Full Length Research Paper
ABSTRACT
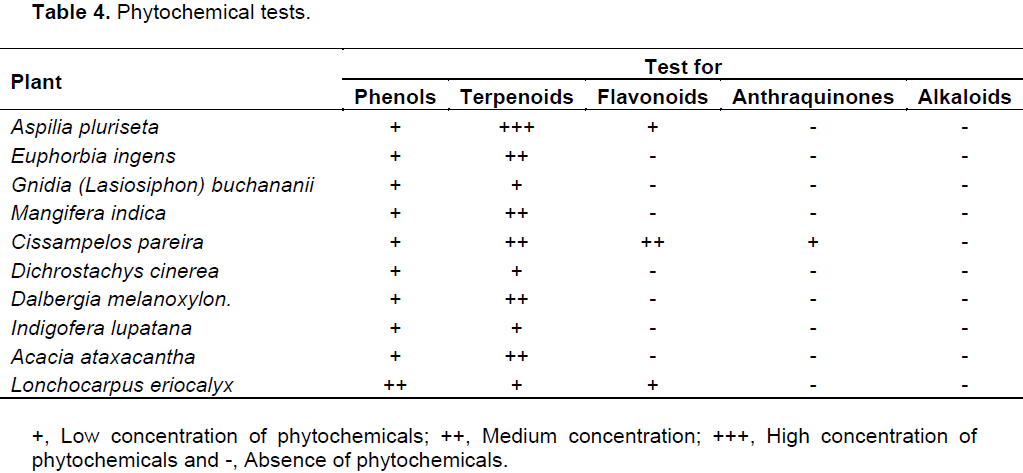
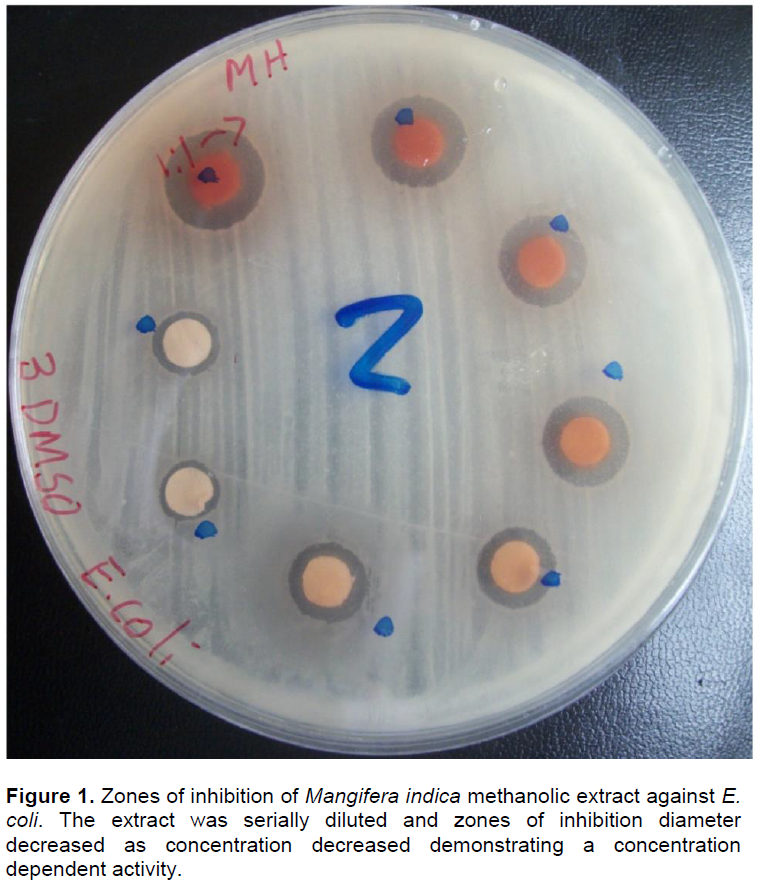
Tuberculosis is a serious chronic infectious disease affecting large global population. While efforts to control tuberculosis have intensified, they are challenged by rapid drug resistance development. For this reason, prospecting for compounds with potential antituberculous activity have been stepped up. The current study was done in a participatory appraisal manner to identify ten plants commonly used for management of “persistent coughs”. Bioassays were conducted against Mycobacterium tuberculosis (H37Rv ATCC 27294) using the BACTEC MGIT™ 960 system. This was followed by assay of toxicity of the extracts towards Vero cells (ATCC CCL-81). Six extracts showed remarkable antitubercular activity. Four extracts had complete inhibition (0 GU- Growth Units) of Mycobacterium tuberculosis. The extracts were tested for their general antimicrobial activity and found to be broad spectrum antimicrobials. The highest activity against Escherichia coli (15.3 mm) was by Cissampelos pareira, while Mangifera indica yielded the highest activity against Staphylococcus aureus (11.7 mm) and Candida albicans (12.0 mm). In addition, six crude methanolic extracts were found to be within the acceptable toxicity limit (CC50<90 µg/ml). The observed activity is attributable to phytochemicals in the extracts, including: phenols, terpenoids, flavonoids and anthraquinones. These findings could partly explain observed “positive” treatment outcome by indigenous people using these plant formulations.
Key words: Antibacterial activity, antituberculous activity, BACTEC MGIT™ 960 system, cytotoxicity, flavonoids, phytochemicals, terpenoids, Vero cells.
INTRODUCTION
RESEARCH DESIGN
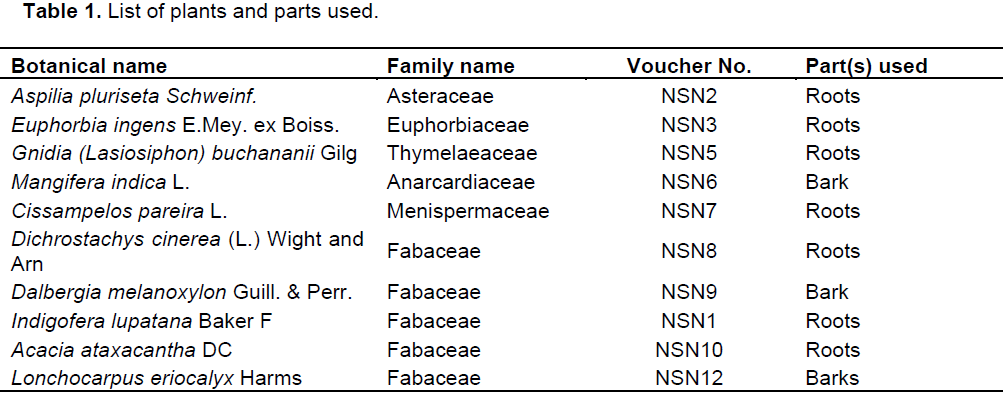
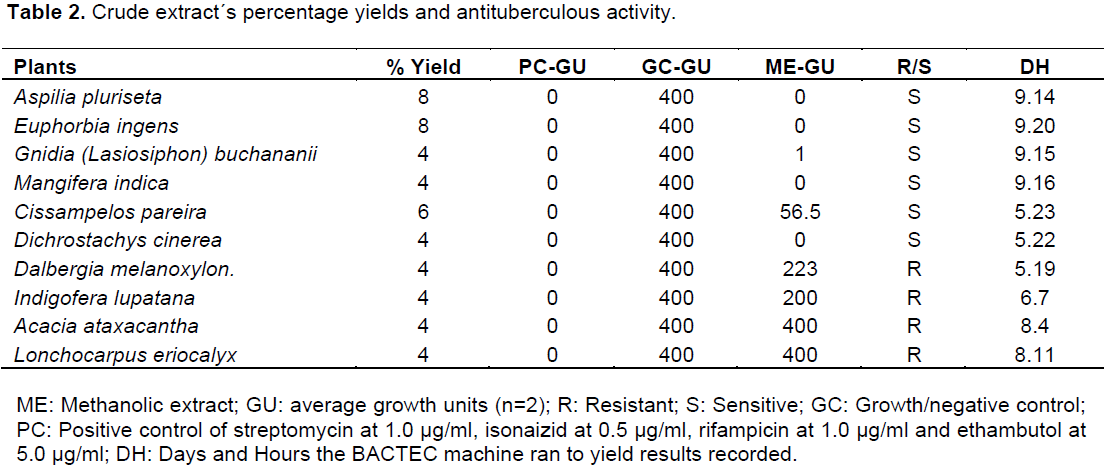
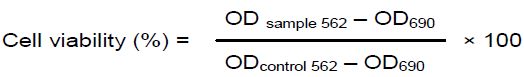
RESULTS
DISCUSSION
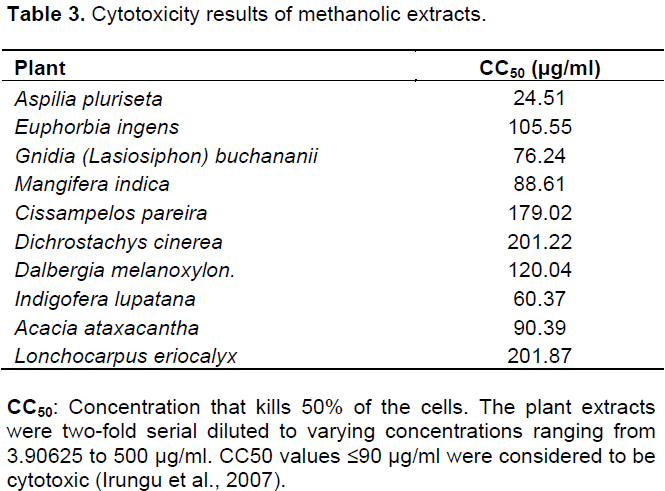
CONCLUSION
CONFLICT OF INTERESTS
ACKNOWLEDGEMENTS
REFERENCES
|
Abubakar EM (2009). Antibacterial activity of crude extracts of Euphorbia hirta against some bacteria associated with enteric infections. J. Med. Plants Res. 3(7):498-505. |
|
|
Al-Bayati FA, Al-Mola HF (2008). Antibacterial and antifungal activities of different parts of Tribulus terrestris L. growing in Iraq. J. Zhejiang Univ. Sci. 9:154-159. |
|
|
Antoun MD, Ramos Z, Vazques J, Oquendo I, Proctor GR, Gerena L, Franzblau SG (2001). Evaluation of the flora of Puerto Rico for in vitro antiplasmodial and antimycobacterial activities. Phytother. Res. 15:638-642. |
|
|
Aworet-Samseny RRR, Souza A, Kpahé F, Konaté K, Datté JY (2011). Dichrostachys cinerea (L.) Wight et Arn (Mimosaceae) hydro-alcoholic extract action on the contractility of tracheal smooth muscle isolated from guinea-pig. BMC Complement. Altern. Med. 11:18. |
|
|
Ayo RG, Amupitan JO, Zhao Y (2007). Cytotoxicity and anti-microbial studies of 1,6,8-trihydroxy-3-methyl-anthraquinone (Emodin) isolated from the leaves of Cassia nigricans Vahl. Afr. J. Biotechnol. 6:1276-1279. |
|
|
Becton Dickinson Company (2007). BBL MGIT Mycobacteria growth indicator Manual. Maryland, USA. P 123. |
|
|
Brennan PJ, Draper P (1994). In Tuberculosis: Pathogenesis, protection, and control (ed. Bloom, B. R.) (American Society for Microbiology, Washington DC). pp. 271-284. |
|
|
Cateni F, Zilic J, Falsone G, Hollan F, Frausin F, Scarcia V (2003). Preliminary biological assay on cerebroside mixture from Euphorbia nicaeensis. Farmaco 58:809817. |
|
|
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393(6685):537-544. |
|
|
Cole ST, Telenti A (1995). Drug resistance in Mycobacterium tuberculosis. Eur. Respir. Rev. 8:701S-713S. |
|
|
Cowan MM (1999). Plant products as anti-microbial agents. Clin. Microbiol. Rev. 12:564-582. |
|
|
Denizot F, Lang R (1986). Rapid colorimetric assay for cell growth and survival. J. Immunol. Meth. 89:271-277. |
|
|
Earl EA, Altaf M, Murikoli RV, Swift S, O'Toole R (2010). Native New Zealand plants with inhibitory activity towards Mycobacterium tuberculosis. BMC Complement. Altern. Med. 10:10-25. |
|
|
Edwards PA, Ericsson J (1999). Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 68:157-185. |
|
|
Glickman MS, Jacobs WR (2001). Microbial pathogenesis review of Mycobacterium tuberculosis: Dawn of a discipline. Cell Press 104:477-485. |
|
|
Gupta VK, Shukla C, Bisht GRS, Kumar S, Thakur RL (2010). Detection of anti-tuberculosis activity in some folklore plants by radiometric BACTEC assay. Lett. Appl. Microbiol. 52:33-40. |
|
|
Harborne JB (1984). Phytochemical methods: A guide to modern techniques of plant analysis, 2nd Edition. Chapman and Hall, New York, USA. |
|
|
Idu M, Erhabor JO, Efijuemue HM (2010). Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop. J. Pharm. Res. 9:110-118. |
|
|
Ighodaro OM, Agunbiade SO, Omole JO, Kuti OA (2012). Evaluation of chemical, nutritional, antimicrobial and antioxidant-vitamin profiles of Piliostigma thonningii leaves (Nigeria species). Res. J. Med. Plant 6:537-543. |
|
|
Irungu BN, Rukanga GM, Mungai GM, Muthaura CN (2007). In vitro antiplasmodial and cytotoxicity activities of 14 medicinal plants from Kenya. S. Afr. J. Bot. 73:204-207. |
|
|
Kaur GJ, Arora DS (2009). Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 9:30-41. |
|
|
Khalil IA (2003). Antimicrobial activity of extracts from leaves, stems and flowers of Euphorbia macroclada against plant pathogenic fungi. J. Phytopathol. 42:245-250. |
|
|
Lawn SD, Zumla AI (2011). Tuberculosis. Lancet 378:57-72. |
|
|
Lawson L, Emenyonu N, Abdurrahman ST, Lawson JO, Uzoewulu GN, Sogaolu OM, Ebisike JN, Parry CM, Yassin MA, Cuevas LE (2013). Comparison of Mycobacterium tuberculosis drug susceptibility using solid and liquid culture in Nigeria. BMC Res. Notes 6:215. |
|
|
Mann A, Amupitan JO, Oyewale AO, Okogun JI, Kolo I (2007). An ethnobotanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger state, Nigeria. J. Phytomed. Ther. 12:1-12. |
|
|
Mariita RM, Ogol CKP, Oguge NO, Okemo PO (2010a). Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop. J. Pharm. Res. 9:379-385. |
|
|
Mariita RM, Okemo PO, Orodho JA, Kirimuhuzya C, Otieno JN, Magadula JJ (2010b). Efficacy of 13 medicinal plants used by indigenous communities around lake Victoria, Kenya, against tuberculosis, diarrhoea causing bacteria and Candida albicans. Int. J. Pharm. Technol. 2:771-791. |
|
|
Mbaveng AT, Ngameni B, Kuete V, Simo IK, Ambassa P, Roy R, Bezabih M, Etoa F, Ngadjui BT, Abegaz BM, Meyer JJM, Lall N, Beng VP (2008). Anti-microbial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 116:483-489. |
|
|
Mohamed LT, El Nur BS, Abdelrahman MN (2010). The Antibacterial, antiviral activities and phytochemical screening of some Sudanese medicinal plants. EurAsia. J. Biosci. 4:8-16. |
|
|
Mosmann T (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. 65:55-63. |
|
|
Mwitari PG, Ayeka PA, Ondicho J, Matu EN, Bii CC (2013). Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS ONE 8(6):e65619. |
|
|
Ngeny LC, Magiri E, Mutai C, Mwikwabe N, Bii C (2013). Antimicrobial properties and toxicity of Hagenia abyssinica (Bruce) J.F.Gmel, Fuerstia africana T.C.E. Fries, Asparagus racemosus (Willd.) and Ekebergia capensis Sparrm. Afr. J. Pharm. Ther. 2:76-82. |
|
|
Ngoci SN, Matasyoh JC, Mwaniki CG, Mwendia CM (2012). Antibacterial activity of methanol root extract of Indigofera lupatana Baker F. Eastern J. Med. 17:11-16. |
|
|
Ngoci SN, Mwendia CM, Mwaniki CG (2011). Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. J. Anim. Plant Sci. 11(1):1364-1373. |
|
|
Ngoci SN, Ramadhan M, Ngari SM, Oduor PL (2014). Screening for antimicrobial activity of Cissampelos pareira L. methanol root extract. Eur. J. Med. Plants 4:45-51. |
|
|
Parekh J, Chanda S (2006). In-vitro antimicrobial activities of extractsof Launaea procumbens Roxb. (Labiateae), Vitis vinifera L. (Vitaceae) and Cyperus rotundus L. (Cyperaceae). Afr. J. Biomed. Res. 9:89-93. |
|
|
Rao A, Zhang Y, Muend S, Rao R. (2010). Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 54(12):50-62. |
|
|
Samy RP, Gopalakrishnakone P (2008). Review: Therapeutic potential of plants as anti-microbials for drug discovery. J. Evid. Based Complement. Altern. Med. 7:283-294. |
|
|
Snider DE Jr, Raviglione M, Kochi A (1994). In Tuberculosis: Pathogenesis, protection, and control (ed. Bloom, BR) (American Society of Microbiology, Washington DC). pp. 2-11. |
|
|
Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, Whittam TS, Musser JM (1997). Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. |
|
|
Sripathi SK, Sankari U (2010). Ethnobotanical documentation of a few Medicinal plants in the Agasthiayamalai region of Tirunelveli district, India. Ethnobot. Leaflets 14:173-181. |
|
|
Tabuti JRS, Kukunda CB, Waako PJ (2009). Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J. Ethnopharmacol. 127:130-136. |
|
|
Tomczykowa M, Tomczyk M, Jakoniuk P, Tryniszewska B (2008). Antimicrobial and antifungal activities of the extracts and essential oils of Bidens tripartite. Folia Histochem. Cytobiol. 46:389-393. |
|
|
Trombetta D, Castelli F, Sarpietro M, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G (2005). Mechanisms of anti-bacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49:2474-2478. |
|
|
Wheeler PR, Ratledge C (1994). In Tuberculosis: Pathogenesis, protection, and control (ed. Bloom, BR) (American Society of Microbiology, Washington DC). pp. 353-385. |
|
|
World Health Organization (WHO) (2013). Global tuberculosis report 2015. |
|
|
World Health Organization (WHO) (2014). Global Tuberclosis Report. |
|
|
Yuan E, Liu B, Ning Z, Chen C (2009). Preparative separation of flavonoids in Adinandra nitidaleaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 115:1158-1163. |
|
|
Zaleskis R (2006). Adverse effects of anti-tuberculosis chemotherapy. Eur. Respir. Rev. 47-49. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0