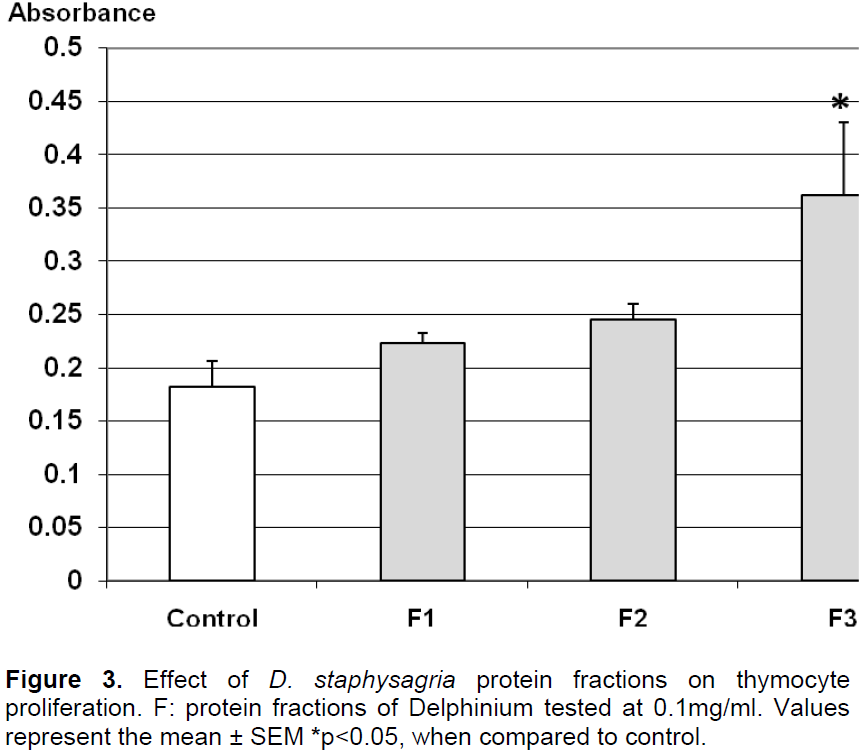
Results obtained in Figure 2 indicate that the three fractions at 100 μg/ml showed no cytotoxicity. In fact, the proliferation of splenocytes was increased under the protein fractions F1 and F3 indicating a mitogenic effect by stimulating splenocytes proliferation of over 40% compared to control, while F2 does not significantly alter this proliferation. According to Figure 3, the number of thymocytes had significantly raised in the presence of the fraction F3 by 50%. In the same conditions, F1 and F2 didn’t significantly alter the proliferation of thymocytes. These results indicated a specific action of F1 on splenocytes proliferation and combined action of F3 on both splenocytes and thymocytes.
Effect on phagocytosis
The results of protein fractions effect on macrophage phagocytosis are presented in Figure 4. The fractions showed a decrease in phagocytosis activity in comparison to control, the phagocytosis activity of macrophages was significantly decreased by 49, 47 and 34%, respectively under the effect of F2, F3 and F1.
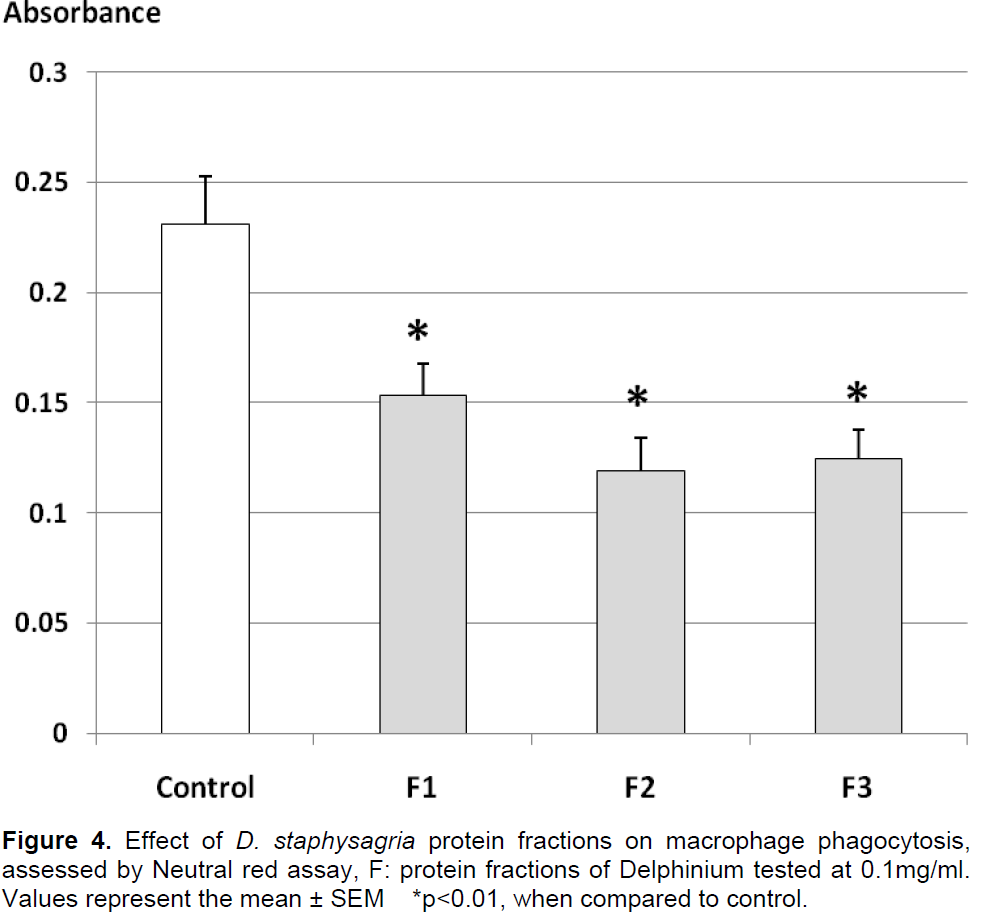
These results indicated a strong inhibitory effect of protein fractions on macrophage phagocytosis activity.
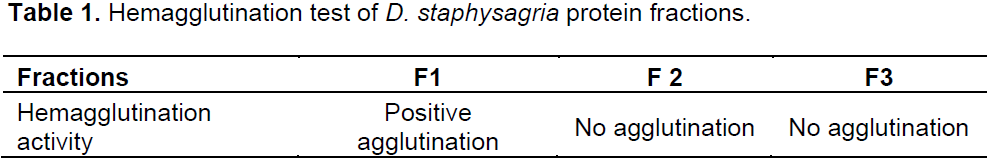
Hemagglutination test
The hemagglutination test was performed with protein fractions using Concanavalin-A as the positive control.
Results represented in Table 1 show the absence of hemagglutination on cells treated by F2 and F3 fractions, where F1 gives a positive agglutination activity. At high concentration of protein extract more than 0.25mg/ml, we have observed a lowest hemolytic effect.
The aim of this study was to explore the immuno-modulating activity of proteins fractions isolated from D. staphysagria seeds. The protein fractions of the plant were tested on rabbit lymphocyte proliferation, on macrophage phagocytosis and for their hemagglutination activities.
The results indicate that the protein fractions of D. staphysagria at 0.1 mg/ml didn’t affect splenocytes and thymocytes viability. This finding completes the study previous results which demonstrated that aqueous and total protein extracts have non cytotoxic effect on lymphocytes (Daoudi et al., 2008, 2012). This suggests that the plant had no cytotoxic effect at the dose used. The toxicity effect of D. staphysagria described by several authors (Benn and Jacyno, 1983; Marin et al., 2011; Wiese, 2013; Faridi et al., 2014a) was probably due to the higher plant concentration as observed during studying acute toxicity indicating for ethanol extract DL50 of 300 mg/kg (Faridi et al., 2014b). This later was confirmed by a low hemolytic action of protein extract observed in vitro at the high concentration.
Furthermore, the study noticed an increase in the proliferative activity of splenocytes and thymocytes, which indicate an action of the extract on both humoral and cellular immunity reactions. This mitogenic effect was observed under F1 fractions on splenocytes and with F3 on splenocytes and thymocytes. This suggests an important mitogenic effect of F3 fraction on both lymphocytes where F1 affect splenocytes indicating an action on B-lymphocytes probably. The mitogenic effects of F1 and F3 could be considered as an immunostimulating action to enhance immune defenses. Furthermore, a slight inhibition of macrophage phagocytosis capacity was observed indicating that antigen presentation may be impaired partially by these fractions. On the other hand, the study investigated the agglutination activity of the protein fractions against rabbit erythrocytes.
The study results showed that F2 and F3 didn’t agglutinate rabbit erythrocytes, whereas F1 of 115 kDa showed an agglutination effect suggesting a lectin aspect of this high protein fraction. This later fraction increased proliferation of splenocytes indicating that the proliferative effect of this fraction is probably mediated by a mechanism including lectin pathway. The fraction F3 also has a mitogenic action on thymocytes and splenocytes but without showing any agglutination activity. The mechanism of this mitogenic effect was certainly not related to lectins.
Three protein fractions (F1, F2 and F3) were isolated from D. staphysagria seeds with no cytotoxic effect at 0.1mg/ml on lymphocytes. A mitogenic effect of F3 was observed on both lymphocytes indicating possible use of this fraction to enhance lymphocytes proliferation, while F1 seems to preferentially stimulate proliferation of B-lymphocytes with impairing antigen presentation by macrophages, which indicate possible use of this fraction to stimulate humoral immunity.
The authors have not declared any conflict of interests.
REFERENCES
|
Bellakhdar J (1997). Moroccan traditional medicine, In: Ibis Press (Eds), France. P 112.
|
|
|
|
Bellakhdar J (2008). Medicinal plants in the Maghreb and basic care for specific modern herbal medicine, In: The Fennec (Eds) Handbook of Modern Phytotherapy. Casablanca. P 186.
|
|
|
|
|
Benn MH, Jacyno JM (1983). The toxicology and pharmacology of the diterpenoid alkaloids: In Alkaloids; Chemical and Biological Perspectives (SW Pelletier, Ed), John Wiley & sons, New York, USA. P 153.
|
|
|
|
|
Daoudi A, Abdel-Satter E, Aarab L (2013). The relationship between lectin compounds and immunomodulatory activities of protein extracted from plants. J. Plant Stud. 3:56-64.
Crossref
|
|
|
|
|
Daoudi A, Aarab L, Abdel-Satter E (2012). Screening of immunomodulatory activity of total and protein extracts of some Moroccan medicinal plants. Toxicol. Ind. Health 29(3):245-253.
Crossref
|
|
|
|
|
Daoudi A, Benbouker H, Bousta D, Aarab L (2008). Screening of fourteen Moroccan medecinel plants for immunomodulating activities. Moroc. J. Biol. 5:24-30.
|
|
|
|
|
EL Hamsas El Youbi A, Ouahidi I, Aarab L (2012). In vitro immunomodulation effects of the aqueous and protein extracts of Berberis hispanica Boiss and Reut. J. Med. Plant Res. 6(25):4239-4246.
|
|
|
|
|
EL Hamsas El Youbi A, Bousta D, Ouahidi I, Aarab L (2010a). Antidepressant effects, antinociceptive and immunomodulatory protein and aqueous extracts of Berberis hispanica Boiss and Reut. Moroc. Phytother. 9:25-32.
|
|
|
|
|
EL Hamsas El Youbi A, Bousta D, Ouahidi I, Aarab L (2010b). Primary pharmacological screening of an endemic plant from the Southern Morocco (Tetraena gaetula [Emb. & Maire] Beier & Thulin). C. R. Biol. 333(10):736-43.
Crossref
|
|
|
|
|
Faridi B, Alaoui K, Alnamer R, Cherrah Y, Zellou A (2013). Analgesic activity of ethanolic and alkaloidic extracts of Delphinium staphysagria seed. Int. J. Univ. Pharm. BioSci. 2(5):102-112.
|
|
|
|
|
Faridi B, Doudach L, Alnamer R, Faouzi MA, Tahiri F, Cherrah Y, Zellou A (2014a). In vitro cytotoxicity and free radical scavenging activity of ethanolic and alkaioidic extracts of Delphinium staphysagria. Int. J. Pharm. 4(1):7-12.
|
|
|
|
|
Faridi B, Zellou A, Touati D, Alaoui K, Cherrah Y (2014b). Toxicité aigue et activité anti-inflammatoire des graines de Delphinium staphisagria. Phytothérapie 12:175-180.
Crossref
|
|
|
|
|
Marin C, Ramírez-Macías I, López-Céspedes A, Olmo F, Villegas N, Díaz JG, Rosales MJ, Gutiérrez-Sánchez R, Sánchez-Moreno M (2011). Delphinium staphisagria against chagas disease. J. Nat. Prod. 74(4):744-50.
Crossref
|
|
|
|
|
Kabat EA, Mayer MM (1961). Experimental Immunochemistry, 2nd Ed, In Charles C.Thomas, Springfield, IL. pp. 149-153.
|
|
|
|
|
Merzouki A, Ed-derfoufi F, Molero Messa J (2000). Contribution to the knowledge of Rifan traditional medicine II: Folk medecine in Ksar Lakbir district (NW Morocco). Fitoterapia 71(3):278-307.
Crossref
|
|
|
|
|
Mossman T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Method 65:55-63.
Crossref
|
|
|
|
|
Patil US, Jaydeokar AV, Bandawane DD (2012). Immunomodulators: A pharmacological review. Int. J. Pharm. Sci. 4(1):30-36.
|
|
|
|
|
Patwardhan B, Kalbag D, Patki, PS, Nagsampagi BA (1990). Search of immunomodulatory agents. Indian Drugs 28:56-63.
|
|
|
|
|
Raza M, Shaheen F, Choudhary MI, Suria A, Atta-UR, Sombati S, Delorenzo RJ (2001). Anticonvulsant activities of the fs-1 subfraction isolated from roots of Delphinium denudatum. Phytother. Res. 15:426-430.
Crossref
|
|
|
|
|
Riedle H, Nasir YJ (1991). Flora of West Pakistan (Ali SI, Nasir YJ, Eds.), National Foundation, Times Press, Karachi, Pakistan, P 41.
|
|
|
|
|
Tang XC, Feng J (1981). Analgesic actions and local anesthetic activity of 3-acetylaconitine hydrobromide. Acta Pharmacol. Sinc. 2:82-84.
|
|
|
|
|
Wiese K (2013). Sierra Nevada Wildflowers: A Field Guide to Common Wildflowers and Shrubs of the Sierra Nevada, Including Yosemite, Sequoia, and Kings Canyon National Parks. Rowman & Littlefield.
|
|